Abstract
Objectives
To investigate absolute and relative risk of serious infections in adult/elderly inflammatory bowel disease (IBD) diagnosed 2002–2017.
Methods
Nationwide, register-based cohort study of Swedish patients with IBD compared with general population matched reference individuals with regard to time to first serious infection, equal to hospital admission. Multivariable Cox regression estimated hazard ratios (HRs) for any serious infection. Secondary outcomes included site-specific infections, opportunistic infections and sepsis.
Results
We identified 47 798 individuals with IBD. During a follow-up of 329 000 person-years, they had 8752 first serious infections (26.6 per 1000 person-years). This compared with an incidence rate of 10.7 per 1000 person-years in matched reference individuals, corresponding to a 2.53-fold increased hazard of serious infections (95%CI = 2.47–2.59). The HR for serious infection in elderly-onset IBD was 2.01 (95%CI = 1.95–2.08). The relative hazard of serious infection was somewhat higher in Crohn’s disease (2.94; 95%CI = 2.81–3.06) than in ulcerative colitis (2.24; 95%CI = 2.17–2.31). The HR for serious infections was high in the first year of follow-up (5.17; 95%CI = 4.93–5.42). Individuals with IBD were at a particularly high relative hazard of gastrointestinal and opportunistic infections. The HR for sepsis was 2.47 (95%CI = 2.32–2.63). The relative rates for serious infections in IBD increased in recent years.
Conclusions
Patients with adult-onset IBD are at increased risk of serious infections, particularly gastrointestinal and opportunistic infections. Relative rates were highest just after IBD diagnosis, and seem to have increased in recent years.
Introduction
Inflammatory bowel disease (IBD), mainly consisting of Crohn’s disease (CD), and ulcerative colitis (UC), is a chronic inflammatory condition. Most medical treatments for IBD may dampen the immune system and both patients and their physicians are often concerned by the risk of serious infection. Almost one-third of hospital admissions for IBD are associated with infections, and these cause a significant morbidity [Citation1]. From genome wide association studies there is increasing evidence of an aberrant immune response including defense mechanisms against microbes in IBD [Citation2,Citation3]. Susceptibility loci involve both the innate and adaptive immune response towards a diminished diversity of commensal microbiota.
In our recent paper on mortality in adult IBD [Citation4], we found a 2.5-fold increased hazard of death from infections and parasitic conditions in patients diagnosed with IBD in 2002 or later. However, valid data on incident infections, and absolute risks of serious infections are missing and earlier studies have typically focused on patients with immunosuppressants rather than the overall IBD population [Citation5–8].
These findings emphasize the potential significance of infections among patients suffering from IBD, while also highlighting the contrasts of earlier research data. For clinicians meeting patients with IBD, accurate data on complications and serious infections are essential for correct patient management. In principle, risks of serious infections in IBD patients can meaningfully be communicated in at least three ways: (1) Absolute risk of serious infections and compared to comparable IBD-free subjects from the general population, with stratum specific estimates for different subtypes, phenotypes, age groups, duration of disease, and calendar periods. (2) Absolute risk of serious infections and compared to IBD-free comparators, with stratum specific estimates by time-varying exposures to different IBD medications. (3) Risk of serious infections in comparable IBD patients but with different IBD treatment exposures. While there are quite a few studies published in the third category [Citation9] and to some extent in the second category [Citation9], studies representing the first category are scant, restricted to tertiary centers and/or not representing more recent IBD care.
In this nationwide study, we therefore linked nationwide validated healthcare data to investigate the absolute and relative risk of first serious infections in patient with IBD compared to matched general population reference individuals overall and by clinically important strata.
Methods
Study design and setting
Through the personal identity number [Citation10] we linked population-based data from Swedish national healthcare registers (Supplementary Table S1) to examine the risk of serious infections among incident patients with adult onset (≥18 years) and elderly (≥60 years) onset IBD, diagnosed between 2002–2017 (exposed cohort) compared to matched reference individuals from the general population (unexposed cohort) using a prospective cohort study design.
Definition of IBD exposure and data sources
We defined IBD as having ≥2 international classification of disease (ICD) codes in the Swedish Patient Registers (ICD codes, or having one such ICD code and ≥1 colorectal biopsy indicating inflammation, or specifically UC, CD or IBD-U (see Supplementary Table S2 for ICD codes and histopathology SnoMed codes). Data were obtained from the Patient Register [Citation11] or the nationwide histopathology cohort ESPRESSO [Citation12].
