 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Increasing clarithromycin resistance has led to the need for an alternative first-line therapy for the eradication of Helicobacter pylori (H. pylori) in Korea, and bismuth containing quadruple therapy (BQT) and tailored therapy (TT) have been proposed as alternative regimens. The aim of this study was to compare the eradication rates of BQT and TT as first-line H. pylori eradication therapies.
Methods
H. pylori infection was diagnosed using the rapid urease test or dual-priming oligonucleotide-based multiplex polymerase chain reaction (DPO-PCR) during endoscopy. Patients positive for H. pylori were divided into two groups; those tested using the rapid urease test received empirical BQT (the BQT group) whereas those tested by DPO-PCR received TT (the TT group). Eradication rates, adverse events, and overall medical costs, which included diagnostic test and eradication regimen costs, were compared.
Results
Three hundred and sixty patients were included in the study (TT group 178, BQT group 182). The modified intention-to-treat eradication rates of BQT and TT were 88.2% (142/161) and 80.3% (118/147), respectively (p = .055), and corresponding eradication rates in the per-protocol population were 88.8% (142/160) and 81.4% (118/145) (p = .07). Compliance and adverse event rates were similar in the two groups. Average medical costs were $90.3 per patient in the TT group and $75.5 in the BQT group (p = .000).
Conclusions
Empirical BQT and tailored therapy were similar in terms of H. pylori eradication rate, safety, and tolerability, but BQT was more cost-effective.
Background
Helicobacter pylori (H. pylori) is the main cause of chronic gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer. Thus, the eradication of H. pylori infections dramatically decreases peptic ulcer recurrence and prevents gastric cancer [Citation1]. Clarithromycin triple therapy regimens consisting of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin for 7–14 days are globally accepted first-line therapies for the eradication of H. pylori [Citation2]. However, eradication rates associated with clarithromycin triple therapy have declined to unacceptable levels and clarithromycin resistance has been reported to be the main reason for eradication failure. Therefore, new therapeutic regimens are needed in areas of high clarithromycin resistance [Citation3].
The recent Maastricht V consensus conference recommended clarithromycin susceptibility testing when considering clarithromycin triple therapy as a first-line therapy, except in areas with low clarithromycin resistance rates (<15%) [Citation4]. On the other hand, in areas with high clarithromycin resistance rates, bismuth quadruple therapy (BQT; bismuth subsalicylate, tetracycline, metronidazole, and PPI) has been recommended as a first-line empirical therapy because its efficacy is unaffected by clarithromycin resistance [Citation4–6]. In Korea, the 3rd Clinical Practice Guideline for H. pylori published in 2013 recommended clarithromycin triple therapy as the first-line therapy, despite falling eradication rates, and also that BQT be considered as the first-line regimen in areas of high clarithromycin resistance [Citation6]. In Korea, clarithromycin resistance varies regionally from 8.5 to 37.5% and has been increasing over recent years [Citation7–9]. Accordingly, testing for clarithromycin resistance is becoming increasingly important prior to treatment decision making.
Clarithromycin acts by binding to 23S ribosomal RNA (rRNA), and, thus, inhibiting protein synthesis, but cannot bind to 23S rRNA harboring a point mutation [Citation10]. In most cases, resistance to clarithromycin is caused by point mutations at the 2142 or 2143 positions of the 23S rRNA gene. Dual-priming oligonucleotide-based multiplex polymerase chain reaction (DPO-PCR) can be used to detect clarithromycin resistance as well as H. pylori, and unlike conventional PCR, DPO-PCR blocks nonspecific binding sites and suppresses imperfect primer annealing, and thus, increases sensitivity and specificity [Citation11,Citation12]. In addition, testing is straightforward and rapid, and thus, DPO-PCR test enables tailored therapy (TT) without H. pylori culture.
