Abstract
Background and aims
Glucagon-like peptide-1 receptor agonist ROSE-010 has been studied for management of irritable bowel syndrome (IBS). ROSE-010 showed promising effects by reducing pain during attacks of IBS. In this exploratory substudy, we cross-analyzed earlier data to identify the most suitable subpopulation for treatment with ROSE-010.
Methods
Data comprising 166 participants (116 females, 50 males) treated by subcutaneous injection with ROSE-010 at 100 µg and 300 µg versus placebo were broken down into subpopulations with recall of historical pain intensity, pain intensity immediately before treatment, gender, age, BMI, IBS subtype as well as pain intensity and pain relief of ROSE-010 with relationship to plasma glucose using visual analogue scores. Statistical cross-analysis was performed to detect optimal responders for adequate pain relief response.
Results
ROSE-010 gave dose- and time-dependent effects with maximum pain relief at 300 µg relative 100 µg and placebo at 120 min post injection. Females had greater pain relief than males; age and BMI did not affect treatment response. IBS pain relief was greatest in constipation-dominant IBS (IBS-C) and mixed IBS (IBS-M) relative diarrhea-dominant and unspecified IBS.
Conclusions
Clinical trial data indicate that female participants are more likely than males to respond to ROSE-010 100 µg and 300 µg to achieve meaningful IBS pain relief. Maximum pain relief was achieved at 120 min with the higher dose, although this was accompanied with higher rates of nausea. Improvement of IBS pain attacks was most pronounced in IBS-C and IBS-M, suggesting these subgroups to be optimal ROSE-010 responders.
Introduction
Irritable bowel syndrome (IBS), a common gastrointestinal (GI) disorder affecting around 5–10% of populations worldwide, is characterized by recurrent abdominal pain, bloating and disturbed bowel habits with either constipation, diarrhea or both [Citation1,Citation2]. The Rome IV diagnostic criteria, which are based on the clinical symptoms of the patient, are currently considered the gold standard for IBS diagnosis [Citation3]. Accordingly, IBS is classified by bowel habits, bowel function and stool consistency into four subtypes: constipation dominant (IBS-C), diarrhea dominant (IBS-D), mixed (IBS-M) and unclassified/unspecified IBS (IBS-U). The majority of individuals with IBS alternate in bowel habits between IBS-C and IBS-D, of which IBS-C is the most commonly diagnosed subtype in the clinic account for more than 25% of cases [Citation4]. Patients classified with IBS-C or IBS-M are reported to exhibit more severe symptoms compared to those with other subtypes [Citation5], whereas IBS-D patients exhibit more frequent pain attacks than IBS-C and IBS-M [Citation6]. Additionally, other IBS accompanying symptoms comprise depression, anhedonia, fear, stress and embarrassment which all impact quality of life [Citation5]. Pathophysiology and underlying mechanisms of IBS are still ambiguous and inadequately understood due to its multifactorial etiology. Pain associated with IBS is assumed to be a consequence of the disrupted smooth muscle activity together with reduced sensory threshold that results in visceral hypersensitivity [Citation2,Citation7]. Lately, additional factors have been identified, including alterations in gut immune activation [Citation8], intestinal permeability and colonic microbiota and microbiome [Citation9–11]. Despite a high incidence, economic and health burden of IBS and its severity, treatment is extremely challenging and feasible therapy strategies are still limited [Citation12]. Currently, the main treatment strategy is to relieve symptoms and improve quality of life. Pharmacological approaches for management of IBS symptoms include smooth muscle relaxants, tricyclic antidepressants, selective serotonin reuptake inhibitors and anticonvulsants [Citation13].
Gastric emptying is the initial step in the metabolic endocrine cascade after food intake. Incretin hormones, particularly glucagon-like peptide-1 (GLP‐1) released postprandially from L-cells lining the gut in response to food ingestion, exert multiple biological actions including stimulation of insulin secretion, inhibition of glucagon and gastric acid secretion and decrease of GI transit and motility [Citation13–16]. Moreover, GLP-1 has been repeatedly shown to relax GI muscle activity through nerve-mediated processes dependent on nitric oxide, both in vitro and in vivo [Citation6,Citation17,Citation18]. Biologically active GLP-1 has a high affinity for the GLP-1 receptor (GLP-1R) [Citation19,Citation20], which, aside from being expressed in peripheral tissues, is also expressed in the central nervous system (CNS) and restricted to neurons in caudal nucleus of the solitary tract (NTS) and the ventrolateral medulla in the brainstem and hypothalamus [Citation21–23]. The GLP-1R has also been detected in both myenteric and submucosal neuronal plexuses in the GI tract [Citation17,Citation22]. Numerous studies have shown a major impact of GLP-1 and its specific analogue ROSE-010 on the motility pattern of the gut. GLP-1 reduced motility in the antro-duodeno-jejunal region and inhibited the migrating motor complex (MMC) in healthy subjects and also in patients with IBS [Citation24]. In a placebo-controlled double-blind crossover clinical trial, administration of the GLP-1 analogue ROSE-010 to a mixed group of IBS patients reduced GI motility and relief of acute pain was reported [Citation6].
