Abstract
Introduction
Autoimmune pancreatitis (AIP) is a special form of pancreatitis that responds well to glucocorticoid (GC) treatment. Relapses of AIP are common. The anti-CD20 antibody rituximab (RTX) has shown promising results in GC refractory cases, but long-term data are scarce. The study aims to determine the clinical and imaging response to RTX and summarize the existing data on RTX therapy in patients with AIP type 1 in the literature.
Patients and methods
Retrospective analysis of electronic medical records was conducted. Additionally, we conducted a systematic review of the literature concerning RTX use in AIP type 1.
Results
Twelve (11.7%) of 103 patients with AIP type 1 were treated with RTX during the study period: eight (66.7%) achieved complete and four (33.3%) partial remission. RTX was discontinued in one patient who developed fever and reactivation of latent tuberculosis. None of the remaining 11 patients relapsed during a median follow-up of 17 months. No significant differences were detected in baseline clinical characteristics or history of relapse between the patients who obtained complete and partial remission. Altogether, eight studies with 110 AIP type-1 patients treated with RTX were analyzed. Adverse effects ranged from 11–43% and the relapse-free period during follow-up (range 2–173 months) ranged from 38–94%.
Conclusions
Our results confirm that RTX is efficacious in the treatment of AIP type 1 by inducing remission and preventing relapse. In addition, there are few adverse effects of the treatment.
Introduction
Autoimmune pancreatitis (AIP) type 1 is a pancreatic manifestation of IgG4-related disease (IgG4-RD). Glucocorticoids (GCs) are the preferred first-line medication for active IgG4-related disease (including AIP), with about 97–100% response rates. However, relapse of AIP after or during initial GC treatment is common and represents a challenging problem in clinical practice [Citation1,Citation2]. European studies show that the frequency of relapses after or during the tapering period of the GC treatment varies from 7-55% [Citation1,Citation3,Citation4]. The presence of other organ involvement (OOI), IgG4-related cholangitis (IRC) and initial need for GC therapy have been suggested as risk factors for relapse [Citation1,Citation3,Citation5]. The indications for maintenance GC therapy and its duration after the initial remission of AIP type 1 remain controversial. The approaches significantly differ across the World since Western researchers opposed the routine use of maintained GC in cases of AIP with initial remission (due to fears of toxicity related to long-term GC use, especially in elderly patients) [Citation6]. This position differs from the Japanese approach since 2010, calling for 3-year maintenance of GC after remission in all patients with AIP type 1 to reduce relapse [Citation7–9]. A recently published systematic review and meta-analysis evaluated the relapse rate in patients with AIP type 1 according to the duration of GC treatment. The meta-analysis found that the relapse rate decreased with an extended duration of GC therapy up to 36 months [Citation7]. European guidelines on IgG4-related digestive disease allow different strategies in treating disease relapse, including consideration of immunomodulating drugs (IMs) in disease relapse as maintenance of remission strategy, and in patients with a high risk of disease relapse, particularly for multi-organ involvement [Citation1]. Among IMs, most evidence is available for azathioprine (AZA) and mycophenolate mofetil (MMF) [Citation1] that, compared to GC monotherapy, failed to provide longer relapse-free survival [Citation10]. Furthermore, both long-term therapies are associated with adverse effects, including osteoporosis, adrenal insufficiency, peptic ulcers, glaucoma, cataract, hyperlipidemia, growth suppression for GCs [Citation11] and flu-like illness, malaise, pancreatitis, myelosuppression (most common mild leukopenia), hepatotoxicity, lymphoma and other malignancies [Citation12,Citation13]. Considering all these clinical dilemmas, finding safe and effective GC sparing drugs in patients with AIP type 1 is essential.
Rituximab (RTX) is a monoclonal antibody that depletes B-cells by targeting their CD20 antigen [Citation1]. Thus, in comparison to IM, it provides a more exact interference into the plasmablast-initiated pathology of IgG4-RD [Citation14]. Moreover, studies have shown induction of remission in 83–94% of patients who are refractory or intolerant to GC or IM treatment [Citation1,Citation3,Citation10,Citation15]. Therefore, according to European guidelines, RTX should be considered if patients are resistant or intolerant to high-dose GC or have failed to respond to IM therapies [Citation1].
The present study aims to determine the treatment efficacy of RTX in the largest AIP cohort in Scandinavia and summarize the existing data in the literature on RTX in AIP type 1.
