Abstract
Background
Strict adherence to a gluten-free diet usually leads to clinical and histological remission in celiac disease. Few studies have investigated the prevalence of persistent symptoms in a celiac population. We aimed to study the impact of gastrointestinal symptoms on general health in a large number of treated celiac patients, and describe the prevalence of persistent gastrointestinal symptoms and investigate associated factors.
Methods
Adults with celiac disease filled out background questions, the Celiac Symptom Index (CSI) and the celiac disease adherence test (CDAT) in a web-based national survey. Participants who reported gastrointestinal symptoms during the previous week also recorded the gastrointestinal symptom rating scale-irritable bowel syndrome version (GSRS-IBS). Statistical analysis included chi-squared test, t-test, correlation, and linear regression.
Results
Of 3834 participants (82% women; mean age 47 years), 54% reported gastrointestinal symptoms the previous week, and 30% of these had CSI score ≥45, indicative of the relatively poor quality of life (vs. 5% among those without gastrointestinal symptoms). The prevalence of persistent gastrointestinal symptoms (GSRS-IBS ≥30) was 40% and the most prominent symptoms were bloating (44%) and pain (37%). Age, sex, symptoms at the time of diagnosis, comorbidity, dietary adherence and CeD-specific health were significantly associated with gastrointestinal symptoms (p < .001).
Conclusion
In this national cross-sectional study among participants with celiac disease, persistent gastrointestinal symptoms were frequent, and were associated with a high symptom burden and reduced CeD-specific health. Several factors were associated with gastrointestinal symptoms, but more research is needed to find the cause of persistent symptoms in patients with celiac disease.
Introduction
Celiac disease (CeD) is an immune disease of the small intestine with local, abdominal and general manifestations. The only current treatment is a gluten-free diet, as gluten proteins from wheat, rye and barley trigger CeD [Citation1]. Strict adherence to a gluten-free diet usually leads to clinical and histological remission, reduced risk of gastrointestinal (GI) malignancies and improvements in nutritional status and quality of life (QoL) [Citation2,Citation3]. Still, many patients have continued GI symptoms and/or reduced general health even though they are dietary adherent [Citation4–8].
Persistent GI symptoms in CeD resemble irritable bowel syndrome (IBS), a common functional GI disorder with an estimated global prevalence of 11% [Citation9]. IBS is characterized by recurrent abdominal pain, bloating and change in frequency and/or appearance of stool [Citation10]. Previous studies found that 20–58% of CeD patients may suffer from persistent IBS-like symptoms [Citation4,Citation7,Citation8,Citation11–13], but there are few large, nationwide studies [Citation7,Citation8,Citation13].
Gluten exposure is a common cause of persistent symptoms in CeD [Citation5,Citation6,Citation14], but rates for strict dietary adherence vary. Strict adherence to a gluten-free diet ranged from 42 to 91% in a 2009 review [Citation15], probably illustrating heterogeneity in dietary adherence assessment and lack of standardized validated methods.
Studies show that CeD patients have reduced QoL compared to the general population, and poor gluten-free diet adherence and IBS-like symptoms seem to be contributing factors [Citation11,Citation16,Citation17]. However, studies assessing QoL and GI symptoms in CeD are sparse, vary in design and often have poorly validated outcomes [Citation1,Citation18,Citation19]. Recently, the disease-specific patient-reported outcome measures (PROMSs) for general health, specific symptoms and dietary adherence have become available, enabling larger samples of patients with appropriate methodology [Citation1,Citation18].
In Norway, 98% of the population has internet access [Citation20], and the use of social media is common, facilitating studies in a larger number of patients via digital patient communities. CeD affects 1–2% of the population [Citation21] (50,000–100,000 Norwegians).
Moreover, Norwegian patients with a verified CeD diagnosis receive governmental reimbursement through the public health care system, meaning that study patients who receive reimbursement have confirmed CeD.
In this national cross-sectional study, our primary aim was to study the impact of GI symptoms on general health in a large sample of treated CeD patients. Our secondary aim was to describe the prevalence of persistent GI symptoms and investigate associated factors.
Methods
Study design and participants
The cross-sectional web-based ‘Survey about celiac disease and health’ was performed in October 2018. We invited adult CeD patients (age 18-75) on a strict gluten-free diet for at least 12 months, and with biopsy verified diagnosis, or serology verified in childhood/adolescence [Citation22]. We also asked whether they received governmental reimbursement. Participants were invited through digital patient communities, and the Norwegian Celiac Association (∼9500 members) invited participants by e-mail and the association’s journal.