Decreased serum GLP-1 concentrations [Citation25] and mucosal expression of GLP-1R were associated with constipation-predominant IBS. Moreover, this was correlated with severity of abdominal pain [Citation25], which led to the hypothesis that lower concentrations of GLP-1 might cause loss of the pro-kinetic effects of GLP-1 in the colon [Citation26], resulting in constipation and abdominal pain. Circulating concentrations of bioactive GLP-1 were also decreased in a rat model of visceral pain sensitivity [Citation27]. Recent studies showed that GLP-1 and ROSE-010 inhibit postprandial GI motility, likely through GLP-1R at myenteric neurons, this requiring functional nitrergic and cAMP signaling [Citation17].
Administration of ROSE-010 is generally well tolerated but adverse events (AEs) occur. Expected reactions to the drug that include nausea, vomiting, headache and decreased blood glucose concentration are rarely severe [Citation6,Citation26].
In this study, combining case report forms (CRFs) and data files (software SAS/HTML) from patients who participated in the GLP-1 analogue ROSE-010 clinical trial, an exploratory cross-analysis was performed on several variables of the IBS population including gender, age, weight, treatment injection order, pain profile and score, concurrent medications and IBS subgroup in order to specify the most suitable sub-population for ROSE-010 treatment.
Materials and methods
Study design
The material for this investigation is an excerpt from a clinical trial in IBS patients receiving cross-over treatments with placebo and the GLP-1 analogue ROSE-010 at 100 or 300 µg doses () [Citation6]. Patient outcomes were recorded as pain relief and pain intensity ratings at timepoints 0, 10, 20, 30, 40, 50, 60, 90 and 120 min post injection. In preparation for an upcoming study of ROSE-010 in IBS management, the previous study was analyzed to identify optimal patient outcomes defining adequate treatment result.
Figure 1. The flowchart of the study showing the total number of randomized subjects included in the full analysis set (n = 166) and the subjects included in the modified analysis set (n = 99).
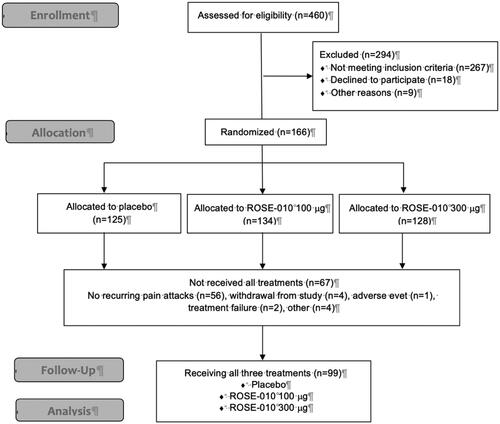
The protocol (#GL61-001) was approved by the Ethics review boards of Stockholm, Göteborg, Linköping, Lund, Marburg and Køge University Hospitals for the ethics permit KI Dnr 03-387 (2003-11-03). All patients provided signed informed consent.
The present report consists of two parts. First, several characteristics of the study population were studied in depth, such as gender, age, body weight, IBS subtype as determined in retrospect from CRFs, historical pain intensity and pain intensity immediately before injection of ROSE-010 and treatment results extracted from the clinical trial database, thus generating new experimental readouts. Second, pain relief and pain intensity response as a result of treatment with the two different doses of ROSE-010 measured at three time points, were cross-analyzed. In order to validate the reliability of the clinical response to ROSE-010, a Bland–Altman scatterplot of the difference between pain intensity and pain relief at 120 min post injection in relation to standard deviation was constructed.
Drug formulation and treatments
Vials containing freeze-dried ROSE-010 (originator LY307161) were delivered to the central pharmacy for labeling. The reconstitution of ROSE-010 and the preparation of all study treatment syringes was performed by unblinded registered nurses who were not further involved in the study. Reconstituted study drug was transferred to syringes in a fixed volume of 0.3 mL. For placebo treatment, 0.3 mL of isotonic saline (NaCl) solution was transferred to identical syringes ().