Patients and methods
We retrospectively analyzed medical records of patients treated for AIP in our Pancreas Outpatient Clinic at the Department of Upper Abdominal Disease at Karolinska University Hospital in Stockholm, Sweden, between January 2001 and December 2020.
Inclusion and exclusion criteria
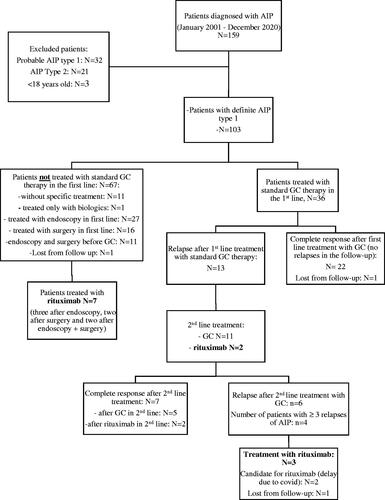
shows the patient selection process. We included patients with definite AIP type 1 according to the International Consensus Diagnostic Criteria (ICDC) [Citation15]. Patients diagnosed before the publishing of the ICDC (2011) were reviewed by two senior pancreatologists retrospectively to assure a definitive diagnosis. We excluded patients <18 years, AIP type 2 and probable AIP type 1 (according to the ICDC).
Standard GC treatment and rituximab protocol
The standard protocol of GC treatment in our institution included a starting dose of 0.6–0.8 mg/kg (most commonly 40 mg per day) for the first 4 weeks with tapering of 5 mg per week with the supplementation of calcium and vitamin D, proton pump inhibitors in protective dose (20 mg per day) and routine follow-up of blood glucose at least once per month. Since the publication of European guidelines on IgG4-related digestive disease, we prolonged the tapering period on 5 mg every two weeks. However, all patients presented in this study were included and treated with GC before the publication of the guidelines.
In IgG4-related digestive disease RTX proved effective when administered both at 375 mg/m2 weekly for 4 weeks followed by maintenance infusions every 2–3 months (onco-hematological protocol) or at two 1000 mg infusions 15 days apart every 6 months (immunological/rheumatoid arthritis protocol) [Citation1,Citation3]. Pursuant to the protocol on RTX treatment in our institution, RTX is given in two 500 mg infusion doses biweekly in the first month and every 6 months after that. CRP, erythrocyte sedimentation test, full blood count, plasma alanine aminotransferase, plasma creatinine and dipstick urinalysis are taken 10 days prior to each RTX infusion. Pre-treatment with a 50 mg tablet of prednisolone, a 500 mg tablet of paracetamol and 10 mg of cetirizine is routinely given before the start of RTX infusion. Blood pressure is controlled before and during the infusion period. Doses and intervals for maintenance treatment are determined for each case by disease activity [Citation1].
Exposure and outcome definitions
We collected data on demographics, history of AIP (including the follow-up period since both AIP diagnosis and the start of RTX treatment), other organ involvement (OOI), previous treatment, number of AIP relapses, history of pancreatic surgery, long-term consequences, such as pancreatic exocrine insufficiency (PEI), diabetes mellitus (DM) and osteoporosis, symptoms at the time of diagnosis (asymptomatic, new-onset diabetes, acute pancreatitis, obstructive jaundice, weight loss, abdominal pain) and IgG4 serology.
Relapse was defined as symptoms typical for AIP or typical imaging results of pancreatic or extra-pancreatic involvement, both presenting any time after the initial resolution while other differential diagnoses have been excluded [Citation16].
GC refractory disease was defined as continued disease activity despite the start of full-dose GC therapy [Citation2].
The primary outcome was a clinical and radiological response to RTX treatment evaluated 6 months after the initiation of treatment. Complete remission was defined as the complete absence of both clinical symptoms and imaging findings (on magnetic resonance imaging). Remission was considered partial if a patient had a complete absence of clinical symptoms and a partial imaging response.
Statistical analysis
Data are expressed as median, minimum and maximum for numerical data or percentage for categorical data. Comparison of data was obtained using appropriate nonparametric statistical tests. For categorical data, chi-square or Fisher’s exact test was used and for numerical data, the Mann–Whitney U-test. The analyses were performed using the IBM SPSS Statistics 27. A p-value <.05 (two-sided) was considered statistically significant.
Ethics
The study adhered to the latest version of the Declaration of Helsinki and was approved by the Clinic Ethical Committee in Stockholm, Sweden (Dnr. 2016/1571-31 and 2020-02209).