Variables
Background characteristics were obtained from the survey. The Norwegian counties were categorized according to the Regional Health Authorities: Northern, Central, Western and Southeastern [Citation23].
GI symptoms were assessed by the question: “Did you experience GI symptoms during the previous week?” Answering “yes” on this question created a subgroup that was asked to fill out the Gastrointestinal Symptom Rating Scale, IBS version (GSRS-IBS) [Citation24]. A total score ≥ of 30 was empirically set as the cut-off for GI symptoms (range 13–91) based on our previous experience [Citation25,Citation26]. A mean score ≥ of 4 defined moderate to severe symptoms for the five sub-dimensions; satiety, abdominal pain, diarrhea, constipation and bloating [Citation24,Citation26].
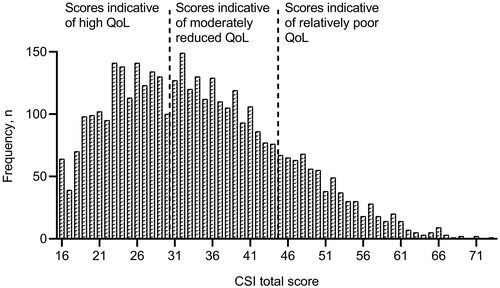
General health and disease-specific symptoms were assessed by celiac symptom index (CSI) [Citation27]. CSI total score range is 16–80 and ≤30 indicates high QoL and excellent gluten-free diet adherence, while ≥45 indicates relatively poor QoL and worse gluten-free diet adherence [Citation27]. Scores 31–44 were interpreted as moderately reduced QoL.
Gluten-free diet adherence was measured using the 7-item celiac disease adherence test (CDAT), where a total score of ≤12 is considered ‘adequate adherence’ (range 7–35) [Citation28]. Self-rated dietary adherence was also assessed by the question: ‘How well do you adhere to a gluten-free diet?’ (very well, pretty well, fair, not well, do not adhere).
The Norwegian version of the questionnaires CSI and CDAT were previously translated, re-translated and approved by the original author Dr. Leffler. The survey was pilot tested in 17 Norwegian Celiac Association members, and minor changes were made in CSI and CDAT. In CSI: ‘hunger pain’ was changed to ‘cramps associated with an empty stomach’ in item seven, and ‘food cravings’ was changed to ‘urge to eat’ in item ten. In CDAT: ‘generally intended’ was added in parenthesis in items four and five.
Statistical methods
Descriptive results are presented as mean (standard deviation (SD), range) and frequency (%). Independent samples t-test and chi-squared test were used to compare two groups. We estimated Pearson’s correlation coefficient, r, with 95% confidence interval (CI). Logistic regression was used to compare participants with and without GI symptoms when adjusting for age, sex and comorbidity. Multiple linear regression analysis was used to study associations between GSRS-IBS and years since diagnosis, symptoms at the time of diagnosis, comorbidity, CDAT and CSI. We adjusted for age and sex, and analyses of CDAT and CSI were also adjusted for comorbidity. All tests were two-sided, and p < .05 was considered statistically significant. IBM SPSS Statistic version 27 (SPSS Inc. Chicago, IL) was used for statistical analyzes.
Ethics, approval and pretesting
The study was conducted in accordance with the Helsinki Declaration and approved by the Regional Committee for Medical and Health Research Ethics (REC) [Citation29] 24 August 2018, with the identification 2018/1055, REC South East B.
All authors have reviewed and approved the final manuscript.
Results
After exclusion of 293 persons without definite CeD diagnosis or with disease duration <1 year, the final study sample consisted of 3834 participants. There was a female predominance (82%), and the mean (SD) age was 47.0 (range 19–79) years (). Biopsy verified CeD diagnosis was reported by 97%, while 2.6% reported a diagnosis based on serology tests from childhood/adolescence, and 99% received governmental reimbursement. GI symptoms the previous week were reported by 54%. Females and younger participants were most likely to report GI symptoms (p < .001). There were also differences between the groups for ’region of residence’, ‘first-degree family members with CeD’, ‘years since diagnosis’, ‘symptoms at the time of diagnosis’, ‘self-reported severity of symptoms after exposure to gluten’ and ‘comorbidity’.
Table 1. Participant characteristics and gastrointestinal (GI) symptoms the previous week.