Table 1. Details on drug formulation, solutes, reconstitution, concentrations and injection volumes.
Within one hour of an acute typical abdominal pain attack, screened patients were instructed to go to their study site for treatment. Patients received one treatment with placebo, one treatment with ROSE-010 100 µg and another treatment with ROSE-010 300 µg in a random order; hence, a total of three treatments were performed on three different occasions. Subcutaneous injections of study drug were given by a registered nurse in the patients’ gluteal region to prevent any increased abdominal pain from injection in the abdominal area. Treatments were given over a maximal period of three months, with a minimum of 24 h between each study drug administration, not being administered later than one hour after the patients’ arrival to the study site.
Blinding of the study
For blinding purposes, randomization in blocks was performed by Clinical Data Care AB (Lund, Sweden) and stratified by site. The subjects were randomized to one of six treatment sequences [Citation6]. Eligible patients were numbered in sequential order and sealed envelopes individually labelled with the unique identification number were provided to each study site. All patients, the responsible investigator of the study as well as the statistician responsible for the study analyses were blinded to the performance of the study. Each dose was of identical appearance to ensure a double-blind design.
Rating of pain
Using a visual analogue scale (VAS) 0–100, pain intensity was compared with both historical and baseline abdominal pain intensity, as well as pain intensity at 30, 60 and 120 min after treatment in order to investigate a change of the VAS by subcutaneous injection of placebo or ROSE-010 at 100 µg or 300 µg doses. In similar fashion, pain relief responses to placebo or ROSE-010 at either dose at the same time points were evaluated.
Pain relief response to the three different treatments was determined according to the order (first, second or third) of each given injection. In this way, pain relief response at all three time points in subjects who were administered placebo as first treatment, was compared with pain relief response of those who received placebo as the second or third treatment. The same analysis was implemented on both doses of ROSE-010 (). In the placebo group, the first order of treatment included 59 subjects, the second 36 and the third order of treatment 30 subjects, whereas with ROSE 100 µg the first order treatment group included 54 subjects, the second 46 and the third 34, and with ROSE-010 300 µg the first order treatment group included 53 subjects, the second 40 and the third 30 subjects. This approach is designed to determine whether the order of treatment would influence the results.
The response to treatment with placebo and ROSE-010 was studied in four different subtypes of IBS, namely IBS-C, IBS-D, IBS-M and IBS-U. This was evaluated in two ways: first as pain relief according to the VAS scores; second as number and fraction of responders to treatment. Patients with at least 50% total pain reduction at 60 or 120 min after treatment were classified as responders. Those with a pain reduction less than 50% were classified as non-responders.
The age of participating patients was sorted into five subgroups according to the following: 18–25, 26–35, 36–45, 46–55 and 56–70 years of age. Four subgroups of patients were sorted according to body mass index (BMI), e.g., underweight 15–18.4, normal 18.5–24.9, overweight 25–29.9 and obese 30–41 kg/m2. These subgroups were compared to each other in terms of maximum pain relief response at 120 min post injection of ROSE-010 300 µg. Adverse events were documented in the database. Observed cases with AEs were tabulated with only endometriosis and chest pain reported as serious AEs, assessed as not related to study treatment.
Plasma glucose concentrations
Plasma glucose concentrations were related to treatment effect of GLP-1, since pain intensity would be expected to be related to high plasma glucose concentrations. Reduction of plasma glucose as a theoretical AE to ROSE-010 (being a GLP-1 analogue) was analyzed in all patients, at baseline and at 60 and 120 min post injection to determine if ROSE-010 significantly affected plasma glucose concentration and had any relationship to the pain relief response.
Statistical analysis
Student’s t-test was employed to assess differences in pain response between genders. One-way ANOVA fitting a mixed model was used to compare differences in pain intensity and pain relief, as well as pain relief response with different injection order. The Bland–Altman test was used to visualize coherence between pain intensity and the pain relief as measurements of treatment response. The proportion of pain relief responders among the different IBS groups was evaluated by chi-square test for trend. Values are presented as mean ± SEM.
