Abstract
Objective
Assessment of the upper gastrointestinal tract (UGI) may enable more personalized treatment strategies in pediatric inflammatory bowel disease (IBD). However, data on the frequency and significance of these findings remain limited.
Methods
Data on 132 pediatric IBD patients with systematic UGI sampling were collected and the baseline characteristics and presence of complications compared between those with and without histological UGI findings. The control group comprised 162 children who received no diagnoses.
Results
Seventy-six children had ulcerative colitis (UC), 47 Crohn’s disease (CD) and nine IBD unclassified. UGI findings were more common in IBD patients than controls (69.7% vs. 30.9%, respectively, p < .001), particularly in the stomach (62.1% vs. 16.8%; p < .001). Among IBD patients, findings were more common in CD than in UC (80.9% vs. 63.2%; p = .038), particularly in the duodenum (21.3% vs. 2.6%, p = .001). Four patients had UGI granulomas consistent with CD. Hypoalbuminemia (OR 3.22; 95% CI 1.18–8.79) and failure to thrive (2.82; 1.17–6.78) increased the likelihood of UGI findings in IBD. In CD, perianal morbidity was less common in those with than in those without UGI findings (13.2% vs. 44.4%; p = .032) whereas in UC, UGI findings increased the risk for co-morbidities (18.8% vs. 3.6%; p = .059). The long-term outcomes did not differ between patients with or without UGI findings.
Conclusions
Histologic UGI findings were more common in children with IBD than in children with no gastrointestinal diagnoses. In CD, UGI findings were more frequent than in UC, especially in the duodenum. In UC, UGI findings were associated with more complex disease.
Introduction
Inflammatory bowel diseases (IBD) comprise Crohn’s disease (CD), ulcerative colitis (UC) and IBD unclassified (IBDU). In recent decades, the prevalence of pediatric IBD has increased and diagnostic methods have improved [Citation1,Citation2]. One of the main goals of the current scientific research in this field is to improve clinicians’ ability to make better prognostic predictions and subsequently enable more individual approaches to the treatment of pediatric IBD [Citation3,Citation4]. It would be particularly desirable to facilitate early recognition of CD, as this could, for example, reduce the need for subsequent surgical interventions [Citation1,Citation5]. The continuously increasing use of efficient but highly immunosuppressive biological treatments makes the optimal targeting of treatments as timely as ever [Citation6].
One way to achieve more precise disease classification in IBD could be assessment of histopathologic findings of the upper gastrointestinal tract (UGI). However, although esophagogastroduodenoscopy (EGD) is nowadays considered an integral part of the diagnostics, actual data on the frequency and significance of UGI findings in children with IBD are still limited. Moreover, the results published so far are difficult to interpret due to variation in study designs and cohorts. The patients may, for example, have already received treatment, blurring the distinction between drug- and disease-related histologic findings [Citation7]. Additional challenges are posed by often nonsystematic mucosal sampling during endoscopies and lack of a non-IBD control group [Citation3,Citation4].
We have long experience of performing EGD in all children with suspected IBD. In addition, mucosal biopsies are taken during each gastrointestinal endoscopy from pre-defined anatomical sites regardless of the visual findings. This systematic approach, together with carefully maintained electronic patient records, enabled us to study the prevalence and long-term significance of UGI findings in pediatric IBD.
Methods
Patients and study design
The study was conducted at the Tampere Center for Child, Adolescent and Maternal Health Research, Tampere University and at the Department of Pediatrics, Tampere University Hospital. The patient cohort was formed by collecting comprehensive medical data on all consecutive children (age <17 years) who had undergone upper and/or lower gastrointestinal endoscopies in the Department of Pediatrics between January 2007 and October 2014. Only first diagnostic endoscopies and subjects who had undergone both EGD and colonoscopy were accepted for further analyses. From this series, 132 children were diagnosed with IBD, including 76 with UC, 47 with CD and 9 with IBDU. The diagnosis of IBD was set according to the European Society for Paediatric Gastroenterology and Nutrition guidelines at the timepoint in question [Citation1,Citation8]. The control group included 162 children from the same endoscopy series who did not receive any gastrointestinal diagnoses in EGD, colonoscopy or other investigations carried out initially or during subsequent surveillance. The prevalence, nature and location of histopathologic UGI findings were compared between the study groups. Further, the IBD children were divided into those with and without histopathologic UGI findings and the study data was compared between these groups. The main analyses were conducted only for children with confirmed UC or CD. A follow-up data was available for 2–11 years. It comprised the possible appearance of strictures, abscesses or fistulas and/or need of biological therapy or surgery in IBD patients, and possible later gastrointestinal endoscopies and diagnoses in controls.
The study design and the collection of the medical data were granted institutional approval by the Department of Pediatrics, Tampere University Hospital. All data were analyzed anonymously without any personal contact to patients. The Ethical Guidelines of the Declaration of Helsinki were strictly followed.