Ongoing symptoms and general health
The mean (SD) CSI score was 34.2 (11.0), and 41% had scores indicative of high QoL and excellent gluten-free diet adherence, while 19% had scores indicative of relatively poor QoL and worse gluten-free diet adherence (). Participants with GI symptoms the previous week had significantly higher CSI scores than those without GI symptoms (mean 39.4 vs. 28.1), also after adjustment for age, sex and comorbidity (p < .001). Among participants with GI symptoms, 30% had CSI scores indicative of relatively poor QoL and worse gluten-free diet adherence (vs. 5% among those without GI symptoms).
Figure 1. Celiac Symptom Index (CSI, total score range 16-80), n = 3834. QoL, quality of life. Score ≤30 is associated with high QoL and excellent gluten-free diet adherence, score ≥45 is associated with relatively poor QoL and worse gluten-free diet adherence and score 31–44 is associated with moderately reduced QoL.
Adherence to a gluten-free diet
The mean (SD) CDAT score was 13.4 (3.5) (), and 44% had ≤12, indicating adequate adherence. Self-rated adherence was very well in 89%. Participants with GI symptoms the previous week had higher CDAT scores than those without (mean 14.2 vs. 12.5), also after adjustment for age, sex and comorbidity (p < .001). In contrast, participants with GI symptoms reported significantly better self-rated adherence compared to those without GI symptoms, also after adjustment for age, sex and comorbidity (p < .001).
Table 2. Coeliac disease adherence test (CDAT) and self-reported adherence to a gluten-free diet, by GI symptoms the previous week, n = 3834.
Severity and type of GI symptoms
Among the 2057 (54%) participants who reported GI symptoms the previous week, 1545 had a total GSRS-IBS score ≥ of 30, giving a prevalence of persistent GI symptoms of 40% in the total study sample. The most prominent symptoms were bloating and pain ().
Table 3. Mean (standard deviation, SD) per sub-dimension of gastrointestinal symptom rating scale-irritable bowel syndrome version (GSRS-IBS), and frequencies (%) of moderate to severe discomfort (GSRS-IBS ≥4), n = 2057.
Factors associated with severity of GI symptoms
Age was weakly negatively correlated with GSRS-IBS (r=–0.07), and GSRS-IBS was lower in men than in women (mean difference −3.50, 95% CI (–5.00, −2.00), p < .001). GSRS-IBS scores were significantly higher in participants with symptoms at the time of diagnosis and with comorbidity (adjusted regression coefficients (95% CIs) were 5.45 (3.56, 7.33) and 4.74 (3.60, 5.88), respectively). GSRS-IBS was significantly positively associated with CDAT and CSI (p < .001) ().
Table 4. Simple linear regression (univariable) and multiple regression (multivariable) analysis for the association between GSRS-IBS and listed variables.
Discussion
This national cross-sectional survey among almost four thousand treated CeD patients showed that persistent GI symptoms were frequent (40%), and were associated with a high symptom burden and reduced QoL.
Our findings are partly in concordance with previous studies, depending on the assessment methods. A US study found that 52% had IBS-like symptoms at the time of diagnosis, and 22% still had symptoms one year after initiating a gluten-free diet [Citation8]. A Finnish study found that 23% of long-term well-treated CeD patients had persistent GI symptoms [Citation7]. Furthermore, the prevalence of persistent GI symptoms in our study was higher compared to a meta-analysis from 2013, which showed a pooled prevalence of IBS-like symptoms (ROME criteria) [Citation30] of almost 30% among CeD patients strictly adherent to a gluten-free diet [Citation31].
Among participants with GI symptoms, as many as 30% had CSI scores indicative of reduced CD-specific health, which is associated with poor QoL and worse gluten-free diet adherence. These findings are in line with previous smaller studies reporting reduced QoL and ongoing symptoms in treated CeD patients [Citation4,Citation11], although there seems to be a gender difference [Citation16]. However, Silvester et al. found reduced health and ongoing symptoms in only 8% one year after initiating a gluten-free diet [Citation8]. This contrasts with our finding, possibly due to methodologic differences.