Results
Pain relief and pain intensity response in relation to history, dose, time and treatment order
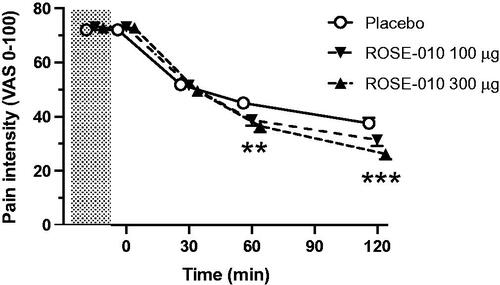
Further analysis showed an overall significant reduction of pain intensity with ROSE-010. Historical pain intensity as well as baseline abdominal pain intensity immediately before injection was clearly reduced after ROSE-010 treatment at all three time points (p < .0001) (). When comparing the different treatments, there was a significantly stronger response to 300 µg ROSE-010 at 60 min (p = .007) and 120 min (p = .0004) minutes as compared to placebo.
Figure 2. Historical pain intensity (shaded area), baseline pain intensity immediately before injection of ROSE-010 and further pain intensity reduction at 30, 60 and 120 min after ROSE-010 (placebo; n = 125, ROSE-010 100 µg; n = 134 and ROSE-010 300 µg; n = 128). Values are mean ± SEM. **p = .007, ***p = .0004.
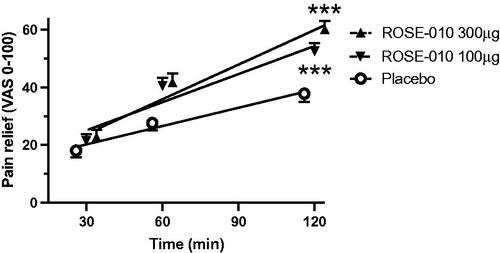
Dose and time response curves for pain relief with subcutaneous ROSE-010 and placebo are illustrated in . After subcutaneous injection of ROSE-010, there was a time related increase of the pain relief reaching maximum at 120 min. There was a significant difference between the sequential time points (p < .0001), as well as regards the maximum pain relief response between placebo versus ROSE-010 at 100 or 300 µg (both p < .0001), but not between the two doses of ROSE-010 (p = .39).
Figure 3. Linear regression of pain relief response at 30, 60 and 120 min after subcutaneous injection of placebo (r = 0.26; n = 125), ROSE-010 100 µg (r = 0.36; n = 134; p) and ROSE-010 300 µg (r = 0.41; n = 128). Values are mean ± SEM. ***p<.001.
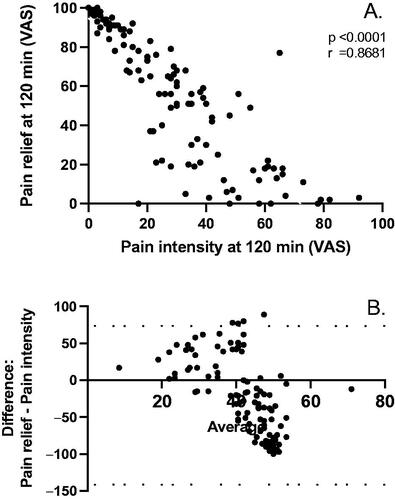
By comparing pain relief with reduction in pain intensity, we found an inverse correlation between these two parameters (p < .0001; r = 0.8681) (). This was further analyzed with a Bland–Altman scatterplot which showed the excursion of four out of 128 (2%) observations outside the 95% confidence interval for the average difference between pain relief and pain intensity at 120 min (). Similar results were obtained at 60 min after treatment with two out of 134 observations (1.5%) outside the 95% confidence interval (data not shown). Hence, either way pain relief was analyzed, ROSE-010 improved IBS pain attacks.
Figure 4. (A) Correlation between pain relief and pain intensity. (B) Bland–Altman’s plot showing agreement between the two estimates of pain at 120 min where the horizontal axis shows the average of the two measurements, and the vertical axis shows the difference between the two measurements with the 95% confidence interval for the difference (dotted lines).
When comparing pain relief at 30, 60 and 120 min post injection of the first, second and third order of each treatment, no significant differences were found between interchanging orders of ROSE-010 treatment. At the 120 min readout, the pain relief with placebo at the first, second and third injection was 24.5 ± 3.3%, 36.2 ± 5.1% and 22.6 ± 2.9% (p = .65), while the corresponding pain relief reduction with ROSE-010 100 µg was 36.7 ± 3.9%, 40.6 ± 4.1% and 37.2 ± 5.1% (p = .99), and with ROSE-010 300 µg 47.0 ± 4.3%, 41.3 ± 4.5% and 33.6 ± 4.2% (p = .82).
