Abstract
Objectives
The use of biologic therapy in inflammatory bowel disease (IBD) is likely to increase with lower costs and more biologics and biosimilars becoming available. Our aim was to estimate the trends in use of first-line biologics during the first year after diagnosis in a Norwegian IBD population from 2010 to 2016.
Methods
Data were collected from the Norwegian National Patient Registry and Norwegian Prescription Database. Patients defined as incident IBD cases between 2010 and 2016 were included and followed for 12 months. Patients were stratified by year of diagnosis to examine change over time. Chi-square test was used for calculations on proportions. Time from diagnosis to first biologic was calculated by Kaplan-Meier failure estimates.
Results
14,645 patients were included, 5283 (36%) with Crohn’s disease (CD) and 9362 (64%) with ulcerative colitis (UC). In the 2010 and 2016 cohort, the proportion initiating biologics increased from 17% to 33% (p < .001) for CD and 7% to 13% (p < .001) for UC. The most frequently used first-line biologics were infliximab (CD: 64% and UC: 82%) and adalimumab (CD: 36% and UC: 15%). The highest registered use of adalimumab was in the 2012 cohort (CD: 56% and UC: 39%). In the 2014–2016 cohorts, infliximab was the most used first-line biologic for both CD and UC.
Conclusions
The proportion of IBD patients initiating biologics within 12 months after diagnosis increased between 2010 and 2016. The use of infliximab as first-line biologic increased after the approval of biosimilar infliximab in 2013.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-mediated diseases in the gastrointestinal tract. Biologics have been used in the treatment of moderate to severe IBD since the late 1990s [Citation1–3] and have traditionally been expensive drugs. The use of biologics in IBD populations differs widely between countries, ranging from low single digit percentages and up to 30%, and strongly correlates with gross domestic product (GDP) [Citation4]. In Norway, the time from diagnosis to initiation of biologic therapy varies between regional health authorities [Citation5], demonstrating that treatment varies both at a national and international level.
Current European guidelines recommend biologic treatment if use of conventional therapy such as corticosteroids, 5-aminosalisylic acids and immunomodulators fail, often referred to as the “step up” approach [Citation6,Citation7]. There is evidence suggesting that early use of biologics (“top down” approach) for patients diagnosed with severe disease or risk of progression might alter disease outcome by inducing remission and reducing corticosteroid use [Citation8], delay the need for surgery [Citation9] and reduce health care costs [Citation10]. In 2013, the European Medicines Agency (EMA) approved the first biosimilar infliximab (CT-P13/Remsima, Celltrion, Budapest, Hungary), which entered the market in Norway in 2014 as a lower-cost alternative to originator infliximab (Remicade, Janssen Biologics, Leiden, The Netherlands). Lower costs improve the cost-effectiveness of biologic therapy [Citation11] and may move biologics to an earlier line in therapy [Citation12], such as in the “top down” approach.
Both at patient and societal levels, real world data are important for describing trends in the use of biologics over time and evaluating the effects of increased use. We aimed to assess if there was a change in the proportion of patients receiving biologics within the first year after diagnosis and in the use of first-line biologics from 2010 to 2016 in a Norwegian IBD population.
Materials and methods
Data and source population
The source population included all patients who received an IBD diagnosis (International Statistical Classification of Diseases 10th Revision [ICD-10] code K50 or K51) at least once between 2008 and 2017 in the Norwegian National Patient Registry (NPR). Data from the NPR were linked to the Norwegian Prescription Database (NorPD) using unique personal identification numbers, which made it possible to follow individual patients over time. NPR comprises all information on inpatient and outpatient hospital contacts including mandatory individual diagnosis codes and procedure codes for both private and public hospitals. Expensive drugs administered at or prescribed from hospitals are registered in the NPR after the Anatomical Therapeutic Chemical Classification System (ATC). NorPD comprises information on all dispensed drug prescriptions by Norwegian pharmacies and dates back to 2004.
Case definition and study population
Patients with their first IBD code registration in the NPR between 2010 and 2016 were included in the study and were followed for 12 months. Patients with an IBD code registration between 2008 and 2009 were excluded due to the risk of misclassifying prevalent cases as incident cases. Patients diagnosed in 2017 were excluded due to follow-up less than 12 months. In order to avoid including false positive IBD cases, several IBD related events (hospital or prescription) had to be present before a patient was included. Patients were included as incident cases if they had two IBD hospital events, or at least one IBD hospital event and at least two IBD prescriptions [Citation13]. Our definition of an IBD prescription included pharmacy claims with ICD-code K50 or K51, ICPC-code D94, and/or 5-ASA or budesonide prescriptions. The date of diagnosis was set to the earliest record of an IBD diagnosis in the NPR or an IBD prescription with a maximum limit of 60 days prior to the first NPR event with an IBD diagnosis code. Patients with IBD prescriptions prior to the 60 days limit were excluded as incident cases. For patients registered with both UC and CD, we used the last observed ICD-10 code in the NPR to determine the diagnosis. Age was only available as ten-year birth cohorts.