The Swedish Patient register started in 1964, and contains nationwide data since 1987. In 1997, daycare surgery was added, and in 2001 hospital-based outpatient data. The ESPRESSO cohort contains data on 2.1 million unique individuals undergoing a gastrointestinal (GI) biopsy that was evaluated in any of Sweden’s 28 pathology departments [Citation12]. Previous validations have found that 93% of patients with ≥2 records of IBD [Citation13], and 95% of those with ≥1 IBD listing and a colorectal biopsy with a relevant SnoMed code representing inflammation have IBD [Citation14].
Because we wanted to examine modern-day patients, we restricted our follow-up to 2002 and later. The Swedish National Patient Register added data on hospital-based outpatient care in 2001, but individuals appearing in the Register as new cases of IBD in 2001 may have represented prevalent cases that had never been inpatients and were therefore identified for the first time in the outpatient register during 2001. Since we aimed to examine truly incident cases, we therefore restricted the study to patients diagnosed 2002–2017. Data on emigration and death were obtained from the Swedish Total Population Register [Citation15]. This register has a high completeness for both death and emigration [Citation15].
IBD subtypes were categorized into UC, CD or IBD-U according to the first two IBD records [Citation16] or according to first ICD code when patients were identified through 1 ICD code and 1 biopsy record. Further, during follow-up IBD patients were classified according to the Montreal classification () (Supplementary Table S3).
Table 1. Baseline characteristics of incident adult inflammatory bowel disease (≥18 years) in Sweden 2002–2017.
Reference individuals
Statistics Sweden identified up to ten reference individuals per IBD individual. Reference individuals were retrieved from the Total Population Register, which covers the complete Swedish population. Matching criteria included age, sex, county, and year of birth.
Exclusion criteria
We applied the following exclusion criteria (living outside Sweden in the last five years, history of any of the following in the last five years: diagnosis/procedure code of organ transplantation, HIV, cancer, congenital immunodeficiency, tuberculosis, and hepatitis B/C) (Supplementary Table S4). These individuals were excluded since they had chronic infections that may increase the risk of serious infectious events after IBD, but also because they may influence the management of IBD per se.
Bowel surgery
We examined validated procedures related to bowel surgery [Citation17]. Inpatient surgery has been recorded through the Patient Register since 1964, with daycare surgery being added in 1997. Surgery data are recorded using NOMESCO Classification of Surgical Procedures codes (Supplementary Table S5).
Outcome: serious infections
Our main outcome was time to first serious infection after IBD diagnosis (or corresponding dates in reference individuals), defined as hospitalizations with an infection as the main or contributory diagnosis (Supplementary Table S6). Patients were considered at risk of first infection from their second record of IBD diagnosis (or the corresponding index date for reference individuals) until first infection or censoring (emigration, change of IBD status, i.e. reference individuals who were later diagnosed with IBD, death, or December 31, 2017, whichever occurred first). An individual could hence enter the study as reference individual (matched to an IBD patient), and would then contribute events and person-years to the reference cohort. Later this same individual could develop IBD and would then stop being a reference individual and contribute information to the IBD cohort. Secondary outcomes included site-specific serious infections (GI, respiratory, skin, urinary and other infections), opportunistic infections, and sepsis (for definitions see Supplementary Table S6).
Statistics
We calculated absolute incidence rates per 1000 person-years and reported the cumulative incidence of serious infections by age of IBD onset. To compare the rates of infection in IBD cases and reference individuals we computed hazard ratios (HRs) and 95% confidence intervals (CIs) using multivariable Cox proportional hazards models adjusted for sex, age, index date, and place of residency [Citation18]. The proportional hazards assumption was checked for the main results by testing the Schoenfeld residuals followed by visual inspection of the complementary log-log survival curves. Marked departures from the proportional hazards assumption were observed, therefore we report HRs separately by length of follow-up (0–<1, 1–<5, 5–<10, ≥10 years). In addition, we estimated time varying relative survival probabilities stratified by calendar period (2002–2006, 2007–2011, 2012–2017) of IBD onset. We used a Wald test to examine a potential interaction for differences in HRs according to calendar period.