Pain relief response in relation to demographics and plasma glucose concentrations
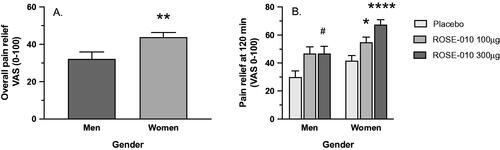
Comparison of the pain relief by ROSE-010 showed a higher overall response in women than in men at both doses (p = .0069) (). When comparing the maximum pain relief at 120 min of ROSE-010 to placebo, men showed borderline response to 100 µg (p = .0560), a marked response to 300 µg (p = .0407), but no difference between the doses. In women, there was a dose-related response to 100 µg (p = .0201) and 300 µg (p < .0001) versus placebo. Also, in women there was a significant difference between the two doses (p = .0343) ().
Figure 5. (A) General responsiveness to ROSE-010 in men and women. (B) The response characteristics of men and women to treatment with ROSE-010. Values are mean ± SEM. #p = .0407; *p = .0201; **p = .0069; ****p<.0001.
The age of the patients showed no correlation to maximum pain relief achieved at 60 or 120 min after ROSE-010 administration treatment (data not shown). Neither did body weight influence the response to treatment (data not shown).
In terms of plasma glucose, concentrations under treatment with ROSE-010 300 µg showed a slight drop from 5.3 ± 0.1 mmol/L at baseline to 4.9 ± 0.1 at 60 min (p < .0014), thereafter rising to 5.1 ± 0.1 mmol/L at 120 min which was not different from baseline values (p = .4056). There was no correlation between plasma glucose levels and pain relief.
Pain relief response in different IBS subtypes
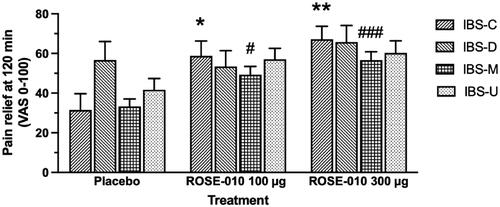
When the four subtypes of IBS were compared in terms of the maximum pain relief at 120 min after treatment with placebo, ROSE-010 100 µg and ROSE-010 300 µg there was a clear-cut response in patients with IBS-C and IBS-M, whereas IBS-D and IBS-U were less responsive ().
Figure 6. Pain relief at 120 min after subcutaneous injection of placebo and GLP-1 at 100 and 300 µg in different subgroups of IBS. Subtypes of irritable bowel syndrome (IBS): IBS-C: constipation-dominant; IBS-D: diarrhea-dominant; IBS-M: mixed; IBS-U: unspecified IBS. Values are mean ± SEM. *p = .0434, **p = .0065, #p = .0236, ###p = .0004.
In a similar manner, based on the ratio of pain relief responders in each IBS subtype, a chi-square test for trend showed significant response to ROSE-010 in IBS-C (p = .0105) and IBS-M (p < .0001), but only with a tendency in IBS-U (p < .0786) and none in IBS-D (p = .2661) ().
Table 2. Pain relief responders with a pain reduction of at least 50% within 1 h of ROSE-010 injection subcutaneously in different IBS subtypes.
Adverse events
Frequently occurring AEs that occurred after injection of ROSE-010 are shown in . There was a dose-relationship with 45% of AEs occurring after the low dose and 66% after the high dose of ROSE-010. Nausea, vomiting and dyspepsia were expected AEs, related to an inhibition of gastric emptying by ROSE-010 and were found in 69 subjects. Nausea was more common in women (72%) and than in men (28%), as was vomiting (women 91%; men 9%). Hypoglycemia was also more frequently seen in women (62%) than in men (38%), but with no plasma glucose below 3.3 mmol/L at 60 min post-dose (analysed only at select sites), while seven subjects had plasma glucose below 3.3 mmol/L at 120 min, one of which after placebo. Plasma glucose of 3.3–3.9 mmol/L was seen in 13 subjects after administration of ROSE-010, eight of which at 60 min and six at 120 min. However, in six subjects plasma glucose below 3.9 mmol/L was found already under baseline conditions, unrelated to plasma glucose reductions during treatment. Dizziness or vertigo was reported by 18 participants, in half of which related to nausea, but otherwise unrelated to any tachycardia or blood pressure changes. Headache, likely related to circulatory effects of ROSE-010, occurred in 14 participants. Similarly, feelings of coldness, possibly related circulatory effects, was found in 7. Other AEs such as abdominal pain, back pain, electrolyte disturbances, palpitations, insomnia, depression, dry mouth, constipation and diarrhea were only found in single individuals but of mild or moderate severity. Three serious AEs were encountered, two cases of chest pain and one endometriosis, all appearing about two weeks after the ROSE-010 injection ().
