Abstract
Objectives
Non-alcoholic fatty liver disease (NAFLD) is more common in patients with type 2 diabetes mellitus (T2DM) compared to individuals without. Recent guidelines recommend screening for NAFLD in patients with T2DM. Our aim was to investigate the prevalence of NAFLD in patients with T2DM in a Swedish primary health care setting, how they are cared for and assess the risk of biochemical signs of advanced fibrosis.
Material and methods
In this cohort study, patients with T2DM from five primary health care centers were included. Medical records were retrospectively reviewed and living habits, medical history, results of diagnostic imaging and anthropometric and biochemical features were noted in a standardized form. The risk of steatosis and advanced fibrosis was assessed using commonly used algorithms (FLI, HSI, NAFLD-LFS, NAFLD ridge score, FIB-4 and NFS).
Results
In total 350 patients were included. Diagnostic imaging had been performed in 132 patients and of these, 34 (26%) had steatosis, which was not noted in the medical records in 16 (47%) patients. One patient with steatosis had been referred to a hepatologist. Of assessable patients, 71–97% had a high to intermediate risk of steatosis and 29–65% had an intermediate to high risk of advanced fibrosis according to the algorithms used.
Conclusion
This study indicates a high prevalence of NAFLD among T2DM patients in Swedish primary care. Patients with known NAFLD were followed up to a very low extent. Using fibrosis algorithms in primary health care would result in many patients needing further assessment in secondary care.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in developed countries with an estimated global prevalence of 25% [Citation1]. Despite the high estimated global NAFLD prevalence, a minority of patients are diagnosed with fatty liver. In a recent European study, based on more than 17 million primary care patients, less than 2% of patients had a NAFLD diagnosis [Citation2].
Type 2 diabetes mellitus (T2DM) and insulin resistance play a critical role in the pathophysiology of NAFLD [Citation3], reflected in the intertwined relationship between NAFLD and T2DM, i.e., individuals with NAFLD have an increased risk of developing T2DM, and vice versa [Citation4,Citation5]. In a recent meta-analysis, the global prevalence of NAFLD among T2DM patients was estimated to 55%, with a higher prevalence (68%) observed in Europe [Citation6]. Moreover, the estimated prevalence of advanced fibrosis amongst individuals with T2DM was 17% [Citation6]. The high prevalence of advanced fibrosis is alarming since fibrosis stage is strongly associated with increased mortality and liver related morbidity and patients with T2DM, compared to patient without T2DM, have a higher mortality rate from liver diseases [Citation7–10].
The European Association for the Study of Diabetes (EASD), Obesity (EASO) and the Liver (EASL) proposed in 2016 screening for NAFLD and advanced fibrosis in patients with T2DM [Citation11]. The gold standard for diagnosing NAFLD is liver biopsy. Because of its invasive nature and high inter- and intra-observer variability, it is not seen as a feasible option for screening. Therefore, EASD, EASO and EASL have recommended non-invasive screening in individuals with metabolic risk factors. However, to date, there is no clear consensus on how to implement these guidelines.
There are several non-invasive tests for diagnosing steatosis or advanced fibrosis. Among them are validated algorithms for the assessment of steatosis such as Fatty Liver Index (FLI), Hepatic Steatosis Index (HSI), NAFLD Liver Fat Score (NAFLD-LFS) and NAFLD ridge score. Furthermore, for the assessment of advanced fibrosis, there are several algorithms. Fibrosis-4 (FIB-4) and NAFLD Fibrosis score (NFS) are the two most validated algorithms for NAFLD. Albeit, the sensitivity is more than adequate, the specificity is poor, and, therefore, these algorithms are useful for ruling out advanced fibrosis, with negative predictive value ranging between 92% and 93% [Citation12–15].