We report estimates for clinically relevant strata, such as IBD subtype, age of IBD onset (young adult onset (18–<40 years), middle age onset (40–<60 years), and elderly onset IBD (60-)). We also created strata based on time-varying exposures such as the Montreal classification (E or L class), and IBD surgery performed during follow-up, in which a patient and his/her matched comparators contributed follow-up time for that category from the date of the corresponding register listing.
Finally, we calculated the risk of any infection minus GI infections in IBD. GI infections may suffer from detection bias, and sometimes represent a flare from IBD rather than a clinically significant infection.
Statistical analyses were performed using R statistical software (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria) and the survival package (version 2.38, Therneau, T (2015), https://CRAN.R-project.org/package=survival). An HR whose 95%CI excluded 1.0 was considered statistically significant.
Ethical considerations
This study was approved by the Ethics Review Board in Stockholm. Informed consent was not deemed necessary because the study was strictly register-based [Citation19].
Results
Background data
The main analysis of this paper was based on 47 798 individuals with adult onset IBD (UC: n = 27,045, CD: n = 13 249, and IBD-U: n = 7504). The female-male ratio was 1:1, and the median age at IBD diagnosis was 41 years (interquartile range: 28-59). One in five patients were diagnosed at the age of 60 years or above (“elderly”; n = 11 059) (). During follow-up, and 5103 (10.7%) individuals with IBD underwent bowel surgery (). More than 13 000 patients (28.0%) had a follow-up of at least ten years.
Any serious infection
During a follow-up of 329,000 person-years, we observed 8752 first serious infections in the IBD group (26.6 per 1000 person-years) (; ). This compared with an incidence rate of 10.7 per 1000 person-years in matched reference individuals, corresponding to a crude rate difference of 15.9/1000 (i.e., approximately 1.6 additional serious infections among 100 IBD patients followed for a year). Absolute rates of serious infections and rate differences were higher in CD vs UC () and (for all IBD subtypes) older patients versus younger ones (; ). The rate difference (IBD vs matched reference individuals) in elderly onset IBD patients corresponded to approximately 3.1 additional serious infections per 100 IBD patients followed for a year.
Figure 1. Kaplan–Meier curves; proportion of patients with adult IBD (≥18 years) and matched reference individuals at risk of first serious infection during follow-up.
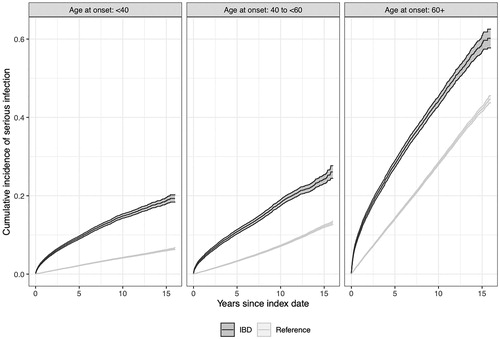
Table 2. Absolute incidence of serious infections (any infectious agent) per 1000 person-years in adult onset inflammatory bowel disease (≥18 years) in Sweden 2002–2017.
The overall HR of serious infection was 2.53 (95%CI = 2.47–2.59) (). The relative hazard of serious infection was higher in patients with CD (2.94; 95%CI = 2.81–3.06) than in patients with UC (2.24; 95%CI = 2.17–2.31). In UC, we observed the lowest HR in patients with proctitis (1.53; 95%CI = 1.39–1.69) and HRs for serious infection in IBD-U were close to that of CD (). While the HR was highest in the youngest age-group (HR = 3.89), it was lowest in elderly-onset IBD (HR = 2.01, ).
Table 3. Hazard ratio of serious infections (any infectious agent) in adult inflammatory bowel disease (≥18 years) in Sweden 2002–2017 compared to matched reference individuals without IBD.
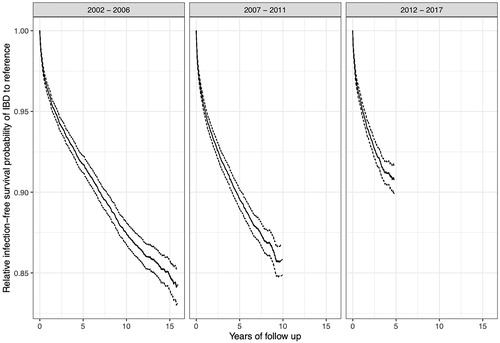
The HR of serious infections associated with IBD increased over calendar periods (), and HRs were highest in the first year of follow-up (5.17; 95%CI = 4.93–5.42) (see for HRs beyond first year after IBD diagnosis). The relative first serious infection free survival declined rapidly during the first year or two of follow-up, and thereafter declined less rapidly at an approximately constant rate (). This pattern was similar for the three different calendar periods, though the relative infection free survival was lower in patients diagnosed during the later calendar periods compared to the earlier (I.e. infection rates were higher) (p < 0.001).