Table 3. Number of patients with adverse events related to treatment with ROSE-010 at doses 100 µg and 300 µg compared with placebo.
Discussion
Results from this cross-analysis, conducted on 166 patients with four subtypes of IBS, verified as hypothesized, that the GLP-1R agonist ROSE-010 mitigates acute pain attacks in IBS, which is consistent with previously published results [Citation6]. Effects of ROSE-010 treatment, in terms of pain relief and pain intensity, was found to be dependent on both dose- and time-response relationships with the maximum pain relief response at 120 min post injection with 300 µg ROSE-010. A marked gradual decline in VAS scoring of the abdominal pain intensity was observed from both the retrospective historical pain intensity as well as the pain intensity immediately before the ROSE-010 injection to 30, 60 and 120 min post treatment with the maximum pain reduction at 120 min post treatment. Comparison of each ndividual's scored pain intensity and meaningful pain relief was found to be inversely correlated. By adding a Bland–Altman plot, we found that all points were scattered, both below and over the zero line suggesting that no consistent bias of one approach versus the other could be perceived. Few points were dispersed outside the 95% confidence interval of the comparison. Therefore, the reduction in pain intensity and the pain relief response would indicate a similar clinical treatment response, i.e., meaningful pain relief.
Gender demonstrated a considerable impact on the treatment outcome in which female participants responded more favourably than males with greater pain relief. Although IBS is more prevalent among women [Citation28], an explanation of why is not obvious. It has been suggested that females are more prone to IBS due to hormonal factors since sex hormones display a crucial role in regulating gut motility or peristalsis, perception of visceral pain and stress, as well as inhibiting contraction of the smooth muscles. Therefore, any disturbances in this system have been suggested to promote the development of IBS-associated pain attacks [Citation28]. Other individual characteristics such as age and BMI were found not to influence pain relief to ROSE-010. Furthermore, no relationship was found between pain relief with ROSE-010 and plasma glucose concentration, which fell at the 60-minute readout but rapidly regained normal levels and remained stable after treatment.
As regards possible significance of the interchanging injection order for the pain relief of the three subcutaneous treatments; placebo, ROSE-010 100 µg and ROSE-010 300 µg as the first, second or third injection in the cross-over design, showed that alternating the order of the treatment did not impact the action of the other involved treatments nor the effect of the drug itself. In addition, by comparing the pain intensity, both before and after treatment at three time points and at each order of the same treatment, proved that reduction of the pain intensity did not differ with the treatment order.
IBS has been classified by Rome criteria into the four subtypes (IBS-D, IBS-C, IBS-M and IBS-U) [Citation3]. Patients participating in this study were sorted and classified according to the dominating IBS symptoms to investigate if a certain subtype was more susceptible to the treatment. The results showed that patients with IBS-C, and even more so with IBS-M, were more prone to respond with higher pain relief than other IBS subtypes, both in terms of pain relief as well as the number and fraction of responders where pain intensity was halved at 60 min of treatment. Thus, in this study, the IBS-C and IBS-M subtypes stood out as the most susceptible responders to treatment with ROSE-010.
ROSE-010 acts as an agonist on the neuronal GLP-1R eliciting a neuronal signal which relaxes GI smooth muscle and motility. This slows gastric emptying and intestinal transit. GLP-1 is suggested to primarily exert its effects on motility via direct local actions in the periphery, which may coexist with indirect actions mediated through the CNS, likely through vagal afferents originating in the GI tract or by binding to receptors within the CNS [Citation17]. The response to ROSE-010 was estimated and evaluated by two different clinical endpoints, either as a reduction of the pain intensity score or increase of the pain relief score using the VAS scale ranging from 0 to 100. These two estimates were found to have a high degree of agreement showing that either one can be used for determining the clinical response to ROSE-010.
Several performed and ongoing studies have forwarded ROSE-010 or GLP-1 in human as well as animal research for the treatment of IBS [Citation4,Citation26,Citation29,Citation30]. Still, further follow-up studies are required to relate the efficacy of ROSE-010 with other parameters that affect IBS improvement. Therefore, more studies and further investigations for ROSE-010 association on IBS patients are needed involving other study populations, such as post-infectious IBS [Citation31], migraine [Citation32], Parkinson’s disease [Citation33,Citation34] and Ehlers-Danlos syndrome [Citation35] where IBS symptoms are common. The current pharmaceutical approaches with GLP‐1 receptor agonists as treatment in diabetes type 2 and obesity show a clear relationship to gut motility‐regulating effects such as gastric emptying [Citation36]. Upcoming pharmaceutical analogues include oral GLP-1 formulations [Citation37] suggesting that future possibilities for treatment of IBS are at hand.