Clinical and laboratory data
The clinical information collected involved demographic and anthropometric data, symptoms leading to diagnostic investigations, presence of other possible previously diagnosed chronic diseases and IBD in first-degree relatives. In addition, data on complications (perianal fistulas, fissures, skin tags, abscesses, fistulas, strictures or disease-related surgery) and co-morbidities (e.g., autoimmune hepatitis, primary sclerosing cholangitis, uveitis, pancreatitis, ankylosing spondylitis or peripheral arthritis, pyoderma gangrenosum, thrombosis and gastrointestinal malignancy) were collected if present.
The laboratory parameters collected included hemoglobin (reference values (Rf) from 100–141 g/l to 130–160 g/l, depending on age and sex), erythrocyte sedimentation rate (ESR, Rf <15 mm/h), albumin (Alb, Rf from 35–46 g/l to 37–51 g/l, depending on age) and fecal calprotectin (Rf <100 μg/g) [Citation9,Citation10].
Endoscopic and histologic findings
In our settings, a standard biopsy collection protocol has been in use in both EGDs and colonoscopies for a long time [Citation11]. During EGD, mucosal samples are systematically taken from the esophagus, both gastric body and antrum and from the duodenum. During the colonoscopy a minimum of two biopsies are collected from the rectum, sigmoid/descending colon, ascending colon, cecum and terminal ileum. Additional endoscopic biopsies are taken depending on the clinical scenario and macroscopic abnormalities. The biopsies are cut and stained using standard histopathology methods and evaluated by a pathologist with expertise in the pediatric alimentary tract. Specific histological methodology is used as appropriate.
The possible macroscopic findings and their site as perceived by the endoscopist in EGD and/or colonoscopy were carefully recorded in each case, likewise the microscopic findings as reported by the pathologist. The latter included, e.g., presence and type of inflammation and presence of crypt abscesses, granulomas, villous atrophy, ulceration, metaplasia and infectious agents, and all other abnormal histopathologic findings.
Statistical analysis
Clinical characteristics and prevalence of abnormal laboratory values or histopathological findings are presented as percentage distributions. The skewness of quantitative variables was assessed by the Shapiro-Wilk method and most of the variables were not normally distributed. Thus, for the sake of simplicity, all quantitative data is expressed as medians with lower and upper quartiles. Chi-squared test or Fisher’s exact test were used to compare the qualitative variables between the groups. Laboratory values between groups were compared using the Kruskal-Wallis one-way analysis of variance or by Fisher’s exact test. The association between clinical features and presence of UGI findings was analyzed by binary logistic regression analysis and presented with odds ratios (OR) with 95% confidence intervals (CI). p values ≤.05 were considered statistically significant. All the statistical analyses were performed using SPSS Statistics version 25 (IBM Corp, Armonk, NY, USA).
Results
Children with UC or CD were older and had more often bloody diarrhea, failure to thrive, anemia, elevated ESR, high calprotectin and low Alb levels, and less often abdominal pain and heartburn than the control children who received no specific diagnosis (). In more detailed analysis, bloody diarrhea was more common (63.2% vs. 23.4%, p ≤ .001) and failure to thrive less common (28.9% vs. 48.9%, p = .025) in UC than in CD patients.
Table 1. Baseline characteristics in children with UC, CD and in control children with no diagnoses in gastrointestinal endoscopies
Mucosal biopsies were available in >95% of the cases from each UGI anatomical site. Abnormal UGI findings were more common in children with IBD than in controls without specific diagnosis (69.7% vs. 30.9%, p < .001), as well as in separate analysis of children with CD than in those with UC (80.9% vs. 63.2%, p = .038). The difference was particularly pronounced in ventricular findings, which were more common in both UC and CD patients than in the controls (). Duodenal findings were more common in CD than in the two other groups and there were no differences between the groups in esophageal findings (). H. pylori negative gastritis was the most frequent finding in stomach and nonspecific mucosal inflammation at the other biopsy sites (Supplementary Table S1). Of note, three children had duodenal granulomas and one-child esophageal granulomas consistent with CD. Macroscopic abnormalities during UGD were reported by the endoscopist in 51.5% of IBD children and in 38.3% of controls (p = .023).
Figure 1. Histological UGI findings in 47 children with CD, 76 children with UC and 162 control children who underwent upper and lower gastrointestinal endoscopies but were not diagnosed with any organic condition.