To date, there are no studies regarding the prevalence of NAFLD in the Swedish population or how these patients are cared for in Swedish primary care. Guidelines, based on the guidelines from EASO-EASD-EASL, for risk assessment and referral decision making in patients with NAFLD were recently published from The Swedish Society of Gastroenterology [Citation16]. In these guidelines, screening for advanced fibrosis is recommended for patients with hard-to-treat obesity and patients with T2DM. Risk assessment using age adjusted FIB-4 or NFS is recommended and patients with intermediate to high score should be further assessed using transient elastography.
A recent study showed that preparedness in Europe to address the challenges linked to NAFLD varied greatly between countries and was generally low [Citation17]. Considering this it is necessary to study how the condition is handled in routine healthcare today. Our aim was to assess the prevalence of NAFLD and advanced fibrosis in patients with T2DM by the use of screening algorithms, as well as investigate if these patients had been clinically evaluated for concomitant NAFLD by their general practitioner.
Materials and methods
Study population
This retrospective cohort study of 350 randomly selected patients with T2DM was conducted in five primary care centers in the county of Östergötland, Sweden, between 2018 and 2019. The included primary care centers were selected to get a generalizable population sample with primary care centers having a medium to large sized patient population and geographically covered both urban and rural areas. All adult patients (i.e., ≥ 18 years) with T2DM were eligible for inclusion. Exclusion criteria were previously diagnosed acute or chronic liver diseases (other than NAFLD) or excessive alcohol consumption defined as AUDIT-C ≥ 4 for men or ≥3 for women and/or phosphatidylethanol (PEth) >0.3 μmol/L, carbohydrate deficient transferrin (CDT) ≥2.5%, and/or medical records indicating alcohol abuse, including alcohol use disorder diagnoses (ICD-10: F10.0-F10.9).
ICD-10 usage
All patients with a T2DM diagnosis (ICD-10 E11.0-9) noted in the digital medical records were identified and compiled in a list for each participating primary care center. Patients with multiple E11 diagnoses were identified and it was ensured that they only appeared once on the list. Study participants were then randomly selected for participation by use of Excel (Microsoft Excel, version 2012, Redmond, Washington, DC).
Data collection
The participants’ digital medical records were retrospectively reviewed. Data were collected by four resident physicians, two specialist physicians and one medical student. The review of the medical records was done standardized based on written instructions using a standardized form (Supplementary Table 1). All data were then compiled and analyzed by the same researcher. The information sought included sex, age, year of T2DM diagnosis (ICD-10 E11.0-9) as well as treatment for T2DM, including lifestyle treatment, oral antidiabetics, insulin and liraglutide. The medical history was reviewed focusing on co-morbidities, medications and lifestyle measures including patients reporting physical activity and if so hours per week, tobacco use and alcohol consumption. Clinical parameters and anthropometric data; height, weight, body mass index (BMI), waist circumference, waist-hip ratio, waist-to-height ratio, systolic and diastolic blood pressure were recorded. In addition, findings of retinopathy on fundus screening examinations as well as diagnostic imaging of the liver were recorded. If imaging had been undertaken, findings of steatosis on ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI) were noted as well as the reason for the examination.
Laboratory results from date of T2DM diagnosis were recorded, see Supplementary Table 1. If there were multiple blood or urine samples, the most recent was recorded. All included variables in the algorithm had to be registered within a 6-month interval to be valid for calculation.
The prevalence of NAFLD was estimated by the FLI, HSI, NAFLD-LFS and NAFLD ridge score algorithms () and the prevalence of advanced fibrosis was estimated by the FIB-4 and NFS algorithms () [Citation12–14,Citation18–22]. Only participants with findings of steatosis on imaging or/and intermediate to high risk for steatosis according to any of the NAFLD algorithms were assessed with algorithms for advanced fibrosis.
