?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Most endoscopists routinely perform moderate or deep sedation for esophagogastroduodenoscopy (EGD). Considering that there is no consensus on the optimal sedation depth and it varies from country to country, our study aims to compare the effectiveness, cost and safety of these two sedation methods in the Chinese population.
Methods
This quasi-experimental study included a total of 556 eligible patients from July 2020 to June 2021, and they entered the moderate sedation group or deep sedation group based on their choices. Baseline information, scores of Patient Satisfaction with Sedation Instrument (PSSI) and Clinician Satisfaction with Sedation Instrument (CSSI), examination time, sedation time, recovery time, expenses before medicare reimbursement, hypoxaemia and hypotension were compared between the two groups. Propensity Score Matching (PSM) analysis was conducted to balance the confounding factors.
Results
After PSM, 470 patients were involved in the analysis, with 235 for each group. The moderate sedation was clearly superior to the deep sedation group in terms of PSSI score (98.00 ± 0.94 vs. 97.29 ± 1.26), CSSI score (98.00 ± 0.78 vs. 97.67 ± 1.30), sedation time (11.90 ± 2.04 min vs. 13.21 ± 2.75 min), recovery time (25.40 ± 3.77 min vs. 28.0 ± 4.85 min), expenses (433.04 ± 0.00 Yuan vs. 789.85 ± 0.21 Yuan), with all p < .001. Examination time was not significantly different between the two groups (p = .124). In addition, the moderate sedation group had a lower occurrence rate of hypoxaemia (0.36% vs. 3.27%, p = .010) and hypotension (17.44% vs. 44.00%, p < .001) compared to the deep sedation group.
Conclusions
Moderate sedation presented better effectiveness and safety and lower cost, and thereby it should be recommended as a widely used sedation method in clinical practice in China. Trial registration: This trial was registered on http://www.chictr.org.cn/index.aspx (ChiCTR2000038050).
Introduction
Esophagogastroduodenoscopy (EGD) is an important method to diagnose upper gastrointestinal diseases [Citation1]. It is an invasive operation and often causes nausea, vomiting, and other discomforts difficult for patients to tolerate, which are not conducive for endoscopists to operate and observe subtle mucosal changes [Citation1]. To improve these discomforts, effective sedation and analgesia are considered integral components of the EGD procedure [Citation2].
In clinical practice, most endoscopists routinely perform moderate or deep sedation to reduce patients’ anxiety and discomforts, thereby improving surgical tolerance and satisfaction and providing an ideal environment for endoscopists with a thorough exam [Citation2,Citation3]. Regarding the optimal sedation depth for EGD, there is no consensus and it varies from country to country. In the United States, the use of deep sedation nearly increases by triple in a decade [Citation4]. In China, during the EGD, physicians expect the patient to be unresponsive, and patients expect no memory, which also often results in deep sedation [Citation5]. Evidence has shown that deep sedation does not perform a higher patient satisfaction degree or operational success rate [Citation6,Citation7]. Other studies showed deep sedation is costly and unnecessary in low-risk procedures that may be completed by moderate sedation [Citation8–10]. In addition, deep sedation brings more cardiovascular complications than moderate sedation [Citation11]. An American guideline for endoscopic sedation or anesthesia in 2018 explicitly mentioned that although the patients’ wishes and medical conditions need to be considered during sedation, moderate sedation is expected for endoscopic examination [Citation12]. Existing studies have reported the effectiveness and safety of moderate sedation for routine EGD [Citation13,Citation14]. In China, moderate sedation remains a low popularity rate and a study comparing moderate and deep sedation has not been reported previously.
Therefore, we designed a study to compare the effectiveness, cost, and safety between moderate sedation and deep sedation based on the Chinese population who accepted EGD.
Methods
Study design
This study was a quasi-experimental study to compare the effectiveness, cost, and safety of moderate sedation and deep sedation in EGD based on the Chinese population. Our study followed the principles in the Declaration of Helsinki. All patients have signed the informed consent, and the study has been approved by the Ethics Committee of Shangluo Central Hospital (approval number: KY2020013). This trial was registered on http://www.chictr.org.cn/index.aspx (ChiCTR2000038050).