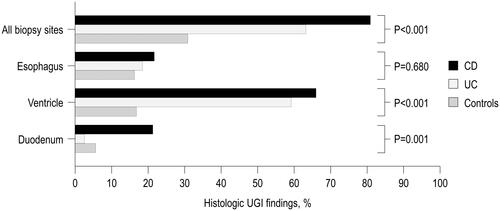
UGI findings in control children without specific diagnosis included mild unspecific inflammation in esophagus, H. pylori negative unspecific gastritis or gastropathy in stomach and lymphangiectasia or lymphoid hyperplasia and mild intraepithelial lymphocytosis in duodenum. None of the control patients with UGI findings received a gastrointestinal diagnosis during the later follow-up. Repeat EGD was conducted in two, colonoscopy in one and a wireless capsule endoscopy in three controls.
In children with IBD, failure to thrive and low Alb levels were associated with higher and likelihood of UGI findings, whereas there was no association between other clinical or demographic features or laboratory parameters and UGI findings studied ().
Table 2. Relationships between clinical features and presence of histologic UGI findings in 132 children with IBD.a
In CD, perianal morbidity was less common in patients with than without UGI findings (). In UC, co-morbidities were more common in patients with than without UGI findings with borderline significance, whereas no differences in other complications or co-morbidities were seen.
Table 3. Presence of complications and co-morbidities in 123 children with CD or UC with and without UGI findings
Ninety-seven percent of children with IBD had abnormal histologic findings in biopsies taken during colonoscopy. Of the four cases with normal colonoscopy, one had oral CD and three isolated small-bowel CD confirmed by wireless capsule endoscopy or magnetic resonance enterography. Neither the site nor extent of the colonic involvement had any effect on the presence of UGI findings in EGD (Supplementary Table S2).
During the long-term follow-up, there were no significant differences between IBD patients with and without UGI findings in the initiation of biological therapy (CD 26.3% vs. 11.1%, respectively, p = .333; UC 8.3% vs. 10.7%, respectively, p = .729) or need of intestinal surgery (CD 10.5% vs. 33.3%, p = .084; UC 8.3% vs. 3.6%, p = .419), or in CD patients in the presence of strictures, fistulas and abscesses (21.1% vs. 22.1%, respectively, p = .939).
Discussion
We observed more UGI findings in children with IBD than in the controls, particularly in the stomach and in the duodenum in CD. By contrast, there was no difference between the groups in esophagus. The most common abnormality in all sites was unspecific inflammation. In earlier studies, nonspecific gastritis has been present in 33%–92%, duodenitis in 23%–33% and esophagitis in 26%–72% of children with CD [Citation12–14], and in 19%–69%, 3%–28% and 11%–50% of children with UC, respectively [Citation12–14]. Contrary to many earlier reports, we found only one case with H. pylori gastritis, which is consistent with the rapid decrease in the prevalence of H. pylori infections [Citation15].
These inconsistent figures could be explained by differences in patient selection and endoscopic procedures. For example, some studies have included only adolescents [Citation3,Citation13,Citation14] and ethnic differences could also play a role [Citation16]. Lack of systematic sampling is also important due to the weak concordance between endoscopic and histological abnormalities [Citation17] Comparisons are further hampered by heterogeneous definitions of histological abnormalities [Citation14,Citation18–21] and selection of controls has often been poorly described or there has been no control group at all. It must be born in mind that there is always a reason for endoscopy—which explains the high frequency of symptoms and unspecific UGI findings among our controls. Sometimes it is also difficult to distinguish between the effects of IBD and treatment [Citation3].
Among the IBD patients, hypoalbuminemia and failure to thrive were more common in those with UGI findings. In comparison, Castellaneta et al. reported more abdominal pain, nausea and weight loss in patients with than without UGI findings [Citation22], whereas Kovacs et al. observed no association between UGI findings and clinical features in either all IBD patients or those with CD [Citation23]. These inconsistencies could again be explained by methodological differences. As a plausible mechanism, UGI abnormalities may cause deficiencies in essential nutrients and/or indicate a more extensive inflammatory state. In general, however, it remains unclear why UGI abnormalities are so common in IBD.
Children with CD and UGI findings had perianal disease less often, whereas in UC the findings predicted complications at the time of diagnosis. In contrast, no significant difference between UGI and no UGI groups were seen in the number of strictures, fistulas or abscesses, or in the frequency of later initiation of biological therapy or surgery, although this could be partly due to rather low number of cases. Previously, Kim et al. found no difference in severe complications between CD children with and without UGI findings [Citation16], whereas such an association has been reported in adults [Citation24–26]. Sullivan et al. found the presence of active gastritis and duodenitis to predict inadequate treatment response in UC [Citation27]. These discrepancies could be explained by the differences in the rate and nature of complications between children and adults, by inconsistent definitions, or by variable length of follow-up. In light of our findings, UGI investigations could be used to determine the intensity of initial treatment.
Our strengths were the availability of comprehensive patient data, systematic sampling from pre-defined sites and use of representative control group. The retrospective design was a weakness. In addition, we did not apply validated IBD activity scores or included mode of initial treatment. Finally, a single-center design may limit the generalizability of the results.