Figure 1. Steatosis algorithms. Note: Steatosis algorithms (FLI, HSI, NAFLD-LFS and NAFLD Ridge score) with variables and cut off values, used for assessment of patients. Metabolic syndrome defined according to the International Diabetes Foundation criteria. NAFLD: non-alcoholic fatty liver disease; NAFLD-LFS: Non-Alcoholic Fatty Liver Disease Liver Fat Score; HSI: Hepatic Steatosis Index; FLI: Fatty Liver Index; T2DM: type 2 diabetes mellitus; BMI: Body Mass Index; ɣGT: gamma-glutamyl transferase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; HDL: high-density lipoproteins; WBC: white blood cell count; HbA1c: hemoglobin A1c.
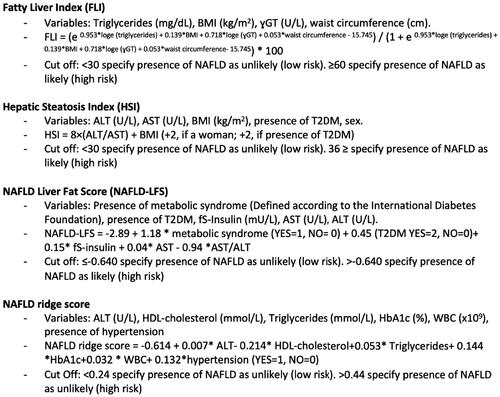
Figure 2. Advanced fibrosis algorithms. Note: Advanced fibrosis algorithms (FIB-4 and NFS) with variables and cut offs, used for assessment of patients with known steatosis or high to intermediate risk for steatosis according to steatosis algorithms. FIB-4: fibrosis-4; NFS: NAFLD Fibrosis Score; T2DM: type 2 diabetes mellitus; PC: platelet count; ALT: aspartate aminotransferase; AST: alanine aminotransferase; BMI, Body Mass Index.
Statistical analysis
Categorical variables are presented as number of patients with corresponding percentage. The Kolmogorov–Smirnov test was used to assess the distribution of continuous variables. Continuous variables are presented as mean (standard deviation [SD]) or, for those with a skewed distribution, median (interquartile range [IQR]). The Students T-test was used comparing normally distributed data and the Mann–Whitney U test was used when comparing data with skewed distribution between groups. The Chi-squared test was used for categorical data. A p-value of <.05 was considered significant. Excel (v2004, Microsoft, Redmond, Washington, DC) and SPSS version 26.0 (SPSS Inc., Chicago, IL) were used for statistical calculations.
Ethics
The participants were de-identified, and data were analyzed on group level. This study has been approved by the Regional ethics approval board in Linköping. Registration number: 2018/365–31.
Results
In total, 381 patients were initially included into the study. Of these, 31 were excluded due to acute or chronic liver diseases other than NAFLD (14 patients), excessive alcohol consumption (10 patients) or an inaccurate T2DM diagnosis (7 patients) when reviewing the individual medical records. Of the remaining 350 patients, 153 (44%) were women. Median age was 71 (range 23–96) years. The median duration from T2DM diagnosis to inclusion in the study was 9 (range 0–61) years. The most common comorbidities were hypertension (83%) and dyslipidemia (82%). In total, 83% had pharmacological T2DM treatment. Baseline data for the study population are shown in .
Table 1. Baseline characteristics of 350 patients with type 2 diabetes mellitus with and without probable steatosis.
Steatosis algorithms
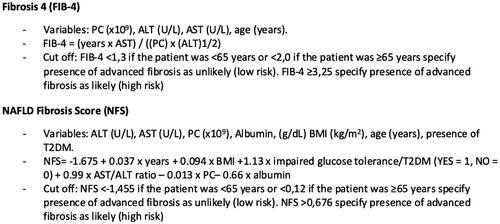
For 220 patients (63%), at least one algorithm could be calculated. NAFLD-LFS could only be calculated in 7 patients, FLI, NAFLD ridge score and HSI were calculated in 31, 165 and 175 patients respectively, see .