Over half of our participants reported comorbidity, most commonly thyroid disease, IBS, rheumatic disorder and fatigue. The association between CeD and other autoimmune conditions is well known [Citation3], including type 1 diabetes [Citation32] and autoimmune thyroid disease [Citation33]. We asked about actual diagnoses, however, there is a possibility that some of the reported comorbidities are self-perceived. Additionally, the true occurrence of some of the comorbidities is questionable. Both fatigue and IBS could possibly be a symptom of CeD rather than a diagnosis by itself. According to the ROME criteria [Citation30], organic GI disease should be excluded before a diagnosis of IBS can be made. Two previous and comparable surveys, the Canadian Celiac Health Survey [Citation16] and Characteristics of Adult Celiac Disease in the USA: Results of a National Survey [Citation34], found that many participants received one or more diagnoses before the CeD diagnosis was made, most commonly anemia, stress, IBS and chronic fatigue syndrome. Furthermore, in the US survey, nearly 40% of the participants were previously diagnosed with IBS, which contrasts with our finding of 11% [Citation34]. CeD might predispose to symptoms resembling IBS [Citation4], possibly due to low-grade inflammation [Citation8,Citation31,Citation35,Citation36].
Adequate adherence to a gluten-free diet according to CDAT was unexpectedly low (44%). In comparison, two US surveys reported adequate adherence of 76% and 96%, by use of CDAT [Citation8,Citation37]. On the other hand, we found a very high rate of self-reported adherence (89% very well). However, self-reported adherence is known to overestimate compliance [Citation38,Citation39]. Furthermore, we found that self-rated adherence was higher in participants with GI symptoms the previous week compared to participants without GI symptoms. In contrast, participants with GI symptoms had lower adherence according to CDAT, which is in line with previous studies who found that gluten intake is the most common cause of persistent symptoms [Citation5,Citation6,Citation14]. The variation in dietary adherence can be explained by the different assessment tools. CDATmeasures non-specific symptoms, self-efficacy, risk behaviour and perceived ability to adhere to a gluten-free diet [Citation28], and thereby measure the risk of gluten consumption rather than actual gluten intake. Additionally, the tool is validated in a US population, and one can speculate whether the tool fits more to a society where eating outside of the home is more common than in Norway.
Adequate gluten-free diet adherence was associated with a lower GSRS-IBS score, which is in line with previous findings [Citation5,Citation14]. GI symptoms are also associated with poorer QoL, and a positive correlation between dietary adherence and QoL is previously found [Citation11,Citation17]. In contrast, a clinical study by Silvester et al. did not find a significant correlation between patients fulfilling ROME criteria and adherence to a gluten-free diet [Citation8]. Furthermore, we found that women had a significantly higher GSRS-IBS score than men, which is also in line with previous findings [Citation4,Citation7]. Similarly, IBS is also shown to be more common in females [Citation9]. Comorbidity also predicted GSRS-IBS score in our study, as participants suffering from comorbidity were more likely to have a higher GSRS-IBS score than those without comorbidity. Others have also reported an association between comorbidity and reduced QoL [Citation40]. Furthermore, we found a lower GSRS-IBS score with a longer duration of CeD, which supports previous findings [Citation4,Citation7]. Lastly, symptoms at the time of diagnosis were associated with a higher GSRS-IBS score.
Previously, a range of different methodologies has been used to assess GI symptoms in CeD [Citation1,Citation18]. The standardized validated tool GSRS-IBS is commonly used [Citation24]. However, a cut-off for the presence or severity of GI symptoms is not established, as GSRS-IBS is developed to assess responsiveness to change. Furthermore, studies exploring QoL in CeD also use a variety of different tools for assessment, and generic instruments like the short form 36 health survey questionnaire are widely used [Citation41]. Comparability of GI symptoms and QoL in CeD patients is therefore difficult, and generic instruments may possibly not adequately reflect challenges in CeD. A strength in our study is the use of the disease-specific questionnaire CSI [Citation27], which includes both intestinal and extraintestinal symptoms. We found a significant positive association between CSI and GSRS-IBS, which is not surprising, as several of the questions in CSI relate to GI symptoms. Obviously, another reason for this association could be the direct impact of GI symptoms on general health. Others have found that abdominal symptoms can impair QoL in CeD [Citation4,Citation11,Citation12], and opposite, it has also been suggested that reduced QoL in CeD is a predictor for abdominal symptoms [Citation42].