Systematic review of the literature
Search strategy and study selection
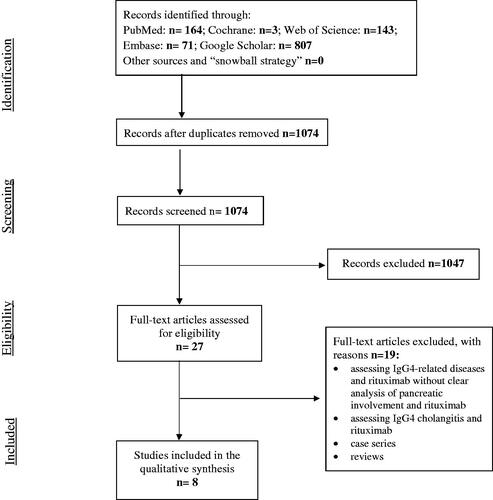
A literature search following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [Citation17] was conducted in order to identify all relevant original articles referring to the treatment of AIP with RTX. Cochrane, Embase, Google Scholar, PubMed and Web of Science databases were searched until 11 May 2021 using the following search terms: (‘autoimmune pancreatitis’ OR ‘autoimmune pancreatitis type 1’ OR ‘IgG4-related disease’) AND (‘relapse,’ ‘rituximab’). Eligibility assessment was performed by screening the titles, and consequently, the abstracts and full articles by three independent reviewers (SN, ESH, NP) and all disagreements were resolved by MV and JML. Review articles, editorials, conference reports, comments on other studies, animal studies, non-English language articles, book sections and theses, overlapping articles and articles that did not entail individual data on the outcomes of patients with AIP type 1 treated with RTX were excluded.
If the study did not exclusively refer to AIP type 1 but the entire group of IgG4-related diseases, it was eligible for inclusion only if individual outcomes of AIP-affected patients treated with RTX could be extrapolated from the available data. Additional studies were identified among the article references included using a snowball strategy. The selection process of the articles for the review is summarized in [Citation17]. Finally, a descriptive analysis was performed on eight identified articles. These data were subsequently compiled and presented in .
Table 1. Studies on rituximab in AIP type 1.
Results
Some 159 patients were treated at the Pancreas Outpatient Clinic at Karolinska University Hospital between January 2001 and December 2020. As shown in , 103 patients met the inclusion criteria. Eleven patients did not receive specific treatment due to spontaneous regression of symptoms and imaging manifestations. Thirty-six patients were treated with standard GC therapy in first-line and 13 (36%) relapsed during the follow-up. In contrast, 56 patients received an alternative first-line treatment detailed in . Third-line treatments included GC (n = 4), AZA (n = 3), cyclophosphamide (n = 2) and methotrexate (MTX, n = 1).
Baseline characteristics of patients treated with RTX
Eleven patients received RTX and one received another monoclonal antibody, siltuximab (anti-IL-6 chimeric monoclonal antibody) due to Castleman’s disease [Citation18]. However, because of the similarity between siltuximab and RTX, the patient given siltuximab was included in the study and subsequently analysed and described with the patients given RTX therapy. Baseline demographic and clinical characteristics, response to treatment and long-term consequences of AIP in the 12 patients that received RTX were listed in . The median age at diagnosis was 57.7 years and the median follow-up time after AIP diagnosis was 72 months (range 12-144 months). The median follow-up after the start of RTX treatment was 17 months (range 6–132 months). The male/female ratio of patients who received RTX was 2:1. The most common symptoms at diagnosis were abdominal pain in 10/12 patients (83.3%), obstructive jaundice in 8/12 patients (66.7%) and weight loss in 5/12 patients (41.7%). Eight patients (66.7%) had elevated serum IgG4 levels at diagnosis. Of the 12 patients, 11 had OOI (91.7%), of which IRC was the most common manifestation, present in 8/12 patients (see Table S1 in the Supplement). Of the 12 patients with PEI, IRC was present in 11 (91.7%) even before RTX. Seven of 12 patients (58.3%) had DM before RTX.
Table 2. Characteristics of patients with rituximab.
Indication for RTX treatment
Previous treatments, indication for RTX treatment, response to RTX treatment, possible side effects and follow-up of the 12 patients that received RTX are reported in Supplement (Table S1). Four patients had ≥3 relapses before RTX treatment. Disease relapse was an indication for the start of RTX treatment in six cases, GC refractory disease in three cases, IM or GC dependence in two cases and a haematological disease in one case. Most patients (n = 8) had RTX as second-line (n = 3, 25.1%) or third-line (n = 5, 41.7%) treatment.