Figure 3. Risk assessment by use of algorithms for high risk (light grey), intermediate risk (medium grey) and low risk (dark grey) for steatosis and advanced fibrosis in patients with type 2 diabetes mellitus. Note: Risk for steatosis and advanced fibrosis was assessed with algorithms HSI, FLI, NAFLD Ridge Score, FIB-4 and NFS. FIB-4 and NFS were calculated for all assessable patients with known steatosis or intermediate to high risk for steatosis in steatosis algorithms. NAFLD: non-alcoholic fatty liver disease; HSI: Hepatic Steatosis Index; FLI: Fatty Liver Index; FIB-4: fibrosis-4; NFS: NAFLD Fibrosis Score.
In the 220 patients with at least one steatosis algorithm, 89% had a high risk for steatosis in one algorithm, 9% an intermediate risk in one or more algorithms and 2% had a low risk in all calculable algorithms, see . When imaging results were added, another 31 patients could be evaluated concerning present liver steatosis.
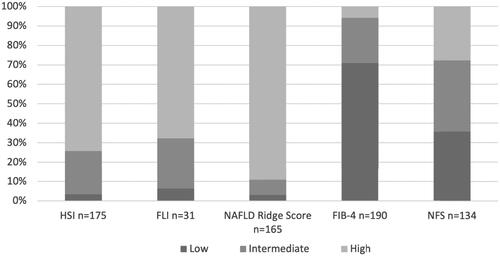
Figure 4. Panel A shows an assessment of steatosis in 350 participants with type 2 diabetes mellitus, imaging and algorithms compiled. Panel B shows an assessment of advanced fibrosis in 191 of the participants, a compilation of algorithms for advanced fibrosis. Note: Panel A: Compilation of NAFLD results for imaging and risk assessment by steatosis algorithms NAFLD-LFS, HSI, FLI and NAFLD Ridge Score for all 350 study participants. Panel B: Compilation of risk assessment with algorithms for advanced fibrosis FIB-4 and NFS for patients with known steatosis or intermediate to high risk for steatosis according to steatosis algorithm. Low risk in panels A and B is defined as low risk in all calculable algorithms, intermediate risk is defined as intermediate risk in 1–4 algorithms and none high risk assessments and high risk is defined as high risk in 1–4 algorithms. NAFLD: non-alcoholic fatty liver disease; NAFLD-LFS: Non-Alcoholic Fatty Liver Disease Liver Fat Score; HSI: Hepatic Steatosis Index; FLI: Fatty Liver Index; FIB-4: fibrosis-4; NFS: Non-Alcoholic Fatty Liver Disease Fibrosis Score.
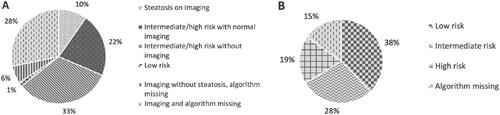
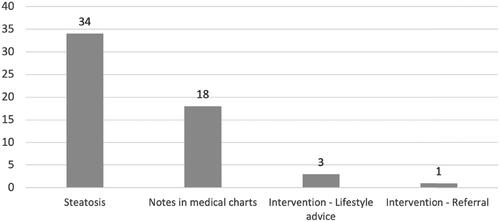
Figure 5. Results of imaging and clinical management of confirmed steatosis in this cohort of 350 patients with type 2 diabetes mellitus. Note: Present steatosis was a compilation of findings of steatosis on diagnostic imaging (US, CT and MRI) and the clinical management of known steatosis. A total of 132 (37.7%) patients had done one or more diagnostic imaging of the liver. US: ultrasound; CT: computer tomography; MRI: magnetic resonance imaging.
Advanced fibrosis algorithms
FIB-4 and NFS could be assessed in 134 and 190 patients, respectively, and of these 29% and 64% had an intermediate to high risk of advanced fibrosis, see . When results from all advanced fibrosis algorithms were summarized, data were available for 191 patients, see .