As regards AEs, the predominant findings nausea, dyspepsia and vomiting seem clearly related to an inhibition of gastric emptying as a well-recognized effect of GLP-1R agonists [Citation6,Citation36]. Nausea and vomiting have been shown to be important factors in influencing patients’ choice of IBS medications and specifically ROSE-010. In an on-line patient survey, Almario et al. [Citation38] assessed patient preferences and trade-offs when considering an IBS pain medication with ROSE-010’s profile. It was found that the most important criterion for patient selection was efficacy followed by side effects of nausea. Thus, there may be a balance and trade-off in maximizing efficacy whilst reducing side-effects. Due to the ability of GLP-1R agonists to release insulin [Citation39], ROSE-010 interference with the plasma glucose was investigated. In two participants, a moderate hypoglycemia was found, whereas another 13 had a mild hypoglycemia, six of which already under baseline conditions, and hence no relationship to the ROSE-010 administration. Furthermore, no hypoglycemia occurred at the maximum pain relief speaking for hypoglycemia as an effect separate from that of pain relief. In line with this, at 120 min when the maximum pain-relieving effect was reached, the reduced plasma glucose was passed and non-significant. The dizziness and vertigo described in some patients were weakly related to nausea but not vomiting, and was also reported in the placebo group at the same frequency as the lower dose group. At the higher dose, it may reflect a passing reduction of systolic blood pressure without any alteration of diastolic blood pressure as previously described [Citation40,Citation41], but not captured in our trial as carried out in an out-patient setting. Headache that occurred in few could also be due to circulatory effects. Other AEs, even if severe, were only seen in single subjects and with no relationship to the effects of a GLP-1R agonist.
In summary, all data and results clarified that both high and low dose of ROSE-010 were effective and tolerable for controlling IBS symptoms, particularly in women with IBS-C and IBS-M independent of age, BMI and injection order. For future studies of ROSE-010 pinpointing this information on patient characteristics may be relevant for the inclusion criteria of patients suffering from IBS.
Acknowledgements
The study was initiated as a master degree project for MSc Aya Touny, Uppsala University. Authors Enda Kenny is an employee of NV Rose where Maria Månsson previously has been consultant. Source data verification and database monitoring was performed by Clinical Reports AB, Stockholm, Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hellström PM, Benno P. The Rome IV: irritable bowel syndrome – a functional disorder. Best Pract Res Clin Gastroenterol. 2019;40–41:101634.
- Hellström PM. Pathophysiology of the irritable bowel syndrome – reflections of today. Best Pract Res Clin Gastroenterol. 2019;40–41:101620.
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–1279.e2.
- Mosińska P, Salaga M, Fichna J. Novel investigational drugs for constipation-predominant irritable bowel syndrome: a review. Expert Opin Investig Drugs. 2016;25(3):275–286.
- Drossman DA, Chang L, Schneck S, et al. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig Dis Sci. 2009;54(7):1532–1541.
- Hellström PM, Hein J, Bytzer P, et al. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29(2):198–206.
- Farzaei MH, Bahramsoltani R, Abdollahi M, et al. The role of visceral hypersensitivity in irritable bowel syndrome: pharmacological targets and novel treatments. J Neurogastroenterol Motil. 2016;22(4):558–574.
- O'Malley D. Neuroimmune cross talk in the gut. Neuroendocrine and neuroimmune pathways contribute to the pathophysiology of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2016;311(5):G934–G941.
- Benno P, Norin E, Midtvedt T, et al. Therapeutic potential of an anaerobic cultured human intestinal microbiota, ACHIM, for treatment of IBS. Best Pract Res Clin Gastroenterol. 2019;40–41:101607.
- Myneedu K, Deoker A, Schmulson MJ, et al. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7(8):1033–1041.
- Schoenfeld PS. Advances in IBS 2016: a review of current and emerging data. Gastroenterol Hepatol. 2016;12(8 Suppl. 3):1–11.
- Radovanovic-Dinic B, Tesic-Rajkovic S, Grgov S, et al. Irritable bowel syndrome – from etiopathogenesis to therapy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162(1):1–9.