Sample size
The sample size was estimated based on the Patient Satisfaction with Sedation Instrument (PSSI) and calculated as the formula:
In this formula, α and β were 0.05 and 0.1, respectively. = 1.96,
= 1.282, and
= 0.3. Therefore, a sample size of 235 patients should be recruited for each group. Considering a dropout rate of 15%, the sample size required for each group was 275, and the total is 550.
Participants
We finally enrolled 556 patients in Shangluo Central Hospital from July 2020 to June 2021. Patients were included if they met all the criteria: (a) age ≥ 18 years (either male or female), (b) with American Society of Anesthesiologists (ASA) physical classification status I–II, (c) with indications and selected to receive EGD, and (d) volunteer to participate and sign the informed consent. Patients were excluded if they: (a) with contraindications for routine endoscopic procedures or refuse sedation, (b) with potentially life-threatening circulatory and respiratory diseases if not under proper control, such as uncontrolled severe hypertension, severe arrhythmias, unstable angina pectoris, acute respiratory infections, and asthma attacks, etc., (c) with other serious heart and lung diseases, (d) with liver dysfunction (Child–Pugh C or above), acute upper gastrointestinal bleeding with shock, severe anemia, gastrointestinal obstruction with gastric contents retention, (e) with an allergy to sedative and analgesic drugs and other serious anesthesia risks, (f) with long-term use of sedative and analgesic drugs or addiction to alcohol, sedative and analgesic drugs, (g) pregnant and lactating women, and (h) without an escort or guardian. The enrolled patients were divided into the moderate sedation group (midazolam + fentanyl) or the deep sedation group (propofol + sufentanil) according to their choices. Baseline information was collected and included gender, age, nationality, height, weight, ASA grade, history of drug allergy, other diseases, history of surgery, diastolic blood pressure (DBP), systolic blood pressure (SBP), mean arterial pressure (MAP), heart rate (HR) and oxygen saturation (SaO2) before sedation. The body mass index (BMI) was calculated as weight (kg)/height2 (m2), and divided into underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI < 24 kg/m2), and overweight (BMI ≥ 24 kg/m2) based on Chinese Adult BMI criteria [Citation15].
Interventions
Before the procedure, patients were fasting and entered the endoscopy room for routine preparation of painless EGD. The venous access to the right upper limb was established, and electrocardiograph, respiration, HR, blood pressure (left upper limb), pulse oxygen saturation, and mental status were monitored. All patients were given tropisetron (2 mg) to prevent vomiting before sedation. During the procedure, the moderate sedation group was administered intravenously by an endoscopist specially trained in sedation in the absence of an anesthesiologist. The deep sedation was performed by a trained anesthesiologist. Up to the prescribed sedation depth, EGD was performed. Standard EGD was performed by two experienced endoscopists. The sedation level was assessed every 3 min to help adjust the titration infusion rate. The mandible was raised or mask oxygen inhalation was used when the respiration rate was less than 10 times/min and the SaO2 was less than 90%. Atropine was used when the HR was below 55 beats/min, and dopamine (1–2 mg) was intravenously injected when blood pressure was reduced by 30%. After the procedure, patients were admitted to the recovery room for observation and left the hospital until reaching the standard of discharge that was evaluated by the physician.
The sedation level was assessed and monitored using the Observer’s Assessment of Alertness/Sedation (OAA/S) scale, which ranged from 1 point (deep sedation) to 5 points (waking state). The scale consisted of five levels: patients with fast response to calling their names with normal intonation (5 points), slow response to calling their names with normal intonation (4 points), the only response to calling their names loudly or repeatedly (3 points), the only response to mild stimuli or shaking (2 points), and no response to mild stimulation or shaking (1 point). Moderate sedation was defined as 3 points on OAA/S scale and deep sedation was defined as 1 point [Citation16,Citation17].