A large number of participants is a strength of the present study, and all Norway counties were represented. To limit selection bias, we invited people through different channels and used a general title (‘Survey about celiac disease and health’). We believe that the study sample resembles a population of ‘true’ CeD because as many as 99% of the participants received reimbursement, meaning they had biopsy or serology (from childhood/adolescence) verified CeD.To compare, the previously mentioned Canadian and US surveys found a prevalence of 86% and 75% of biopsy verified CeD, respectively [Citation16,Citation34]. Moreover, our participants were not recruited from health care, and thereby more likely to represent a real-life CeD population.
A standardized dietetic assessment is considered the most objective and non-invasive method for monitoring adherence to a gluten-free diet [Citation43,Citation44]. CDAT is found to be highly correlated with expert dietitian evaluation and performs better than serology anti‐tissue transglutaminase antibodies [Citation35]. However, biopsy histology is considered the gold standard to measure CeD activity [Citation44], but due to the nature of the design in this study, we were not able to compare self-reported adherence with nutritional, serological or histological assessment. Therefore, gluten intake cannot be excluded as the cause of ongoing symptoms in our study. Another limitation of this study is the lack of assessment of dietary intake, for example, known triggers of IBS symptoms like FODMAPs. Others have shown that a gluten-free diet is lower in fiber and several micronutrients, and higher in saturated fat and as compared to a gluten-containing diet [Citation45–47]. However, it is unknown whether this less healthy profile of the gluten-free diet is related to ongoing GI symptoms in patients with celiac disease.
In conclusion, this study highlights the high prevalence of persistent GI symptoms and high symptom burden in treated adult CeD patients. There is a need for follow-up of patients with ongoing symptoms and reduced general health despite a strict gluten-free diet and should include evaluation of dietary adherence by a clinical dietician, assessment of serology and histology, and investigation of other causes of ongoing symptoms like refractory CeD and malignancies. More research is needed to find possible causes and treatment methods for the supposedly large group of patients with ongoing symptoms despite remission of CeD. Large randomized trials are needed to find out whether IBS treatment, like for example the low FODMAP diet, can treat ongoing symptoms in CeD.
| Abbreviations | ||
| CeD | = | celiac disease |
| CDAT | = | celiac disease adherence test |
| CI | = | confidence interval |
| CSI | = | celiac symptom index |
| GI | = | gastrointestinal |
| GSRS-IBS | = | gastrointestinal symptom rating scale-IBS version |
| IBS | = | irritable bowel syndrome |
| PROMs | = | patient reported outcome measures |
| QoL | = | quality of life |
| REC | = | Regional Committee for Medical and Health Research Ethics |
| SD | = | standard deviation |
| STROBE statement | = | strengthening the reporting of observational studies in epidemiology |
Acknowledgements
We thank all participants for the valuable sharing of their experience. We would also like to thank the Norwegian Celiac Association for contributions to the development of the survey, and for distributing the survey.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ludvigsson JF, Ciacci C, Green PH, et al. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut. 2018;67:1410–1424.
- Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115(3):191–195.
- Haines ML, Anderson RP, Gibson PR. Systematic review: the evidence base for long-term management of coeliac disease. Aliment Pharmacol Ther. 2008;28(9):1042–1066.
- O’Leary C, Wieneke P, Buckley S, et al. Celiac disease and irritable bowel-type symptoms. Am J Gastroenterol. 2002;97(6):1463–1467.
- Leffler DA, Dennis M, Hyett B, et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5(4):445–450.
- Dewar DH, Donnelly SC, McLaughlin SD, et al. Celiac disease: management of persistent symptoms in patients on a gluten-free diet. World J Gastroenterol. 2012;18(12):1348–1356.
- Laurikka P, Salmi T, Collin P, et al. gastrointestinal symptoms in celiac disease patients on a long-term gluten-free diet. Nutrients. 2016;8(7):429.
- Silvester JA, Graff LA, Rigaux L, et al. Symptoms of functional intestinal disorders are common in patients with celiac disease following transition to a gluten-free diet. Dig Dis Sci. 2017;62(9):2449–2454.
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721.
- Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407.
- Usai P, Manca R, Cuomo R, et al. Effect of gluten-free diet and co-morbidity of irritable bowel syndrome-type symptoms on health-related quality of life in adult coeliac patients. Dig Liver Dis. 2007;39(9):824–828.
- Barratt SM, Leeds JS, Robinson K, et al. Reflux and irritable bowel syndrome are negative predictors of quality of life in coeliac disease and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23(2):159–165.
- Hauser W, Gold J, Stein J, et al. Health-related quality of life in adult coeliac disease in Germany: results of a national survey. Eur J Gastroenterol Hepatol. 2006;18(7):747–754.