Coherence of steatosis and advanced fibrosis algorithms
Coherence of the steatosis algorithms for the individual patients was assessed. Of the patients 125 were assessable with two or three algorithms. A total of three patients were assessable with four algorithms and these were excluded from this analysis because of low assessment rate. The risk for steatosis in the patients that were assessable with two or three algorithms was concordant in 65–70% of the cases. Data were also compiled for the individual patients on how consistent the advanced fibrosis algorithms were. For 132, both FIB-4 and NFS could be calculated. The calculated risk was concordant in 45% of the assessable patients.
Imaging and clinical management of NAFLD
Abdominal imaging had been performed in 132 patients. A total of 167 examinations (US, CT or MRI) had been done and of these 31 were performed on suspicion of liver disease. Of the 132 patients, 34 (26%) had signs of steatosis on imaging. Notes of steatosis or NAFLD (diagnosis code, mention in free text or copied imaging results) was present, in the medical records, for 18 (53%) of these 34 patients. A recommendation of an intervention was given for four of the 18 patients. The interventions recommended were weight loss, intensified diabetic treatment and referral to a hepatologist, see .
Screening test
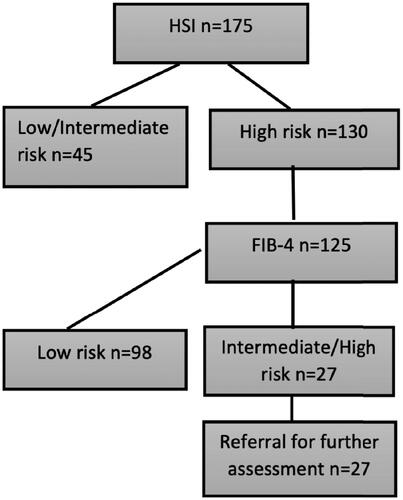
The EASL, EASD and EASO guidelines was theoretically tested using HSI and thereafter FIB-4. Of the assessable 125 patients, five (4%) had a high risk, 22 (18%) an intermediate risk and 98 (78%) a low risk for advanced fibrosis. Using the EASL, EASD and EASO guidelines for screening, resulted in a need of referral for 22% of the assessable patients, see .
Figure 6. Screening for steatosis and advanced fibrosis in this cohort of 350 patients with type 2 diabetes mellitus according to the proposed screening algorithms from the European Association for the Study of Obesity, Diabetes, and the Liver. Note: HSI is used for assessing risk of steatosis and thereafter FIB-4 for advanced fibrosis in appliable patients. A theoretical assessment of the results of the proposed screening algorithm from the European Association for the Study of Obesity, Diabetes, and the Liver. HSI: Hepatic Steatosis Index; FIB-4: fibrosis-4.
Characteristics of risk groups for advanced fibrosis
All assessable patients with fibrosis algorithms were divided into low risk and intermediate to high risk for advanced fibrosis. If both algorithms had been assessed the highest risk result was applied. The patients with estimated high to intermediate risk for advanced fibrosis compared to low risk were significantly heavier and had higher waist-to-height-ratio but there were no differences regarding sex, diabetes duration, diabetes treatment or present retinopathy. Patients with hypertension, malignancy, cardiac arrythmia and angina pectoris were more often at high to intermediate risk of advanced fibrosis compared to those without the comorbidities, see .
Table 2. Characteristics and comparison of groups high/intermediate risk and low risk according to algorithms (FIB-4 and NFS) for advanced fibrosis in patients with type 2 diabetes mellitus.
Discussion
In this retrospective study of a primary care cohort of 350 T2DM patients, a majority had NAFLD when estimated with algorithms. However, very few had clinically been assessed for NAFLD and even fewer had been diagnosed with NAFLD. Algorithms for advanced fibrosis showed that 29–65% had intermediate to high risk. If the proposed guidelines for screening had been applied to this cohort, 22% of the assessed patients would need to be referred for further assessment in specialized care.