Treatment outcomes
Of the 12 patients treated with RTX, 10 (83.3%) achieved complete clinical response, but only 8/10 patients achieved complete imaging response (). Thus, eight patients (66.7%) achieved complete remission (complete clinical and imaging response) and four (33.3%) partial remission (complete clinical but partial imaging response). No significant differences in the variables listed in were found between patients with complete remission (n = 8) compared to with partial remission (n = 4). Patients with a history of relapse before RTX seemed to have a higher tendency of achieving complete remission than patients with no history of relapse before RTX. However, the difference was not significant between these patient groups (p = .09). Two of the four patients that attained partial remission had not responded to GCs before RTX (Table S1).
Adverse effects
One patient who received RTX due to OOI relapse (kidney) developed fever; however, because of suspected tuberculosis, RTX was discontinued after 6 months of treatment. The patient attained complete clinical and imaging response of AIP (and stable) after subsequent endoscopic retrograde cholangiopancreatography (ERCP) with stenting and low-dose GC maintenance therapy. Another patient developed fever and skin infection caused by Borrelia, leading to discontinuing RTX therapy after 16 months (Table S1).
Systematic review
Study selection
Some 1074 articles were assessed for inclusion. Of those, 1047 were not eligible for inclusion based on title and abstract. Of the remaining 27 articles with full text, 19 were excluded (). Thus, the final sample included eight studies [Citation3,Citation10,Citation19–24].
Results of the individual studies
synthesizes the main results of each study included in the systematic research. Four of the eight studies sought to investigate IgG4-RD but AIP type-1 patients could be identified and extracted [Citation20,Citation21,Citation23,Citation24].
Outcomes of RTX treatment in AIP type 1
The eight included articles entailed 391 patients with AIP type 1, of which 110 (28.1%) were treated with RTX [Citation3,Citation10,Citation19–24]. In four studies, data on treatment efficacy, rate of relapses and adverse events were [Citation20,Citation21,Citation23,Citation24] extracted from the cohort analysis of IgG4 patients. The primary outcome in most of the selected studies was defined as the prevalence of remission during follow-up or cumulative incidence of relapse-free survival. The secondary outcome was defined as the hazard ratio for AIP relapse after RTX treatment and analysis of risk factors associated with relapse. The rate of relapse-free survival ranged from 38–94% during a follow-up ranging from 2–173 months. The relapse rate was up to 75%. Adverse effects were described in 6/8 studies and occurred in 11–43% of patients. The most common adverse effects were infusion-related reactions and infections [Citation3,Citation10,Citation19–24].
Discussion
IgG4-RD-related AIP type 1 is characterized by a high relapse rate after resistance to GC, which would require other immunosuppressive treatment strategies. Besides immunosuppressive drugs such as thiopurines (azathioprine and 6-mercaptopurine), mycophenolate mofetil, methotrexate or calcineurin inhibitors (tacrolimus and cyclosporine A), RTX has been suggested as an alternative in GC resistance IgG4-RD [Citation1]. The study aimed to present the outcomes of RTX treatment in patients with AIP type 1 and systematically assess the existing evidence on this subject matter.
Twelve patients were treated with RTX during the study period (eight achieved complete and four partial remission) and none relapsed during the 17-month follow-up.
A systematic review identified eight studies on RTX treatment in relapsing AIP type 1 that demonstrated the efficacy of RTX in inducing remission up to 94%. However, significant heterogeneity was observed among the studies, including different sample sizes (14–163 participants), follow-up time (2–173 months) and definition of outcomes [Citation3,Citation10,Citation19–24].
The proportions of complete clinical remissions (83.3%) and complete radiological remissions (66.7%) in our study are comparable to the remission rates observed in studies with similar follow-up times [Citation3,Citation10,Citation19,Citation20,Citation24]. However, definitions of complete and partial remission varied across the studies and were often not explicitly reported [Citation3,Citation10,Citation19–24]. Backhus et al. measured remission according to the IgG4 Responder Index, including only clinical parameters [Citation19]. Majumder et al. defined relapse as worsening radiological or laboratory findings [Citation24] and Ebbo et al. measured clinical, radiological and biochemical responses to treatment [Citation20].