BQT is a recognized, effective second-line therapy for H. pylori according to domestic and international practice guidelines [Citation5,Citation6,Citation13,Citation14]. According to one meta-analysis of primarily western studies, the eradication rate of BQT as a first-line therapy was 77.6% by intention-to-treat (ITT) analysis [Citation15]. However, recent studies on the efficacy of BQT as a first-line treatment are lacking. The aim of this study was to compare the efficacies of first-line BQT and TT for the treatment of H. pylori, and its secondary aim was to compare their safeties and cost-effectiveness. In addition, we also evaluated the efficacy of levofloxacin-based therapy as a second-line treatment after BQT or TT failure.
Methods
Patients and study design
Nine hundred and fifty-seven patients that underwent upper gastrointestinal endoscopy at Inha University hospital and testing for H. pylori from August 2016 to April 2018 were prospectively screened. After exclusions, patients were assigned to one of two groups using a random number generator. For H. pylori testing, one group was tested using a rapid urease test (RUT), whereas the other group was tested by DPO-PCR. Of those found to be H. pylori positive, those tested by RUT received empirical BQT and those tested by DPO-PCR received TT analysis. Patients that had previously received H. pylori eradication therapy and those with a severe illness, such as liver cirrhosis, chronic kidney disease, or heart failure, were excluded, as were pregnant or possibly pregnant patients. Eradication success rates, adverse events related to medications, and compliances with treatments were investigated and compared. All patients provided informed consent, and the study was approved by the Institutional Review Board of Inha University (2016-01-21) and registered as a trial at ClinicalTrials.gov (NCT03361267).
Test for H. pylori
Two gastric tissue specimens were obtained from antrum and corpus RUT (Bio Helicobacter Test, Shinsung Pharmaceutical Co., Ltd, Suwon, Korea) or DPO-PCR. RUT status was assessed within 24 h of sampling. DPO-PCR was performed using Seeplex® H. pylori-ClaR ACE Detection kits (Seegene Inc., Seoul, Korea). This test can detect the A2142G and A2143G mutations in 23S rRNA. If two-point mutations were of the wild type, H. pylori was considered susceptible, but if one of the two-point mutations were present, clarithromycin resistance was deemed positive.
Treatment for H. pylori
In the BQT group, the BQT regimen was prescribed for 7 days. This regimen consisted of rabeprazole 20 mg twice a day, metronidazole 500 mg three times a day; bismuth 300 mg four times a day, and tetracycline 500 mg four times a day for 7 days. In the TT group, clarithromycin triple therapy was prescribed in patients with clarithromycin-sensitive H. pylori, and BQT was prescribed in patients with clarithromycin-resistant H. pylori for 7 days. Clarithromycin triple therapy consisted of rabeprazole 20 mg twice a day, amoxicillin 1000 mg twice a day and clarithromycin 500 mg twice a day for 7 days. Patients that remained positive after the first-line therapy were retreated with a levofloxacin-based regimen, which consisted of rabeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and levofloxacin 500 mg once daily for 7 days.
Follow-up and outcomes
Patients returned to our clinics within 2 weeks after commencing drug administration to assess drug compliance and adverse effects, which were assessed using a self-report questionnaire. Non-compliance was defined as taking <90% of the prescribed study drug. H. pylori eradication was confirmed by 13C-urea breath testing (UBT; Korea Otsuka Pharmaceutical Co., Ltd, Seoul, Korea) at least 4 weeks after completing eradication therapy. The cut-off value used for the 13C-urea breath test was 2.5%.
Medical costs
To assess cost-effectiveness, the two groups were compared with respect to costs of diagnostic tools and of the eradication regimen, excluding common costs such as endoscopy and biopsy. The cost of the RUT was $11.7, and that of DPO-PCR was $33.6, and the costs of the seven-day clarithromycin triple regimen and of the 7-day BQT were $32.0 and $24.2, respectively. The cost of the UBT used for eradication confirmation was $22.6, and of the 7-day levofloxacin based triple therapy was $25.6.