The strengths of this study are that all included patients at the primary health care centers in the county council of Östergötland share the same digital medical records, making the data collection standardized and data more comparable. Data collection was done using a standardized case report form ensuring uniform collection by the examiners. Another strength is that the vast majority of Swedish patients with T2DM receive treatment in primary health care and the care provided is homogenous due to the structured work according to the Swedish national guidelines for diabetes care. Furthermore, all the included laboratory results were analyzed by the same laboratory service provided by the county council of Östergötland. Primary care centers with medium- to large-sized populations and located in both urban andrural areas were selected to get a generalizable population sample. Some differences were noted in key variables in a comparison of the primary care centers (Supplementary Table 2) but the study was not statistically powered to draw any conclusions from this.
A limitation of the study is that the data were collected by seven different examiners. However, an evaluation of the data collection in a sample of 30 participants showed excellent agreement (Supplementary Table 3). Another limitation of the results is that the prevalence of NAFLD and advanced fibrosis are, in many patients, based on results from NAFLD algorithms alone and only in a minority of cases on imaging or other diagnostic modalities. This could overestimate the prevalence because of the low specificity of the algorithms [Citation12–15]. Further limitations of this study are that presentation of an overall prevalence as a summarization of all the algorithms also probably overestimate the prevalence in the group because of low consistency between algorithms. Patients with an abundance of blood samples and imaging, facilitating the use of the algorithms, are most likely overall sicker, which could render a bias of assessment of patients that may have a higher risk for NAFLD and advanced fibrosis. Of patients that had done diagnostic imaging, a minority was done to evaluate steatosis, therefore, it not certain that the radiologist has commented on steatosis in all the cases where it was present.
In our study, 29–65% of assessable patients had intermediate to high risk for advanced fibrosis which aligns with previous findings. In a large register study, FIB-4 was used to assess the risk of advanced fibrosis in primary health care patients with verified or probable NAFLD diagnosis [Citation2]. Of these patients, 30–36% had intermediate to high risk. In another study of patients with NAFLD in primary health care, 43% had an intermediate to high risk for advanced fibrosis [Citation23]. In these studies, as well as in our own, NFS predicted a greater proportion of patients with intermediate to high risk. In our study, NFS could less often be calculated compared to FIB-4, primarily due to more input variables. Based on this, FIB-4 could be a more practical and resource saving option in primary health care for risk assessment of advanced fibrosis in T2DM patients.
When the group with a low risk of advanced fibrosis was compared with the group with intermediate to high risk, it was noted that cardiac arrhythmias and angina pectoris were significantly more common in the group with higher risk. The link between NAFLD and cardiac arrhythmias and especially atrial fibrillation is known [Citation24] and the risk increases with concomitant T2DM and with more advanced liver disease [Citation25]. The link between NAFLD and cardiovascular disease is well known, and the risk increases with more advanced liver disease [Citation24]. There was also an association between higher risk for advanced fibrosis and malignancies. Previous studies have shown an increased risk of colorectal cancer in patients with more advanced liver disease [Citation26]. Patients with higher risk of advanced fibrosis were significantly heavier, had a higher waist-to-height ratio and women with increased risk had a greater waist circumference. This is in concordance with previous studies showing that obesity, waist circumference and waist-to-height ratio seem to predict the presence of advanced liver disease [Citation27,Citation28] as well as cardiovascular disease risk [Citation29]. Cut-offs for overweight and obesity for anthropometric measurements are difficult to use for cardiovascular risk evaluation in a cohort with type 2 diabetes patients, because of the high prevalence of overweight and obesity in this patient group. Waist-to-height-ratio has shown promising results for cardiovascular disease risk prediction, with a cut-off of 0.6 for patients with type 2 diabetes [Citation29].