Biologics
All prescriptions of biologics (both intravenous (IV) and subcutaneous (SC) administered drugs) are registered by their ATC codes either in the NPR or the Nor-PD. Biologics included in the analyses were infliximab, adalimumab, golimumab, vedolizumab and natalizumab. All patients with at least one registered event of biologic use after their first IBD diagnosis were considered recipients of biologic therapy. There were 102 patients included as incident IBD cases that received biologic therapy for other diagnoses before their first IBD diagnosis code and before the 60 days limit. These patients were included as incident IBD cases, but were excluded as new treatment cases and were removed from further analysis on biologic use (calculation of proportions and time-to-event curves). Twelve patients were registered with concurrent use of two different biologics at their first biologic registration. This was interpreted as coding errors because there was only one registration with double biologic per patient. The patients were assigned to a biologic group based on their following biologic registrations (Table S1, Supplementary). Patients registered with either vedolizumab or natalizumab as first-line biologic were reviewed manually to check for potential errors. Natalizumab has not been approved as treatment for IBD and was therefore omitted from , although included as biologic cases in . The use of natalizumab is listed in Table S1, Supplementary.
Use of biosimilars
In Norway, pharmaceutical tendering aims to contain spending on expensive drugs, such as biologics. The tendering results in annual or biennial treatment line recommendations of the available biologics, with the tender winner as first choice [Citation14]. We were not able to distinguish between use of biosimilars and originators since only ATC codes are registered in the NPR. Therefore, we compared our data with national sales numbers from 2010 to 2016, which includes originator infliximab (Remicade) and biosimilar infliximab (CTP-13: Remsima and Inflectra [Pfizer, Brussel, Belgium]) (Figure S1, Supplementary).
Statistical analysis
Data were analyzed using Anaconda Python 3.X and Stata, version 16.1 (StataCorp LLC, 2019). Cumulative incidence of biologic use was presented by Kaplan-Meier failure estimates. Patients were stratified by the year of diagnosis in order to examine change over time. Patients were censored at 365 days of follow-up or time of death. The Chi-square test was used to test for differences in proportions of patients receiving biologics within 12 months of diagnosis in the 2010 and 2016 cohorts.
Ethical considerations
The study was approved by NPR, NorPD, the Norwegian Data Protection Authority and the Regional Committees for Medical and Health Research Ethics (application number 2016/113-1).
Results
Patient characteristics
A total of 14,645 incident patients were included in the study. There were 5283 (36%) patients with CD and 9362 (64%) patients with UC. Among CD patients, 2501 (47%) were males, and the corresponding number for UC was 5035 (54%). The median age at diagnosis based on ten-year birth cohorts was 36 years for CD and 40 years for UC. Incident IBD cases from 2010 to 2016 are presented in .
Table 1. Incident inflammatory bowel disease (IBD) cases per year from 2010 to 2016 stratified by diagnosis.
Proportion of patients initiating biologics
In total, 25% of CD patients and 9% of UC patients initiated biologics within 12 months after diagnosis between 2010 and 2016. The proportion of patients initiating biologics increased for both CD and UC during the study period (). The proportion of CD patients receiving biologics was consistently higher than for UC patients. For CD patients, 130 (17%) patients received biologics within 12 months in the 2010 cohort compared to 239 (33%) patients in the 2016 cohort (p < .001). For UC patients, the number initiating biologics increased from 93 (7%) patients in the 2010 cohort to 175 (13%) patients in the 2016 cohort (p < .001).
Table 2. Proportion of patients initiating biologics within 12 months after diagnosis stratified by year of diagnosis.