None of our patients treated with RTX relapsed during the follow-up. However, data from the systematic review suggest a relapse rate ranging from 28-75% [Citation3,Citation10,Citation19–24]. The most probable explanation for this discrepancy is the short follow-up in our group (median of 17 months) and also 3/12 (25%) patients were followed up only 6 months after the start of RTX treatment.
In our study 66.7% of the patients had positive IgG4 serology at the AIP type-1 diagnosis. Backhus et al. [Citation19] reported positive IgG4 serology in 54% of patients. In the same study, downregulation of previously elevated IgG4 concentration levels correlated significantly with clinical response to RTX treatment [Citation19]. However, Carruthers et al. [Citation23] did not observe complete normalization of IgG4 serum levels in patients with exemplary disease control, and therefore, the observed decrease was not considered beneficial. Furthermore, studies did not demonstrate IgG4 levels to be pathognomonic for IgG4 RD [Citation25] and failed to anticipate disease relapse after GC therapy [Citation2].
Data on risk factors for relapse after RTX are inconsistent and potentially biased due to small sample sizes. Caruthers et al. [Citation23] identified failure of treatment with IM, previous relapses and the OOI to RTX treatment to be associated with the occurrence of higher relapse. Soliman et al. [Citation23] reported cholangitis and prior therapy with GC as risk factors for IgG4 RD relapse. Majumder et al. [Citation24] added biliary involvement and younger age at diagnosis to these potential risk factors. In contrast, Backhus et al. [Citation19] did not identify any factors with a predictive role, and Ebbo et al. [Citation20] identified only high activity of IgG4-RD disease, previously defined as IgG4-RD responder index >9 to be a predictor of higher relapse occurrence following RTX therapy. High baseline IgG4 serum and blood eosinophil concentrations were independently associated with IgG4 RD relapse in the study by Wallace et al. [Citation26]; however, Ebbo et al. reported no such link [Citation20].
Two of our patients had adverse reactions in the form of infection. The first patient experienced Borrelia reactivation with skin manifestation, which led to the termination of RTX treatment after 16 months. The second patient had fever and reactivation of tuberculosis after the first induction dose, resulting in the cessation of RTX treatment. The adverse effects of RTX have been assessed in larger studies of rheumatoid arthritis and lymphoma [Citation27,Citation28]. Most of the adverse effects (fever, nausea, vomiting, hypotension, chills, a burning sensation, headache, asthenia, pain, throat discomfort, perspiration, pruritus) were moderate and linked to the ongoing infusion [Citation27,Citation29]. However, 20% of patients with rheumatoid arthritis suffered from significant infections (pneumonia, 5.6%; cellulitis, 2.5%; urinary tract infection, 2.1%; bronchitis, 1.6%; sepsis, 1.2%). RTX treatments were terminated in 1.4% of patients. Opportunistic infections were less common (1.9%). The infection rate increased with patient age, body mass index (BMI) and the baseline Health Assessment Questionnaire Disability Index (HAQ DI) score [Citation28]. Of 52 lymphoma patients, greater than grade-2 leukopenia was noted in 52% of patients and neutropenia in 38%. One patient developed dyspnea with bronchospasm after the sixth infusion. Another patient suffered from acute pancreatitis 6.5 months after completion of RTX treatment, and a third was diagnosed with rectosigmoid cancer 9.4 months after completion of RTX treatment [Citation27]. Cardiovascular and thrombotic events were observed in some patients with RA (myocardial infarction,1.8%; deep vein thrombosis, 1.2%; pulmonary embolism, 1.0%; cerebrovascular accident, 1.7%) [Citation28].
Concerning adverse events in the eight selected studies, the above-mentioned infusion reactions and infections were the main adverse events. The prevalence of infections was from 0–33% in selected studies [Citation3,Citation10,Citation19,Citation20,Citation24]. Common infections were pneumonia, urinary tract sepsis, clostridium difficile colitis, dental abscess and sinusitis [Citation24]. Of severe infections, one patient each suffered from recurrent urinary and biliary sepsis with Gram-negative and Staphylococcus aureus bacteremia, recurrent anal abscesses, Staphylococcus hominis mitral endocarditis and recurrent angiocholitis with Gram-negative bacteremia during biliary relapses [Citation20], diverticulitis and severe neutropenia needing treatment with granulocyte colony-stimulating factor [Citation24]. Concerning infections, varicella-zoster virus was observed in two patients [Citation19,Citation21]. One patient with highly aggressive IgG4-RD was given RTX and high-dose steroid pulse as a last resort and later died due to acute cholangiosepsis and pneumonia with multi-organ failure [Citation19,Citation21]. Other side effects described were hemolytic anemia, amaurosis fugax, leading to carotid endarterectomy, unstable angina and surgery for an IgG4-related orbital pseudotumor [Citation23].