Few patients had been assessed using imaging for NAFLD and a low number of physicians asked about steatosis in their referrals. In patients where imaging findings of liver steatosis were present, a suitable diagnosis or mentioning in the medical records was rare, and, furthermore, intervention or follow up was uncommon. This could reflect a low awareness in Swedish primary health care of NAFLD and its potentially serious complications. There are several studies supporting the presence of low awareness in a primary care setting [Citation30–32]. For instance, in a survey about the awareness of prevalence, clinical manifestations, and diagnostics of NAFLD among Australian primary health care physicians, large knowledge gaps were discovered [Citation30]. To determine the prevalence and incidence of NAFLD and advanced fibrosis in Swedish primary care patients with T2DM the EPSONIP-study (Evaluating the Prevalence and Severity Of NAFLD in Primary Care) recently started [Citation33]. The result of this study could help increase the awareness of NAFLD in primary care.
The prevalence of NAFLD is high. However, according to our study and previous studies most patients with the condition are unknown to their primary health care practitioners [Citation2,Citation32]. Albeit, the majority of patients with NAFLD are stable, some will progress to non-alcoholic steatohepatitis (NASH) with progressive fibrosis and consequently cirrhosis [Citation9]. Finding these patients by utilizing the guidelines as proposed by the EASL, EASD and EASO could be feasible. In our study, we showed that 22% needed referral for a hepatologist evaluation. Similar results were shown by Ciardullo et al., where 13% of screened patients were in need of referral for additional evaluation [Citation34]. Furthermore, risk assessment of diagnosed NAFLD in primary health care has been shown to be cost effective [Citation35,Citation36].
In our screening, only participants with intermediate to high risk for steatosis were evaluated with FIB-4. When FIB-4 was calculated for the remaining six patients that had a low risk of steatosis, two had an intermediate risk for advanced fibrosis suggesting that patients with advanced fibrosis could be missed. However, in this population, where few had undergone abdominal imaging, steatosis algorithms were the best available method. Furthermore, it is problematic to use fibrosis scores developed for NAFLD in patients with T2D without known steatosis. Using algorithms for assessing the risk of steatosis and advanced fibrosis in patients in primary health care is an appealing alternative to liver biopsy and transient elastography because of the low cost and easy applicability [Citation11]. Most of the advanced fibrosis algorithms have thresholds for both low and high risk of concomitant advanced fibrosis in NAFLD, and values in between these thresholds are defined as intermediate needing further assessment. Nevertheless, the number of patients assessed as having an intermediate risk for advanced fibrosis can be decreased using age-adjusted thresholds for FIB-4 and NFS [Citation14,Citation34].
Concerns have been raised on whether screening is cost-effective, especially since most available non-invasive tests have low positive predictive values and because of few treatment options [Citation37]. There are for the moment no recommendations of pharmacological treatment of NAFLD but there are several ongoing studies [Citation38,Citation39]. There is, however, evidence that steatosis could be treated with 5–10% weight loss, physical exercise and recommendations against excessive alcohol consumption [Citation37,Citation40]. Regular screening for esophageal varices and hepatocellular carcinoma should be conducted in patients developing cirrhosis [Citation41,Citation42].
To date, there are no guidelines for screening individuals with T2DM for NAFLD in primary health care in Sweden. According to the results in this study, a large proportion of the patients with T2DM would have to be referred to secondary care for further evaluation if screened for NAFLD/advanced fibrosis using existing algorithms. If screening is to be considered, it would be wise to choose algorithms that are easily applied, using blood tests and anthropometrics commonly measured in T2DM care. Two such algorithms are HSI and NAFLD ridge score which both were calculable in a large proportion of patients compared to FLI and NAFLD-LFS in this study.
Conclusion
This study indicates that there are many unknown cases of NAFLD among patients with T2DM in Swedish primary health care. The few patients that did have a NAFLD diagnosis were monitored and treated to a very low extent. A fifth of the patients with T2DM needed further assessment of liver disease in secondary care.