Time from diagnosis to biologic
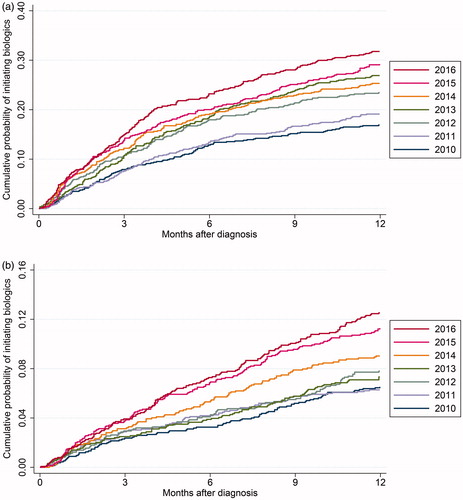
The cumulative probability of initiating biologics during the first 12 months after diagnosis increased from 2010 to 2016 (). In the 2010 cohort, 8% of CD patients and 2% of UC patients received biologics within three months. This increased to 15% (CD) and 4% (UC) in the 2016 cohort. At six months of follow-up, there was an increase from 13% (CD) and 3% (UC) in the 2010 cohort to 23% (CD) and 7% (UC) in the 2016 cohort. The log-rank test was significant for both CD (p < .001) and UC (p < .001).
Figure 1. (a) Kaplan–Meier failure estimate of time from diagnosis to first biologic stratified by year of diagnosis for CD patients. The Y axis shows the cumulative probability of initiating biologics. The X axis shows development over time in months. (b) Kaplan–Meier failure estimate of time from diagnosis to first biologic stratified by year of diagnosis for UC patients. The Y axis shows the cumulative probability of initiating biologics. The X axis shows development over time in months.
Choice of first-line biologic
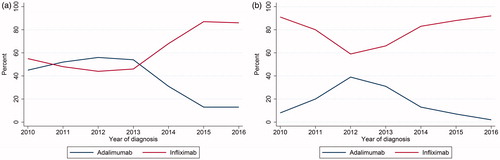
Infliximab and adalimumab were by far the two most common types of first-line biologics (). Golimumab and vedolizumab were rarely used as first-line biologics. The use of adalimumab and infliximab as first-line biologic treatment is presented in (CD) and 2 b (UC). For CD patients in the 2010–2013 cohorts, approximately half of patients started with adalimumab and half started with infliximab. Infliximab was the preferred first-line biologic for CD patients in the 2014–2016 cohorts. The use of infliximab increased from 68% in the 2014 cohort to 86% in the 2016 cohort while the use of adalimumab decreased ( and ). Infliximab was the most used first-line biologic for UC patients throughout the study period ( and ). The highest recorded use of adalimumab for UC patients was 39% in the 2012 cohort. In the 2016 cohort, 92% of UC patients received infliximab as their first biologic and the use of adalimumab had decreased to 2%.
Table 3. The use of first-line biologics stratified by diagnosis and year of diagnosis.
Figure 2. (a) First-line biologic for CD patients initiating biologics stratified by infliximab and adalimumab as first biologic registration. Y axis shows percent of biologic recipients started on either infliximab or adalimumab. X axis shows yearly cohorts from 2010 to 2016. (b) First-line biologic for UC patients initiating biologics stratified by infliximab and adalimumab as first biologic registration. Y axis shows percent of biologic recipients started on either infliximab or adalimumab. X axis shows yearly cohorts from 2010 to 2016.
Discussion
In this nationwide registry study, we showed that the proportion of IBD patients initiating biologics within the first year after diagnosis increased between 2010 and 2016, and that the use of infliximab as first-line biologic increased after the introduction of biosimilar infliximab in 2014.
Compared to previous studies reporting use of biologics, Norway seems to be in the upper range among European countries [Citation4,Citation15,Citation16]. In the Western European population of the Epicom cohort from 2010, 21% of CD patients and 6% of UC patients received biologics during 12 months of follow-up after diagnosis [Citation16]. These numbers correspond with our findings of 17% (CD) and 7% (UC) in our 2010 cohort. In Denmark, 29% of CD patients and 11% of UC patients initiated biologics during a study period from 2003 to 2016 [Citation15]. These numbers are not directly comparable with our results, since we only followed patients during the first 12 months after diagnosis. We found that 25% of CD patients and 9% of UC patients initiated biologics, and the proportion is likely to increase with prolonged follow-up.
The use of biologics has been shown to correlate with affordability [Citation4]. In 2014, biosimilar infliximab was introduced in Norway as an alternative to originator infliximab at a considerably lower price due to pharmaceutical tendering. Our hypothesis was that a lower economic burden would increase the use of biologics. However, the increase began before 2014, most prominently for CD. A more liberal use of biologics might not only be affected by price. A general understanding of changes in treatment strategies might influence clinical decisions, such as a shift from the “step up” to the “top down” approach in patients with potential risk of severe disease outcomes, although this is not actually reflected in current guidelines [Citation6,Citation7]. Reduced medication costs could also lead to gradual dose escalation on patient level. Pharmaceutical tendering ensures lowered medication costs for hospitals in Norway, and patients are financially compensated if medical expenses exceed a certain limit regardless of private medical insurance. The biologic use could be quite different in countries with other reimbursement systems.