Sample size calculation
Based on a review of previous studies, the eradication rates of TT and BQT were assumed to be 91% and 78% [Citation8,Citation15]. Sample size was calculated based on a non-inferiority trial at a both-sided alpha significance level of 0.05, a power of 0.90, and a non-inferiority margin of 0.13:
The calculated sample size was 162 per group, and, thus, assuming a dropout rate of 10%, at least 180 patients were required per group.
Statistical analysis
The primary study end point was H. pylori eradication rate, which was evaluated by ITT analysis, modified intention-to-treat (mITT), and per protocol (PP) analyses. The ITT analysis included all enrolled patients who received an eradication drug, and mITT analysis included patients who took at least one dose of the prescribed drugs and received UBT after eradication therapy. PP analysis was conducted after excluding patients who took <90% of the prescribed drugs, those that did not return for follow up, and those that violated the study protocol. The secondary end points were frequency of adverse events, drug compliance, and medical cost. p-values of <.05 were considered statistically significant, and the analysis was conducted using SPSS ver. 19 for Windows (SPSS Inc. Chicago, IL, USA).
Results
Demographics of the study groups
A flow chart of patients that received either of the two treatment regimens is provided in . Nine hundred fifty-seven patients were initially screened for H. pylori infection by RUT or DPO-PCR, and 493 (51.5%) were positive. Of these, 133 were excluded because they refused treatment or to participate in the study. The remaining 360 patients were allocated to the BQT or TT group according to diagnostic method. Demographic and clinical characteristics are summarized in and were not significantly different in the two groups ().
Figure 1. Study flowchart. TT: tailored therapy; BQT: bismuth quadruple therapy; ITT: intention-to-treat; mITT: modified intention-to-treat; PP: per protocol.
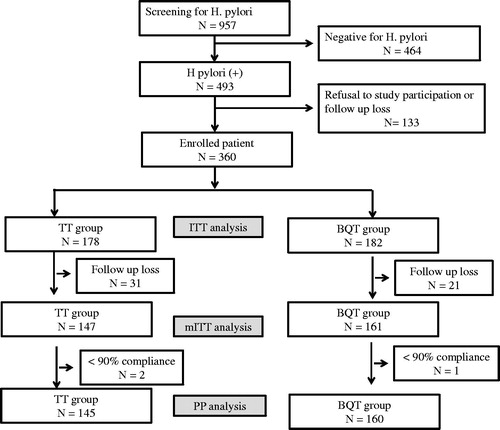
Figure 2. Flowchart of patients in the tailored therapy group. TT: tailored therapy; ITT: intention-to-treat; mITT: modified intention-to-treat; PP: per protocol; BQT: bismuth quadruple therapy.
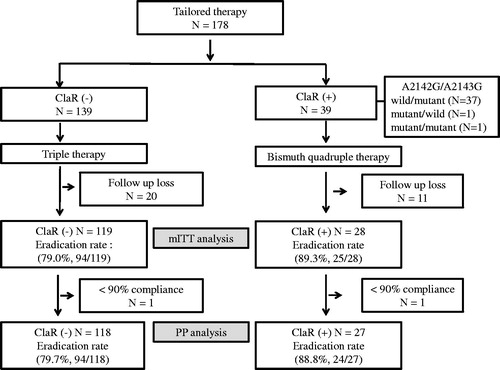
Table 1. Basic characteristics of patients in the two study groups.
Outcomes of BQT and TT
ITT eradication rates in the BQT and TT groups were 78.0 and 66.3%, respectively. Corresponding mITT and PP eradication rates were 88.2 and 80.3% and 88.8 and 81.4%, respectively ().
Table 2. Helicobacter pylori eradication efficacies in the two groups.
Patient compliance and adverse events
Compliance was assessed for 308 patients that completed treatment and was no different in the two groups (98.6% (145/147) in the TT group and 99.4% (160/161) in the BQT group. Most patients did not experience a drug-related adverse or minor side effect (). Two patients discontinued drugs due to adverse effects such as nausea and diarrhea, but recommenced treatment after symptoms improved.
Table 3. Compliance and adverse events in the two groups.