Supplemental Material
Download Zip (43.1 KB)Acknowledgments
The authors thank Ajla Aleckovic, Alexander Zorbas and Axel Lihagen for their help with the data collection.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
- Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–455.
- Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910.
- Nasr P, Fredrikson M, Ekstedt M, et al. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020;40(5):1069–1078.
- Younossi ZM, Golabi P, De Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
- Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611–1625.e12.
- Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273.
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554.
- Campbell PT, Newton CC, Patel AV, et al. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844.
- European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402.
- Sterling RK, Lissen E, Clumeck N, APRICOT Clinical Investigators, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854.
- McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740–751.
- Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501.
- Swedish Society of Gastroenterology. Nationell riktlinje – Utredning och handläggning av fettleversjukdom; [Swedish guidelines for NAFLD]. 2020 [cited 2021 May 5]. Available from: https://svenskgastroenterologi.se/wp-content/uploads/2020/02/2020-utredning-och-handlaggning-av-fettleversjukdom.pdf.
- Lazarus JV, Palayew A, Carrieri P, et al. European 'NAFLD Preparedness Index' – Is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021;3(2):100234.
- Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33.
- Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508.
- Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872.
- Yip TC, Ma AJ, Wong VW, et al. Laboratory parameter-based machine learning model for excluding non-alcoholic fatty liver disease (NAFLD) in the general population. Aliment Pharmacol Ther. 2017;46(4):447–456.
- Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group, et al. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366(9491):1059–1062.
- Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234–240.
- Stahl EP, Dhindsa DS, Lee SK, et al. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(8):948–963.
- Käräjämäki AJ, Hukkanen J, Ukkola O. The association of non-alcoholic fatty liver disease and atrial fibrillation: a review. Ann Med. 2018;50(5):371–380.
- Mantovani A, Scorletti E, Mosca A, et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170.
- Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17(4):e1003100.
- Andreasson A, Carlsson AC, Önnerhag K, et al. Waist/Hip ratio better predicts development of severe liver disease within 20 years than Body Mass Index: a population-based cohort study . Clin Gastroenterol Hepatol. 2017;15(8):1294–1301.e2.
- Rådholm K, Chalmers J, Ohkuma T, et al. Use of the waist-to-height ratio to predict cardiovascular risk in patients with diabetes: results from the ADVANCE-ON study. Diabetes Obes Metab. 2018;20(8):1903–1910.
- Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J. 2018;48(2):144–151.
- van Asten M, Verhaegh P, Koek G, et al. The increasing burden of NAFLD fibrosis in the general population: time to bridge the gap between hepatologists and primary care. Hepatology. 2017;65(3):1078.
- Blais P, Husain N, Kramer JR, et al. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10–14. 134
- Nasr P, Iredahl F, Dahlström N, et al. Evaluating the prevalence and severity of NAFLD in primary care: the EPSONIP study protocol. BMC Gastroenterol. 2021;21(1):180.
- Ciardullo S, Muraca E, Perra S, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8(1):e000904.
- Tapper EB, Hunink MG, Afdhal NH, et al. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One. 2016;11(2):e0147237.
- Tanajewski L, Harris R, Harman DJ, et al. Economic evaluation of a community-based diagnostic pathway to stratify adults for non-alcoholic fatty liver disease: a Markov model informed by a feasibility study. BMJ Open. 2017;7(6):e015659.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357.
- Younossi ZM, Ratziu V, Loomba R, REGENERATE Study Investigators, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196.
- Sumida Y, Yoneda M, Ogawa Y, et al. Current and new pharmacotherapy options for non-alcoholic steatohepatitis. Expert Opin Pharmacother. 2020;21(8):953–967.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5.
- Loomba R, Lim JK, Patton H, et al. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158(6):1822–1830.
- Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases. Hepatology. 2017;65(1):310–335.