Our primary aim included evaluating the choice of first-line biologics. This study showed that both adalimumab and infliximab were common first-line biologics, but the use of adalimumab as first-line biologic decreased from the 2013 cohort and beyond. There are no head-to-head trials comparing infliximab and adalimumab, but they are considered to be equally efficient in the treatment of IBD [Citation17]. A study comparing UC treatment patterns in the US and five European countries reported only minor differences in adalimumab and infliximab use, and in the US, adalimumab was used more than infliximab [Citation18]. The observed reduction of adalimumab in Norway is therefore likely an effect of pharmaceutical tendering and treatment line recommendations rather than a result of clinician and patient preferences. Route of administration and convenience factors have been reported as common reasons for changing treatment regimen from one biologic to another [Citation18]. IV drugs, such as infliximab, are administered at hospitals, and require facilities, equipment and health care professionals. SC drugs, such as adalimumab, can be administered by the patient at home and provide economic benefits to the health care system and increased independence for the patient. The major benefit of pharmaceutical tendering is reduced drug expenses for the hospitals. The drawback with strict treatment line recommendations is potential interference with regard to choice of biologic. IBD mainly affects patients during critical years of schooling and career growth. If an IV drug is the tender winner, patients could be restricted from the convenience of SC administration. On the other hand, self-administered SC drugs could lead to less frequent follow-up at outpatient clinics as well as decreasing adherence, which is not necessarily optimal for patients nor cost-effective [Citation19].
The “Pharmaceutical Strategy of The Norwegian Hospital Procurement Trust” (“Legemiddelinnkjøp på sykehus” [LIS]) is in charge of the pharmaceutical tendering at all Norwegian hospitals [Citation14]. We compared our results with national sales numbers for infliximab from drug statistics from Norwegian hospital pharmacies (“Sykehusapotekenes legemiddelstatistikk” [SLS]). The result from annual tendering is reflected in the use of biosimilars replacing the originator infliximab after 2014 (Figure S1, Supplementary). Switching from originator to biosimilars has been proven safe and feasible in a number of studies [Citation20,Citation21]. However, biosimilars and switching have been met with profound skepticism, resulting in a low uptake of biosimilars in some countries [Citation22]. Pharmaceutical tendering promotes a more liberal use of biosimilars, and due to mandatory switching the uptake of biosimilars in Norway has been substantial after 2014.
Strengths
This study has several strengths. Selection and information bias are limited since the study cohort is based on two nation-wide registries and the data are prospectively collected. Our data has a good coverage of all biologic use in Norway. The NPR includes events from both private (of which very few prescribe biologics) and public hospitals, and registration of biologic IV and SC drug administrations is mandatory due to reimbursement regulations. The NorPD contains electronic registrations of all dispensed drugs from Norwegian pharmacies, which means that biologics dispensed from pharmacies and not administered at the hospitals are included in the data.
Limitations
The NPR is based on reimbursement codes from hospital admissions, and there is a risk of coding errors that could lead to misclassification of patients. The IBD diagnosis codes in the NPR have not yet been validated, but the quality of the data from the NPR is generally considered to be of high standard. Validation studies of the Swedish and Danish NPR showed that the validity of IBD diagnosis codes were high [Citation23,Citation24], and it is reasonable to assume that the quality of the Norwegian NPR resembles that of other Nordic countries. Despite a short minimum look-back period of two years in the NPR (from 2010 to 2008), we suspect the number of false positive incident IBD cases to be very low with our case definition, because the case definition combined data from the NPR with NorPD, which has a look-back period of minimum six years (from 2010 to 2004), and the incidence based on this definition was stable from 2010 to 2017 [Citation13].
In conclusion, with new biologics, biosimilars and other targeted therapies becoming available, the treatment paradigm of IBD is likely to shift towards earlier initiation of biologics. In the present study, we demonstrate that the time from diagnosis until initiation of first biologic decreased over time. The long-term effects of earlier initiation of biologics remains to be discovered, for instance the effect on surgery rates, hospitalization rates and adverse events. Further studies should also pursue whether increased biologic use leads to an increase in dose escalations on patient level.
Supplemental Material
Download PDF (129.6 KB)Acknowledgements
We thank Skule Ingeberg at Dept. of Pharmacology at Oslo University Hospital for analytical assistance of and permission to publish national sales numbers from drug statistics from hospital pharmacies in Norway (“Sykehusapotekenes legemiddelstatistikk” [SLS]).