TT group
The clarithromycin resistance rate in the TT group was 21.9% (39/178). The A2142G mutation was detected in one patient (2.6%, 1/39), while the A2143G mutation was detected in 37 (94.9%, 37/39). Both mutations were detected in one patient (2.6%, 1/39). For the 139 patients with clarithromycin-sensitive H. pylori, the eradication rate of clarithromycin triple therapy was 79.0% by mITT analysis and 79.7% by PP analysis. On the other hand, for the 39 patients with clarithromycin-resistant H. pylori, corresponding eradication rates for BQT were 89.3 and 88.8% respectively.
Levofloxacin triple therapy
In this study, 40 patients (25 patients in the TT group and 15 patients in the BQT group) who failed the first-line therapy were treated with the second-line levofloxacin-based triple therapy. Twenty-one of these 40 patients achieved eradication, but 13 failed second-line eradication. Six of the 40 patients were not followed up. Eradication rates for levofloxacin triple therapy as determined by ITT and PP analysis were 52.5 and 61.8%, respectively.
Medical cost
Average medical costs were calculated by summing the costs of diagnosis and treatment for first-line eradication treatment and by assuming patients that failed first-line therapy received second-line therapy. Average medical costs per patient were then $90.3 and $75.5 for the TT and BQT groups, respectively (p < .001).
Discussion
In Korea, clarithromycin resistance varies regionally, and therefore, the efficacies of H. pylori eradication regimens depend on areas studies. Thus, we sought to determine the regional prevalence of clarithromycin resistance in the Incheon area, which abuts western Seoul. In this region, the clarithromycin resistance rate was previously reported to be 37.4%, though it should be added patients in whom H. pylori eradication had failed, and, thus, clarithromycin resistance may have been overestimated [Citation16]. In the present study, clarithromycin resistance was 21.9% (39/178) which is still high resistant rate and that empirical clarithromycin triple therapy is not a suitable first-line regimen for H. pylori eradication in the region.
According to the Maastricht V guidelines, in a setting with high clarithromycin resistance (>15%), choice of therapy should be considered on the frequencies of metronidazole and of combined clarithromycin and metronidazole resistance. However, antimicrobial susceptibility testing by culture and antibiogram is difficult and time-consuming, and not available in all institutions. In addition, the success rate of H. pylori culture is relatively low at 55–73% [Citation17]. Furthermore, if empirical bismuth-based quadruple therapy is expected to be used, it is not advisable to perform antimicrobial susceptibility testing because the risk of tetracycline resistant strains is extremely low and metronidazole resistance has little effect on eradiation rate [Citation18]. Therefore, empirical BQT provides a good alternative first-line eradication therapy in areas of high clarithromycin resistance.
Based on the previous studies, we considered tailored therapy would have a higher eradication rate than that of empirical BQT [Citation8,Citation15]. However, we found the eradication rates of BQT and TT were not significantly different (88.8 versus 81.4, p = .07). In particular, in patients with clarithromycin-sensitive H. pylori in the TT group, the eradication rate of clarithromycin triple therapy was only 79.4% (94/118), which was lower than that previously reported in a Korean study [Citation8]. Although DPO-PCR can diagnose A2142G and A2143G variants, it does not provide a comprehensive view of clarithromycin resistance and cannot identify resistance to other antibiotics. Nevertheless, point mutation of 23S rRNA, which usually involves the substitution of guanine with adenine at nucleotides 2142 and 2143 is detected by DPO-PCR and is known to be associated with clarithromycin resistance. Substitution of adenine with cytosine at position 2142 is also less frequently observed [Citation19]. Other point mutations in 23S rRNA, such as of A2115G, G2141A, T2117C, T2182C, T2289C, G224A, C2245T, and C2611A, have also been reported, but their clinical significances are unknown. Macrolide resistance caused by the efflux pump system also plays an important role in promoting multidrug H. pylori resistance [Citation19]. Thus, despite the absence of A2142G or A2143G mutations, it can be inferred the eradication rate was lower than expected in the present study because of other point mutations or the involvements of efflux pump systems.