- Abdulkarim AS, Burgart LJ, See J, et al. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97(8):2016–2021.
- Hall NJ, Rubin G, Charnock A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2009;30(4):315–330.
- Cranney A, Zarkadas M, Graham ID, et al. The Canadian Celiac Health Survey. Dig Dis Sci. 2007;52(4):1087–1095.
- Burger JPW, de Brouwer B, IntHout J, et al. Systematic review with meta-analysis: dietary adherence influences normalization of health-related quality of life in coeliac disease. Clin Nutr. 2017;36(2):399–406.
- Hindryckx P, Levesque BG, Holvoet T, et al. Disease activity indices in coeliac disease: systematic review and recommendations for clinical trials. Gut. 2018;67(1):61–69.
- Canestaro WJ, Edwards TC, Patrick DL. Systematic review: patient-reported outcome measures in coeliac disease for regulatory submissions. Aliment Pharmacol Ther. 2016;44(4):313–331.
- Statistisk sentralbyrå. Andel med tilgang til internett (webpage). [cited 2020 Apr 18]. Available from: http://www.medienorge.uib.no/statistikk/medium/ikt/347
- Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63(8):1210–1228.
- Al-Toma A, Volta U, Auricchio R, et al. European society for the study of coeliac disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7(5):583–613.
- Ministry of Health and Care Services. The Department of Hospital Ownership (webpage). [cited 2020 Jul 24]. Available from: https://www.regjeringen.no/en/dep/hod/organisation-and-management-of-the-ministry-of-health-and-care-services/Departments/the-department-of-hospital-ownership/id1413/
- Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38(9):947–954.
- Skodje GI, Sarna VK, Minelle IH, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–539.
- Jorgensen SF, Reims HM, Frydenlund D, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol. 2016;111(10):1467–1475.
- Leffler DA, Dennis M, Edwards George J, et al. A validated disease-specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7(12):1328–1334.
- Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7(5):530–536.
- Regional Committee for Medical and Health Research Ethics (REC) (webpage). [cited 2020 Oct 14]. Available from: https://rekportalen.no/#/omrek
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–1279.
- Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(4):359–365.
- Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123–1132.
- Roy A, Laszkowska M, Sundström J, et al. Prevalence of celiac disease in patients with autoimmune thyroid disease: a meta-analysis. Thyroid. 2016;26(7):880–890.
- Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96(1):126–131.
- Silvester JA, Graff LA, Rigaux L, et al. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2016;44(6):612–619.
- Rubio-Tapia A, Rahim MW, See JA, et al. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105(6):1412–1420.
- Villafuerte-Galvez J, Vanga RR, Dennis M, et al. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment Pharmacol Ther. 2015;42(6):753–760.
- Silvester JA, Weiten D, Graff LA, et al. Is it gluten-free? Relationship between self-reported gluten-free diet adherence and knowledge of gluten content of foods. Nutrition. 2016;32(7–8):777–783.
- Lovik A, Skodje G, Bratlie J, et al. Diet adherence and gluten exposure in coeliac disease and self-reported non-coeliac gluten sensitivity. Clin Nutr. 2017;36(1):275–280.
- Hauser W, Stallmach A, Caspary WF, et al. Predictors of reduced health-related quality of life in adults with coeliac disease. Aliment Pharmacol Ther. 2006;25(5):569–578.
- Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164.
- Lohiniemi Mmkkplpc S. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand J Gastroenterol. 2000;35(9):947–949.
- Ciacci C, Cirillo M, Cavallaro R, et al. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66(3):178–185.
- Leffler DA, Edwards George JB, Dennis M, et al. A prospective comparative study of five measures of gluten‐free diet adherence in adults with coeliac disease. Aliment Pharmacol Ther. 2007;26(9):1227–1235.
- Skodje GI, Minelle IH, Rolfsen KL, et al. Dietary and symptom assessment in adults with self-reported non-coeliac gluten sensitivity. Clin Nutr ESPEN. 2019;31:88–94.
- Myhrstad MCW, Slydahl M, Hellmann M, et al. Nutritional quality and costs of gluten-free products: a case-control study of food products on the Norwegian marked. Food Nutr Res. 2021;65:6121.
- Dennis M, Lee AR, McCarthy T. Nutritional considerations of the gluten-free diet. Gastroenterol Clin North Am. 2019;48(1):53–72.