- Lacy BE, Weiser K, Lee RD. The treatment of irritable bowel syndrome. Ther Adv Gastroenterol. 2009;2(4):221–238.
- Hellström PM. GLP-1: broadening the incretin concept to involve gut motility. Regul Pept. 2009;156(1–3):9–12.
- Hellström PM. Glucagon-like peptide-1 gastrointestinal regulatory role in metabolism and motility. Vitam Horm. 2010;84:319–329.
- Hellström PM. GLP-1 playing the role of a gut regulatory compound. Acta Physiol. 2011;201(1):151–156.
- Halim MA, Degerblad M, Sundbom M, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. 2018;103(2):575–585.
- Rowlands J, Heng J, Newsholme P, et al. Pleiotropic effects of GLP-1 and analogs on cell signaling. Front Endocrinol. 2018;9(672):672.
- Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2012;166(1):27–41.
- Muller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130.
- Graaf C, Donnelly D, Wootten D, et al. Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev. 2016;68(4):954–1013.
- Knauf C, Abot A, Wemelle E, et al. Targeting the enteric nervous system to treat metabolic disorders? "Enterosynes" as therapeutic gut factors. Neuroendocrinology. 2020;110(1–2):139–146.
- Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr Opin Pharmacol. 2013;13(6):964–969.
- Hellström PM, Naslund E, Edholm T, et al. GLP-1 suppresses gastrointestinal motility and inhibits the migrating motor complex in healthy subjects and patients with irritable bowel syndrome. Neurogastroenterol Motil. 2008;20(6):649–659.
- Li Z-Y, Zhang N, Wen S, et al. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. 2017;41(4):459–465.
- Camilleri M, Vazquez-Roque M, Iturrino J, et al. Effect of a glucagon-like peptide 1 analog, ROSE-010, on GI motor functions in female patients with constipation-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G120–G128.
- Yang Y, Cui X, Chen Y, et al. Exendin-4, an analogue of glucagon-like peptide-1, attenuates hyperalgesia through serotonergic pathways in rats with neonatal colonic sensitivity. J Physiol Pharmacol. 2014;65(3):349–357.
- Kim YS, Kim N. Sex-gender differences in irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24(4):544–558.
- O'Brien R, O'Malley D. The glucagon-like peptide-1 receptor agonist, exendin-4, ameliorated gastrointestinal dysfunction in the Wistar Kyoto rat model of irritable bowel syndrome. Neurogastroenterol Motil. 2020;32(2):e13738.
- Siekmeier R, Hofmann T, Scheuch G, et al. Aerosolized GLP-1 for treatment of diabetes mellitus and irritable bowel syndrome. Adv Exp Med Biol. 2015;849:23–38.
- Lindberg G. Pseudo-obstruction, enteric dysmotility and irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2019;40–41:101635.
- Wongtrakul W, Charoenngam N, Ungprasert P. Increased prevalence of irritable bowel syndrome in migraine patients: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;34(1):56–63.
- Mertsalmi TH, But A, Pekkonen E, et al. Irritable bowel syndrome and risk of Parkinson's disease in Finland: a nationwide registry-based cohort study. J Parkinsons Dis. 2021;11(2):641–651.
- Yoon SY, Shin J, Heo SJ, et al. Irritable bowel syndrome and subsequent risk of Parkinson's disease: a nationwide population-based matched-cohort study. J Neurol. 2021.
- Zweig A, Schindler V, Becker AS, et al. Higher prevalence of joint hypermobility in constipation predominant irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(9):e13353.
- Halawi H, Camilleri M, Acosta A, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiol Gastrointest Liver Physiol. 2017;313(5):G442–G447.
- Pratley RE, Crowley MJ, Gislum M, et al. Oral semaglutide reduces HbA1c and body weight in patients with type 2 diabetes regardless of background glucose-lowering medication: PIONEER Subgroup Analyses. Diabetes Ther. 2021;12(4):1099–1116.
- Almario CV, Eberlein S, Khalil C, et al. Determining patient treatment preferences for management of acute pain episodes in irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14145.
- Salehi M, Aulinger B, Prigeon RL, et al. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes. 2010;59(6):1330–1337.
- Viswanathan P, Chaudhuri A, Bhatia R, et al. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13(5):444–450.
- Wijkman MO, Dena M, Dahlqvist S, et al. Predictors and correlates of systolic blood pressure reduction with liraglutide treatment in patients with type 2 diabetes. J Clin Hypertens. 2019;21(1):105–115.