Location and type of infections
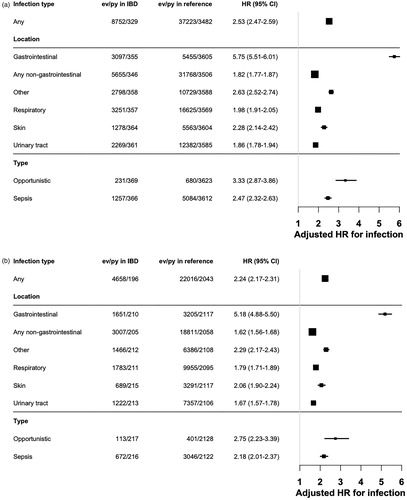
Patients with IBD were at an increased risk of infections at all sites, but especially prone to develop GI infections. Excluding GI infections, the HR for any infection in IBD was 1.82 (95%CI = 1.77–1.87) (). GI infections made up one third of all serious infections both in the first year after IBD and thereafter.
Figure 3. (a) Hazard ratio of serious infections at different sites (opportunistic infections and sepsis regardless of site) in adult inflammatory bowel disease (≥ 18 years) as compared to matched general population reference individuals 2002–2017. Numbers next to the forest plot represent the number of specific first serious infections (in that category) in IBD-patients per multiples of 1000 person-years, Hazard Ratio (95% Confidence Interval). (b) Hazard ratio of serious infections at different sites (opportunistic infections and sepsis regardless of site) in adult ulcerative colitis (≥18 years) as compared to matched general population reference individuals 2002–2017. Numbers next to the forest plot represent the number of specific first serious infections (in that category) in UC-patients per multiples of 1000 person-years, hazard ratio (95% confidence interval). (c) Hazard ratio of serious infections at different sites (opportunistic infections and sepsis regardless of site) in adult Crohn's disease (≥18 years) as compared to matched general population reference individuals 2002–2017. Numbers next to the forest plot represent the number of specific first serious infections (in that category) in CD patients per multiples of 1000 person-years, Hazard Ratio (95% confidence interval).
The HR for opportunistic infections was 3.33 (95%CI = 2.87–3.86) and 2.47 for sepsis (95%CI = 2.32–2.63) []. Opportunistic infections made up 2.9% of all infections in the first year after IBD diagnosis, and 2.5% of infections beyond the first year.
We found similar patterns of relative rates of location and type of infections in UC and CD [].
Discussion
Main findings
This population-based study found an increased risk of serious infections in IBD, but absolute rates were low (roughly 2–3% of IBD patients had a serious infection each year compared with 1% of the reference individuals). While relative hazards were highest in newly diagnosed IBD patients (the first year of follow-up), we also detected a long-term increased risk of serious infections. HRs were increased in all sites and highest for gastrointestinal infections. Patients with IBD were at a 2–3-fold increased rate of opportunistic infections and sepsis. The highest rates in absolute terms were seen in the elderly onset IBD patients, whereas the highest relative rates were seen in young adults. Finally, the relative risk of having a record of infection seems to have increased over calendar periods.
Comparison with earlier literature
The last decade has seen a large number of papers on (serious) infections in IBD according to treatment [Citation8,Citation20–23], or exploring risk factors for infection [Citation24]; however we are unaware of any large-scale study examining the baseline risk of serious infections (including opportunistic infections) across different strata in a modern sample of IBD patients. Our study contained more than 47 000 individuals with IBD diagnosed since 2002. The overall incidence of first serious infections in our study of incident IBD patients was 27 per 1000 person-years (or 2.7% every year). This compares with 8.4 per 1000 person-years in patients unexposed to thiopurines and anti-Tumor Necrosis Factor (TNF) agents to 10.5, 18.9, and 22.4 per 1000 person-years in those exposed to thiopurine monotherapy, anti-TNF monotherapy, and combination therapy in a recent French population-based study of serious infections in a mix of incident and prevalent IBD cases [Citation8].