Maastricht V guideline suggests that H. pylori eradication rate should be more than 80% according to ITT analysis and more than 90% according to PP analysis [Citation4]. In this study, due to by using BQT therapy and clarithromycin based triple therapy as a first line therapy, the eradication rate became below the Maastricht V guideline. We should study further to improve eradication rate and efficacy in aspect to the complication and medical cost. Furthermore, fluoroquinolone resistance has recently been increased in our region. In our present study, RUT was used in the BQT group and DPO-PCR in the TT group to diagnose H. pylori infection, and, thus, H. pylori diagnosis rates may have differed in the two groups. Originally, we intended to test all participants using RUT and DPO-PCR, regardless of treatment regimen. However, our IRB committee determined that it was unethical to select an eradication regimen irrespective of clarithromycin resistance results, and thus, we modified the study design. RUT is reliable and its sensitivity and specificity are 90% and 95–100% [Citation20,Citation21]. False-negatives are more common than false positives, and thus, negative results do not exclude the possibility of H. pylori infection, but false positive are uncommon. The Seeplex® ClaR-H. pylori ACE detection kit has been reported to have a sensitivity of 97.7% and a specificity of 83.1% for the diagnosis of H. pylori versus culture testing [Citation22], and, thus, our diagnostic method is not likely to have biased diagnosis or eradication rates due to false positive results.
In this study, levofloxacin-based triple therapy was used as the second-line therapy following failed the first-line treatment. In the Maastricht V consensus, fluoroquinolone-containing triple therapy, such as levofloxacin, or bismuth quadruple therapy is recommended as second-line treatment after failure of first-line clarithromycin-based. According to a meta-analysis, the eradication rate of levofloxacin-based triple therapy as a second-line treatment was 74.8–79.6% [Citation23]. In the present study, the eradication rate of levofloxacin-based triple therapy was 61.8% by PP analysis, which was lower than those reported previously. This may have been related to a rapid increase in fluoroquinolone resistance over recent years. A recent Korean study reported a resistance rate for levofloxacin of 5.7% for 2003–2005, but a rate of 34.6% for 2009–2012 [Citation12]. This rapid increase in levofloxacin resistance may be due to cross-resistance to other fluoroquinolones such as ciprofloxacin or moxifloxacin. BQT has been recommended as the second-line therapy after failure of conventional triple therapy. However, in our study, levofloxacin-based triple therapy was given after failure of clarithromycin-based triple therapy. To compare the compliance and medical cost between BQT and TT group, we used the same second-line regimen in the failure group.
According to recently published guidelines, the recommended duration for H. pylori eradication therapy is 14 days [Citation4,Citation14], whereas we used a 7-day regimen. When we planned this study, the 14-day regimen was not recommended because the eradication rates of 7- and 14-day regimens were believed to be similar, and, thus, Korean medical insurance only covered 7-day regimens. However, metronidazole resistance can be overcome by long-duration treatment, the eradication success rate of BQT could be improved after 14 days treatment of BQT.
In terms of medical cost, the RUT was less expensive than DPO-PCR and the 7-day BQT regimen was less expensive than the 7-day clarithromycin triple therapy regimen. Thus, based on diagnostic and therapeutic costs, empirical BQT can be preferred. However, in a recent study, conducted in Incheon city, it was reported that DPO-PCR guided target therapy was equivalent or superior to empirical clarithromycin triple therapy in terms of cost effectiveness [Citation16].
Our research has several limitations. First, it was conducted at a single center in Incheon, and thus, it was difficult to generalize our results. Second, as mentioned above, different H. pylori diagnostic methods were used in the two groups, and thus, group diagnosis rates might have different results. However, because the sensitivities of diagnostic methods are both high, we believe this might not influence our results. Third, H. pylori culture along with real antibiotic resistance test and validation study for DPO-PCR have not been performed in this study. Fourth, as mentioned above, our study performed a 7-day regimen of H. pylori eradiation therapy. Even though 10–14 days of treatment is recommended in guidelines, Korean medical insurance is only covered 7-day regimen. Furthermore, to be able to equally compare the eradication efficacy between the BQT group and the TT group, we unified to 7-day regimen in each group. Our findings are considered insufficient to explain that the BQT group is not inferior to the TT group.