In comparison to other autoimmune cohorts (systemic lupus erythematosus, rheumatoid arthritis, immune thrombocytopenia), Ebbo et al. [Citation20] observed a higher rate of severe infections in IgG4-RD patients. It is unclear whether maintenance treatment can lead to a higher infection rate than induction treatment alone [Citation24]. Treating physicians should be aware of the risk of infections, especially attributed to late-onset neutropenia and hypogammaglobulinemia [Citation27].
Earlier studies established RTX as a viable alternative to IM in patients with burdensome IgG4-RD, especially in those with OOI [Citation10]. However, Soliman et al. suggest that RTX was more successful (94.1 vs. 65%) than IM in the management of relapsing AIP type 1 during a similar follow-up time. Likewise, Majumder showed that RTX therapy was better tolerated than IM therapy. However, patients who were mostly on RTX maintenance therapy suffered from serious infections requiring hospitalization [Citation24].
A strength of the study is our well-defined cohort of patients with AIP and that it is the largest in Scandinavia and one of the largest in Europe. Moreover, we tried to avoid selection bias and included only definite AIP type-1 patients according to ICDC criteria [Citation15].
A major weakness of the study is its retrospective design. The initial dose was the same for all patients treated with RTX. However, previous heterogeneous treatment and the absence of a standardized protocol for maintenance treatment are potential confounders of RTX efficacy. Another limitation is the small number of patients treated with RTX and the short follow-up.
The main strength of the systematic review is adherence to the PRISMA statement and the independent search by three authors [Citation17]. A limitation of the review was the low number of studies meeting the inclusion criteria and the retrospective design of most of the included studies (exceptions are the Carruthers et al. [Citation23] and Campochiaro et al. [Citation21] studies). Finally, this review lacks (still not published) data from the first pan-European retrospective registry for AIP [Citation30].
Conclusions
Based on our results and those of this systematic review, RTX therapy seems effective in inducing and maintaining remission of AIP type 1 with a low rate of side effects. However, prospective studies, especially RCTs, are needed to fill the knowledge gap on the proper treatment of AIP.
PRISMA 2009 checklist
The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
| Abbreviations | ||
| AIP | = | autoimmune pancreatitis |
| AP | = | acute pancreatitis |
| AZA | = | azathioprine |
| BMD | = | bone mineral density |
| CP | = | chronic pancreatitis |
| CT | = | computed tomography |
| DM | = | diabetes mellitus |
| DXA | = | dual-energy X-ray absorptiometry |
| ERCP | = | endoscopic retrograde cholangiopancreatography |
| GC | = | glucocorticoid |
| HaPanEU | = | United European Gastroenterology evidence-based guidelines for the diagnosis and treatment of chronic pancreatitis |
| ICDC | = | International Consensus Diagnostic Criteria |
| IgG4-RD | = | IgG4-related disease |
| IgG4-RD RI | = | IgG4-RD responder index |
| IM | = | immunomodulating drugs |
| IRC | = | IgG4-related cholangitis |
| MFM | = | mycophenolate mofetil |
| MRCP | = | magnetic resonance cholangiopancreatography |
| MRI | = | magnetic resonance imaging |
| OOI | = | other organ involvement |
| PEI | = | pancreatic exocrine insufficiency |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| RCT | = | randomised controlled trial |
| RTX | = | rituximab |
| UEG | = | United European Gastroenterology |
| MA | = | monoclonal antibodies |
Supplemental Material
Download MS Word (27.2 KB)Disclosure statement
SN: Ferring (lecture fee), Mylan (lecture fee), Krka (lecture fee); NP: none; WR: none; LD: none; ESH: none; AH: Mylan (lecture fee); CS: none; JML: Abbott (lecture fee), Mylan (lecture fee); MV: Abbott (lecture fee), Mylan (lecture fee).
References
- Löhr JM, Beuers U, Vujasinovic M, UEG guideline working group, et al. European guideline on IgG4-related digestive disease - UEG and SGF evidence-based recommendations. United European Gastroenterol J. 2020;8(6):637–666.
- Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62(12):1771–1776.
- Soliman H, Vullierme MP, Marie F, et al. Risk factors and treatment of relapses in autoimmune pancreatitis: Rituximab is safe and effective. United European Gastroenterol J. 2019;7(8):1073–1083.
- Vujasinovic M, Valente R, Maier P, et al. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatol. 2018;18(8):900–904.
- Khosroshahi A, Wallace ZS, Crowe JL, Second International Symposium on IgG4-Related Disease, et al. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688–1699.
- Pannala R, Chari ST. Corticosteroid treatment for autoimmune pancreatitis. Gut. 2009;58(11):1438–1439.
- Yoon SB, Moon SH, Kim JH, et al. Determination of the duration of glucocorticoid therapy in type 1 autoimmune pancreatitis: a systematic review and Meta-analysis. Pancreatology. 2021. DOI:https://doi.org/10.1016/j.pan.2021.05.303.
- Okazaki K, Kawa S, Kamisawa T, Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society, et al. Japanese consensus guidelines for management of autoimmune pancreatitis: I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2010;45(3):249–265.
- Kamisawa T, Okazaki K, Kawa S, Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society, et al. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45(5):471–477.
- Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo clinic experience. Gut. 2013;62(11):1607–1615.
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–294.
- Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: Pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2(9):731–743.
- Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet (London, England). 2009;374(9701):1617–1625.
- Mattoo H, Mahajan VS, Della-Torre E, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014;134(3):679–687.
- Shimosegawa T, Chari ST, Frulloni L, International Association of Pancreatology, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the international association of pancreatology. Pancreas. 2011;40(3):352–358.
- Lopez-Serrano A, Crespo J, Pascual I, Members of the Autoimmune Pancreatitis in Spain Study Group, et al. Diagnosis, treatment and long-term outcomes of autoimmune pancreatitis in Spain based on the international consensus Diagnostic Criteria: A multi-centre study. Pancreatology. 2016;16(3):382–390.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and Meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric castleman's disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15(9):966–974.
- Backhus J, Neumann C, Perkhofer L, et al. A Follow-Up study of a european IgG4-Related disease cohort treated with rituximab. J Clin Med. 2021;10(6):1329.
- Ebbo M, Grados A, Samson M, et al. Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a french nationwide study of thirty-three patients. PLoS One. 2017;12(9):e0183844.
- Campochiaro C, Della-Torre E, Lanzillotta M, et al. Long-term efficacy of maintenance therapy with rituximab for IgG4-related disease. Eur J Intern Med. 2020;74:92–98.
- Barresi L, Tacelli M, Crino SF, Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), Italian Association for the Study of the Pancreas (AISP), et al. Multicentric italian survey on daily practice for autoimmune pancreatitis: Clinical data, diagnosis, treatment, and evolution toward pancreatic insufficiency. United European Gastroenterol J. 2020;8(6):705–715.
- Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74(6):1171–1177.
- Majumder S, Mohapatra S, Lennon RJ, et al. Rituximab maintenance therapy reduces rate of relapse of pancreaticobiliary immunoglobulin G4-related disease. Clin Gastroenterol Hepatol. 2018;16(12):1947–1953.
- Su Y, Sun W, Wang C, et al. Detection of serum IgG4 levels in patients with IgG4-Related disease and other disorders. PLoS One. 2015;10(4):e0124233.
- Wallace ZS, Mattoo H, Mahajan VS, et al. Predictors of disease relapse in IgG4-related disease following rituximab. Rheumatology (Oxford)). 2016;55(6):1000–1008.
- Tobinai K, Igarashi T, Itoh K, IDEC-C2B8 Study Group, et al. Rituximab monotherapy with eight weekly infusions for relapsed or refractory patients with indolent B cell non-Hodgkin lymphoma mostly pretreated with rituximab: a multicenter phase II study. Cancer Sci. 2011;102(9):1698–1705.
- Winthrop KL, Saag K, Cascino MD, et al. Long-Term safety of rituximab in patients with rheumatoid arthritis: Results of a Five-Year observational study. Arthritis Care Res. 2019;71(8):993–1003.
- Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56(12):3896–3908.
- Lanzillotta M, Vinge-Holmquist O, Overbeek KA, et al. PrescrAIP: a Pan-European study on current treatment regimens of Auto-Immune pancreatitis. Front Med (Lausanne)). 2020;7:408.