Unsurprisingly, we found an increased risk of gastrointestinal (GI) infections. Interestingly our research group has previously found an increased risk of GI infections also before IBD [Citation25]. It is possible that such infections play a role in triggering the disease, but it seems that infections are more common also after IBD diagnosis. We cannot rule out that mucosal damage in IBD predisposes to GI infection but to some extent this risk increase might also be explained by detection bias (GI cultures are very often performed when a patient presents with a flare). Patients with IBD have a defect mucosal immunology and the disrupted epithelial barrier may predispose to local infections [Citation26]. Rodeman et al have previously demonstrated a 2.9-fold increased risk for one such infection, Clostridium difficile [Citation27].
However, GI infections may also be an incidental finding in IBD and may actually represent a flare of IBD. While a recent study found that true CMV (Cytomegalovirus) colitis marks poor prognosis in UC [Citation28], the role of CMV infection in IBD remains under debate [Citation29].
However, even when excluding GI infections, we found a positive association between IBD and serious infections.
Also, the risk of respiratory infections was increased. This confirms earlier reports of an increased risk of pneumonia [Citation30]. With a median follow-up of 2 years, Long et al. noted an 82% increased risk of pneumonia in IBD. Guidelines have encouraged vaccinations against Streptococcus pneumoniae, and also against another respiratory disease (influenza) [Citation31] but despite any such vaccinations, Swedish IBD patients were still at a 98% increased risk of respiratory infection. We also noted an increased risk of urinary tract infections in IBD (HR = 1.86). Data are sparse on urinary tract infections in IBD, but when Varda et al reviewed US emergency department visits 2006–09 they found that IBD is an independent risk factor for urinary tract infections (HR = 1.3) [Citation32].
Most data on sepsis in IBD have been linked to specific treatment regimens, surgery [Citation33] or specific situations. Several population-studies have observed increased mortality from sepsis (or infections, and then most often with a septic component) in patients with IBD [Citation34–36]. The relative risk of infectious death or sepsis in CD have varied between 1.52 [Citation34], 3.15 [Citation36], and 4.27 [Citation35] and for UC between 1.26 [Citation36], 1.28 [Citation35], and 2.08 [Citation34]. While these studies suggest an increased risk of sepsis the risk estimates vary by a factor of almost 3, and they only consider death and not incident disease. From our Swedish cohort, we have previously reported a 2.5-fold increased risk of death from infectious diseases in adults (95%CI = 2.0 − 3.1) [Citation4]. This latter figure was almost identical to our findings on incident sepsis in IBD (HR = 2.47; 95%CI = 2.32–2.63). We confirm earlier research that has noted an increased risk of opportunistic infections but add to that research [Citation31] through larger numbers, longer follow-up and more precise risk estimates. During our follow-up, 231 individuals with IBD and 680 reference individuals had a diagnosis of opportunistic infection, corresponding to an HR of 3.33 (95%CI = 2.87–3.86).
Finally, we can only speculate as to why our study detected an increase in serious infection over time. Among explanations are changes in IBD management, including frequency of healthcare visits, use of immunosuppressive medications, and improved diagnostics for infectious disease.
Strengths and limitations
Our population-based data were obtained from nationwide, extensively validated registers [Citation11,Citation15,Citation37]. Overall the Patient register has a positive predictive value (PPV) of 85–95% for most diagnoses [Citation11]. Of special importance to the current study, having ≥2 ICD codes for IBD had a PPV of 93% [Citation13], and having ≥1 ICD code for IBD and non-specific inflammation on colorectal biopsy had a PPV of 95% [Citation14]. Linkage of registers through the personal identity number [Citation10] allowed us to follow-up patients. Virtually 100% of deaths, and 91% of emigrations are reported to the Population Registers within 30 days and with a higher proportion over time [Citation15]. Our exclusion of individuals who had lived outside Sweden in the last five years, decreases the risk of misclassified disease onset. Patients with IBD were retrieved from all hospitals in Sweden. This minimizes selection bias that may otherwise be a problem in studies from tertiary centers. Likewise, our pathology data used to ascertain IBD were retrieved from all pathology departments in Sweden (n = 28). Furthermore, earlier data have shown that use of pathology data to define disease onset will shift the date of diagnosis in >40% of patients with IBD [Citation38]. We chose to restrict our cohort to patients with an incident diagnosis since 2002. The main reasons for only examining patients diagnosed in the last 15–20 years is that they better reflect the situation and prognosis of today’s IBD patients but also that the sensitivity of the Swedish Patient Register increased significantly in 2001 when hospital-based outpatient data were added to the register.