Disclosure statement
KA reports consultant fees from Takeda outside the submitted work. SSL reports grants and consultant fees from Takeda. AWM reports no conflicts of interest. BM reports consultant fees from Takeda, Janssen, AbbVie, Pfizer; advisory board Takeda, Janssen, AbbVie, Pfizer, Sandoz, Pharma Cosmos; speaker fees from Takeda, Janssen, AbbVie, Sandoz, Orion Pharma. HOM reports grants and consultant fees from Takeda. MLH reports speaker fees from AbbVie, Meda, Tillotts and Takeda; advisory board Takeda.
Data availability statement
The data underlying this article were provided by the Norwegian National patient register (NPR) and the Norwegian Prescription Database (Nor-PD) by permission. Data cannot be shared publicly due to the privacy of the patients listed in the registries. Data are available from the NPR (https://www.helsedirektoratet.no/tema/statistikk-registre-og-rapporter/helsedata-og-helseregistre/norsk-pasientregister-npr) and Nor-PD (http://www.reseptregisteret.no/) through an application process.
Additional information
Funding
References
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340(18):1398–1405.
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350(9):876–885.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476.
- Péntek M, Lakatos PL, Oorsprong T, et al. Access to biologicals in Crohn's disease in ten European countries. World Journal of Gastroenterology. 2017;23(34):6294–6305.
- Lirhus SS, Hoivik ML, Moum B, et al. Regional differences in anti-TNF-α therapy and surgery in the treatment of inflammatory bowel disease patients: a Norwegian nationwide cohort study. Scand J Gastroenterol. 2018;53(8):952–957.
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22.
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784.
- D'Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371(9613):660–667.
- Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–1201.
- Marchetti M, Liberato NL, Di Sabatino A, et al. Cost-effectiveness analysis of top-down versus step-up strategies in patients with newly diagnosed active luminal Crohn's disease. Eur J Health Econ. 2013;14(6):853–861.
- Baji P, Gulácsi L, Brodszky V, et al. Cost-effectiveness of biological treatment sequences for fistulising Crohn's disease across Europe. United European Gastroenterol J. 2018;6(2):310–321.
- Dutta B, Huys I, Vulto AG, et al. Identifying key benefits in european off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34:159–170.
- Lirhus SS, Høivik ML, Moum B, et al. Incidence and prevalence of inflammatory bowel disease in Norway and the impact of different case definitions: a nationwide registry study. Clin Epidemiol. 2021;13:287–294.
- Sykehusinnkjøp HF. Pharmaceutical strategy of the Norwegian hospital procurement trust. 2020. [updated 2020 Feb 19 02; cited 2020 Jul 10]. Available from: https://sykehusinnkjop.no/pharmaceutical-strategy-of-the-norwegian-hospital-procurement-trust-sykehusinnkjop-hf-
- Alulis S, Vadstrup K, Borsi A, et al. Treatment patterns for biologics in ulcerative colitis and Crohn's disease: a Danish Nationwide Register Study from 2003 to 2015. Scand J Gastroenterol. 2020;55(3):265–271.
- Burisch J, Pedersen N, Cukovic-Cavka S, et al. Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: the ECCO-EpiCom cohort. Inflamm Bowel Dis. 2014;20(1):36–46.
- Patil SA, Rustgi A, Langenberg P, et al. Comparative effectiveness of anti-TNF agents for Crohn's disease in a tertiary referral IBD practice. Dig Dis Sci. 2013;58(1):209–215.
- Armuzzi A, DiBonaventura MD, Tarallo M, et al. Treatment patterns among patients with moderate-to-severe ulcerative colitis in the United States and Europe. PloS One. 2020;15(1):e0227914.
- Panaccione R, Colombel J-F, Travis S, et al. Tight control for Crohn's disease with adalimumab-based treatment is cost-effective: an economic assessment of the CALM trial. Gut. 2020;69(4):658–664.
- Jorgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316.
- Buer LC, Moum BA, Cvancarova M, et al. Switching from Remicade® to Remsima® is well tolerated and feasible: a prospective, open-label study. J Crohns Colitis. 2017;11(3):297–304.
- Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a belgian case study. Pharmacoeconomics. 2014;32(7):681–691.
- Jakobsson GL, Sternegård E, Olén O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–221.
- Lo B, Vind I, Vester-Andersen MK, et al. Validation of ulcerative colitis and Crohn's disease and their phenotypes in the Danish National Patient Registry using a population-based cohort. Scand J Gastroenterol. 2020;55(10):1171–1175.