Conclusions
Our findings support the recommendation to perform an upper GI endoscopy with systematic biopsy sampling on children with suspected IBD [Citation1]. The UGI findings may be helpful in targeting additional imaging studies and the distinction between UC and CD, and also in offering better means for prognostic evaluation.
Supplemental Material
Download MS Word (14.4 KB)Supplemental Material
Download MS Word (16.7 KB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Levine A, Koletzko S, Turner D, et al. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795–806.
- Bernstein CN. Review article: changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment Pharmacol Ther. 2017;46(10):911–919.
- Ashton JJ, Coelho T, Ennis S, et al. Endoscopic versus histological disease extent at presentation of paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2016;62(2):246–251.
- Ashton JJ, Bonduelle Q, Mossotto E, et al. Endoscopic and histological assessment of paediatric inflammatory bowel disease over a 3-Year follow-up period. J Pediatr Gastroenterol Nutr. 2018;66(3):402–409.
- Birimberg-Schwartz L, Zucker DM, Akriv A, et al. Development and validation of diagnostic criteria for IBD subtypes including IBD unclassified in children: a multicentre study from the pediatric IBD Porto group of ESPGHAN. J Crohn’s Colitis. 2017;11:1078–1084.
- Sieczkowska-Golub J, Jarzebicka D, Oracz G, et al. Biosimilars in paediatric inflammatory bowel disease. World J Gastroenterol. 2018;24(35):4021–4027.
- Feakins RM. Ulcerative colitis or Crohn's disease? Pitfalls and problems. Histopathology. 2014;64(3):317–335.
- IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis–the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1–7.
- Fimlab Laboratoriot Oy. Tutkimusluettelo; [cited 2021 Dec 10]. Available from: https://fimlab.fi/tutkimusluettelo.
- HUSLAB. Tutkimusohjekirja; [cited 2021 Dec 10]. Available from: https://huslab.fi/ohjekirja/.
- Repo M, Rajalahti T, Hiltunen P, et al. Diagnostic findings and long-term prognosis in children with anemia undergoing GI endoscopies. Gastrointest Endosc. 2020;91(6):1272–1281.e2.
- Ushiku T, Moran CJ, Lauwers GY. Focally enhanced gastritis in newly diagnosed pediatric inflammatory bowel disease. Am J Surg Pathol. 2013;37(12):1882–1888.
- Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):39–44.
- Genta RM, Sonnenberg A. Non-Helicobacter pylori gastritis is common among paediatric patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(11):1310–1316.
- Oona M, Utt M, Nilsson I, et al. Helicobacter pylori infection in children in Estonia: decreasing seroprevalence during the 11-year period of profound socioeconomic changes. Helicobacter. 2004;9(3):233–241.
- Kim ES, Kwon Y, Choe YH, et al. Upper gastrointestinal tract involvement is more prevalent in korean patients with pediatric Crohn's disease than in European patients. Sci Rep. 2020;10(1):19032.
- Sheiko MA, Feinstein JA, Capocelli KE, et al. The concordance of endoscopic and histologic findings of 1000 pediatric EGDs. Gastrointest Endosc. 2015;81(6):1385–1391.
- Basturk A, Artan R, Yilmaz A, et al. Gastritis associated with initially pediatric Crohn's disease and ulcerative colitis. Pediatr Gastroenterol Hepatol Nutr. 2018;21(3):163–169.
- Putra J, Ornvold K. Focally enhanced gastritis in children with inflammatory bowel disease: a clinicopathological correlation. Pathology. 2017;49(7):808–810.
- Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: a pathological review. World J Gastroenterol. 2019;25(16):1928–1935.
- Lin J, McKenna BJ, Appelman HD. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study. Am J Surg Pathol. 2010;34(11):1672–1677.
- Castellaneta SP, Afzal NA, Greenberg M, et al. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257–261.
- Kovacs M, Eszter Muller K, Arato A, et al. Diagnostic yield of upper endoscopy in paediatric patients with Crohn's disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6(1):86–94.
- Chow DKL, Sung JJY, Wu JCY, et al. Upper gastrointestinal tract phenotype of crohn’s disease is associated with early surgery and further hospitalization. Inflamm Bowel Dis. 2009;15:551–557.
- Sun XW, Wei J, Yang Z, et al. Clinical features and prognosis of Crohn's Disease with upper gastrointestinal tract phenotype in Chinese patients. Dig Dis Sci. 2019;64(11):3291–3299.
- Cosnes J, Bourrier A, Nion-Larmurier I, et al. Factors affecting outcomes in Crohn's disease over 15 years. Gut. 2012;61(8):1140–1145.
- Sullivan KJ, Wei M, Chernetsova E, et al. Value of upper endoscopic biopsies in predicting medical refractoriness in pediatric patients with ulcerative colitis. Hum Pathol. 2017;66:167–176.