The purpose of the current study was to examine the risk of infections in IBD and in comparison to the general population. To better estimate that risk we carefully excluded individuals with conditions that have been strongly linked to infectious disease (or that per se may impact on the treatment and follow-up of IBD patients) prior to start of follow-up, including organ transplantation, HIV, cancer, immunodeficiency, tuberculosis, and hepatitis B/C. The large number of patients allowed us to examine subset of IBD patients (for instance patients undergoing surgery or being diagnosed in old age) but importantly also the type of infection (given the large number of infectious events). Patients with IBD have an aberrant innate immune response [Citation3] that is likely to increase susceptibility to opportunistic infections, but also management in terms of immunosuppressive therapies (primarily corticosteroids), surgery, malnutrition, and the use of intravenous catheters/devices may increase the risk of infections.
This study also had some limitations. This was a register-based study and Swedish registers do not contain data on laboratory measures, radiology or endoscopy results. Hence, we were unable to stratify the risk of infection according to disease severity. Our data on disease extent or location and risk of serious infections were limited to ICD code data, classified according to the Montreal classification and need to be interpreted with caution [Citation39]. For instance, we cannot rule out that L1 and L3/LX disease overlap. We did not have data on body mass index, and hence could not examine serious infections according to degree of malnutrition. Neither did we have any data on smoking, which is important to the risk of e.g. respiratory infections.
We found the highest relative rates of infection in the first year of follow-up (HR = 5.17, as opposed to HR = 1.71 after more than 10 years of follow-up). This was expected, since disease activity and use of corticosteroids tend to be high just after diagnosis [Citation40]. However, we cannot rule out that some of the early infections were detected due to the increased surveillance assigned to IBD patients just after diagnosis. Our main comparison group consisted of the general population, who do not undergo close supervision for infections, while most IBD patients have their hematology and CRP regularly checked initially, especially after the initiation of drug treatment. This may explain why the relative risk for incident infections in IBD (HR = 2.53; current study) was higher than the risk of death from infections and parasitic conditions in our paper on mortality in adult IBD (HR = 2.1) [Citation4].
Drug data were not included in our models as such data were not needed to achieve the prespecified aim of this paper and since analyses of IBD drug use and risk of infections compared to the general population would require extensive analyses that should be reported separately. Furthermore, the lack of vaccinations data in Swedish national registers means that we were unable to examine the potential protective effect of such interventions in patients with IBD.
Implications
Our study found a 2.53-fold increased hazard of serious infections in IBD. This relative rate was particularly high in the first year after diagnosis and has increased over time-periods. Physicians and patients should be aware of the high risk of infections in IBD, especially just after IBD diagnosis, and treat infections properly and promptly. Other research from our group has shown that IBD patients are still at an increased risk from dying from infections [Citation4]. Still it should be underlined that while relative risks were high, the absolute risks were limited.
Conclusion
This nationwide population-based study found a 2.53-fold increased rate of serious infection in IBD. Both absolute and relative risks were higher in CD than UC. While the highest relative risks were observed in the young, the highest absolute excess risks were seen in the elderly. The relative rates seem to have increased over calendar periods. The high rates in newly diagnosed IBD patients and in patients undergoing bowel surgery merit extra attention and surveillance.
Author contributions
Guarantor: O.O. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: O.G., J.H., O.O., J.F.L., M.C.S. Acquisition of data: O.O. Analysis: M.S., O.O. Interpretation of data: all authors. Drafting of the manuscript: J.F.L., J.H., O.O., M.S., O.G. Critical revision of the manuscript for important intellectual content and approval of final version: all authors.
Data sharing statement: No additional data available due to Swedish regulation.
Supplemental Material
Download PDF (153.6 KB)Disclosure statement
JFL coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen corporation. OO has been PI for projects (unrelated to the current paper) at KI partly financed by investigator-initiated grants from Janssen and Pfizer. Karolinska Institutet has received fees for lectures (OO) and participation on advisory boards (OO) from Janssen, Ferring, and Takeda. OG coordinates a study on behalf of the Swedish IBD quality register (SWIBREG), this study has received funding from Pfizer. OG have received consultancy fees from Ferring, Janssen, ViforPharma, Takeda and Pfizer.
Additional information
Funding
References
- Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohn's Colitis. 2013;7(2):107–112.