In a high clarithromycin resistance area (Incheon), the H. pylori eradication rates, safeties, and tolerability of tailored therapy and BQT were similar, but overall empirical BQT was preferred over tailored therapy because of its cost-effectiveness.
Ethical approval
This study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and the international Conference on Harmonization Good Clinical Practice guidelines. All patients provided informed consent by written, and the study was approved by the Institutional Review Board of Inha University (2016-01-21) and registered as a trial at ClinicalTrials.gov (NCT03361267).
| Abbreviations | ||
| H. pylori | = | Helicobacter pylori |
| BQT | = | bismuth containing quadruple therapy |
| TT | = | tailored therapy |
| DPO-PCR | = | dual-priming oligonucleotide-based multiplex polymerase chain reaction |
| PPI | = | proton pump inhibitor |
| rRNA | = | ribosomal RNA |
| RUT | = | rapid urease test |
| UBT | = | 13C-urea breath testing |
| ITT | = | intention-to-treat |
| mITT | = | modified intention-to-treat |
| PP | = | per protocol |
Acknowledgment
The authors thank the study participants, without whom the study would never have been accomplished. The funding body had a role in data analysis and interpretation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- McColl KE. Clinical practice Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597–1604.
- Malfertheiner P, Megraud F, O'Morain CA, European Helicobacter Study Group, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence consensus report. Gut. 2012;61(5):646–664.
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153.
- Malfertheiner P, Megraud F, O'Morain CA, European Helicobacter and Microbiota Study Group and Consensus panel, et al. Management of Helicobacter pylori infection – the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239.
- Kim SG, Jung H, Lee HL, Korean College of Helicobacter and Upper Gastrointestinal Research, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol. 2014;29(7):1371–1386.
- Woo H, Park DI, Park H, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 2009;14(1):22–28.
- Lee HJ, Kim JI, Cheung DY, et al. Eradication of helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 2013;208(7):1123–1130.
- Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59(6):1235–1243.
- Occhialini A, Urdaci M, Doucet-Populaire F, et al. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother. 1997;41(12):2724–2728.
- Yoon K, Park SW, Lee SW, et al. Clarithromycin-based standard triple therapy can still be effective for Helicobacter pylori eradication in some parts of the Korea. J Korean Med Sci. 2014;29(9):1240–1246.
- Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18(3):206–214.
- Lee SW, Kim HJ, Kim JG. Treatment of Helicobacter pylori infection in Korea: a systematic review and meta-analysis. J Korean Med Sci. 2015;30(8):1001–1009.
- Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69. e14.
- Venerito M, Krieger T, Ecker T, et al. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45.
- Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 2018;12(6):648–654.
- Smith SM, O'Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20(29):9912–9921.
- Malfertheiner P, Bazzoli F, Delchier J. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial (vol 377, pg 905, 2011). Lancet. 2011;377:905–913.
- Francesco VD, Zullo A, Hassan C, et al. Mechanisms of Helicobacter pylori antibiotic resistance: an updated appraisal. World J Gastrointest Pathophysiol. 2011;2(3):35–41.
- El-Zimaity HM, Al-Assi MT, Genta RM, et al. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol. 1995;90(11):1962–1964.
- Woo JS, Hala M, Genta RM, et al. The best gastric site for obtaining a positive rapid urease test. Helicobacter. 1996;1(4):256–259.
- Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1:19–17.
- Chen P, Wu M, Chen C, for the Taiwan Gastrointestinal Disease and Helicobacter Consortium, et al. Systematic review with meta‐analysis: the efficacy of levofloxacin triple therapy as the first‐or second‐line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44(5):427–437.