- Ek WE, D'Amato M, Halfvarson J. The history of genetics in inflammatory bowel disease. Ann Gastroenterol. 2014;27(4):294–303.
- Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124.
- Olen O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. 2020 69:453–461.
- Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–2339.
- Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679–1686.
- Wheat CL, Ko CW, Clark-Snustad K, et al. Inflammatory Bowel Disease (IBD) pharmacotherapy and the risk of serious infection: a systematic review and network meta-analysis. BMC Gastroenterol. 2017;17(1):52.
- Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155(2):337–46 e10.
- Singh S, Facciorusso A, Dulai PS, et al. Comparative risk of serious infections with biologic and/or immunosuppressive therapy in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18(1):69–81 e3.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450.
- Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–114.
- Jakobsson GL, Sternegard E, Olen O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–221.
- Nguyen LH, Ortqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5(11):P986–P995.
- Ludvigsson JF, Almqvist C, Bonamy AE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136.
- Everhov AH, Sachs MC, Malmborg P, et al. Changes in inflammatory bowel disease subtype during follow-up and over time in 44,302 patients. Scand J Gastroenterol. 2019;54(1):55–63.
- Forss A, Myrelid P, Olen O, et al. Validating surgical procedure codes for inflammatory bowel disease in the Swedish National Patient Register. BMC Med Inform Decis Mak. 2019;19(1):217.
- Cox DR. Regression models and life‐tables. J Royal Statis Soc Series B (Methodological). 1972;34(2):187–202.
- Ludvigsson JF, Haberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508.
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65.
- D'Haens G, Reinisch W, Colombel JF, et al. Five-year safety data from ENCORE, a European Observational Safety Registry for adults With Crohn's disease treated with infliximab [remicade(r)] or conventional therapy. J Crohns Colitis. 2017;11(6):680–689.
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107(9):1409–1422.
- Nyboe Andersen N, Pasternak B, Friis-Moller N, et al. Association between tumour necrosis factor-alpha inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ. 2015;350:h2809.
- Toruner M, Loftus EV, Jr., Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–936.
- Axelrad JE, Olen O, Askling J, et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a Nationwide Case-Control Study. Clin Gastroenterol Hepatol. 2019;17(7):1311–1322 e7.
- Martini E, Krug SM, Siegmund B, et al. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4(1):33–46.
- Rodemann JF, Dubberke ER, Reske KA, et al. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(3):339–344.
- Oh SJ, Lee CK, Kim YW, et al. True cytomegalovirus colitis is a poor prognostic indicator in patients with ulcerative colitis flares: the 10-year experience of an academic referral inflammatory bowel disease center. Scand J Gastroenterol. 2019;54(8):976–983.
- Garrido E, Carrera E, Manzano R, et al. Clinical significance of cytomegalovirus infection in patients with inflammatory bowel disease. World J Gastroenterol. 2013;19(1):17–25.
- Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108(2):240–248.
- Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443–468.
- Varda BK, McNabb-Baltar J, Sood A, et al. Urolithiasis and urinary tract infection among patients with inflammatory bowel disease: a review of US emergency department visits between 2006 and 2009. Urology. 2015;85(4):764–770.
- Mark-Christensen A, Kjaer MD, Ganesalingam S, et al. Increasing incidence of pelvic sepsis following ileal pouch-anal anastomosis for ulcerative colitis in Denmark: a nationwide cohort study. Dis Colon Rectum. 2019;62(8):965–971.
- Bitton A, Vutcovici M, Sewitch M, et al. Mortality trends in crohn's disease and ulcerative colitis: a population-based study in Quebec, Canada. Inflamm Bowel Dis. 2016;22(2):416–423.
- Jussila A, Virta LJ, Pukkala E, et al. Mortality and causes of death in patients with inflammatory bowel disease: a nationwide register study in Finland. J Crohns Colitis. 2014;8(9):1088–1096.
- Bernstein CN, Nugent Z, Targownik LE, et al. Predictors and risks for death in a population-based study of persons with IBD in Manitoba. Gut. 2015;64(9):1403–1411.
- Ludvigsson JF, Svedberg P, Olen O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437.
- Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395(10218):123–131.
- Shrestha S, Olen O, Eriksson C, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish National Patient Register. Scand J Gastroenterol. 2020;55(4):430–435.
- Bewtra M, Kaiser LM, TenHave T, et al. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19(3):599–613.