Outcomes
Primary outcomes
The primary outcomes were Patient Satisfaction with Sedation Instrument (PSSI) and Clinician Satisfaction with Sedation Instrument (CSSI) [Citation18].
The PSSI consisted of 3 subscales: sedation delivery, procedural recall, and sedation side effects. The CSSI consisted of 2 subscales: sedation administration and recovery procedure. The raw score was converted into a 0 to 100 scale using the formula: score = 100 × (Raw sum − Minimum)/(Maximum − Minimum). The score of the scale and subscales ranges from 0 to 100, with a higher score presenting higher satisfaction [Citation18].
Secondary outcomes
The secondary outcomes were examination time, sedation time, recovery time, expenses before medicare reimbursement, hypoxaemia and hypotension.
The examination time was the time from insertion to the removal of the endoscope. The sedation time was the time from the sedation to the wakefulness of patients. The recovery time was the time between the patients’ arriving in the recovery room and leaving the hospital decided by the physician. The hypoxaemia was judged as SaO2 lower than 83% at any time before, during, and after the EGD. The hypotension was judged as DBP or SBP decreased by 20% or more at any time before, during, and after the EGD compared to before sedation.
Statistical analysis
Propensity Score Matching (PSM) analysis was used to balance the confounding factors between the moderate sedation group and deep sedation group with 1:1 nearest neighbor matching. The normally distributed measurement data were described as mean ± standard deviation (mean ± SD), and intergroup comparison was performed using an independent t-test before PSM and paired t-test after PSM. The count data were described as number (n) and percentage (%), and intergroup comparison was carried out using Chi-squared or Fisher’s exact test before PSM and paired chi-squared test after PSM. The statistical analysis was performed using Python 3.7.3 (Python Software Foundation, Delaware, USA). p < .05 was considered statistically significant.
Results
Baseline information of participants
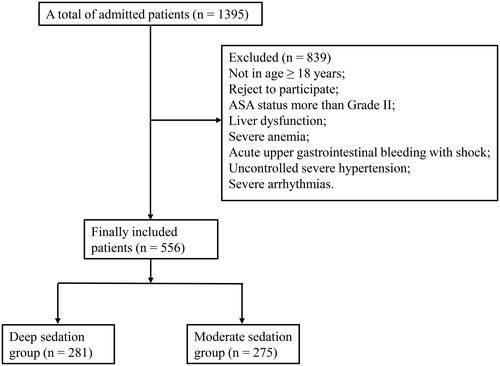
shows that 1395 patients were admitted to our hospital, and 839 of them were excluded because they were not aged more than 18 years, rejected to participate, with ASA status more than Grade II, liver dysfunction, severe anemia, acute upper gastrointestinal bleeding with shock, uncontrolled severe hypertension, and severe arrhythmias. Finally, a total of 556 patients were included in our study, with 281 patients in the deep sedation group and 275 patients in the moderate sedation group. displays that both groups were comparable in terms of gender, nationality, BMI, history of drug allergy, heart rate and oxygen saturation before sedation. However, there was a significant difference in mean age, ASA grade, other diseases, history of surgery, and diastolic blood pressure, systolic blood pressure and mean arterial pressure before sedation (all p < .05). After PSM, 235 patients from each group were included, and there were no significant differences in the baseline information of patients between the two groups (all p > .05), indicating that the two groups were comparable.
Table 1. The baseline information of moderate sedation group and deep sedation group.
Comparison of effectiveness and cost between the two groups
The information related to effectiveness and cost after PSM between the two groups is given in . The total score of PSSI (98.00 ± 0.94 vs. 97.29 ± 1.26) and CSSI (98.00 ± 0.78 vs. 97.67 ± 1.30) were both higher in the moderate sedation group (both p < .001). The deep sedation group showed a little higher score for procedural recall (99.97 ± 0.34 vs. 98.87 ± 1.97, p < .001) and sedation administration (99.93 ± 0.49 vs. 99.72 ± 0.64, p < .001), while moderate sedation group showed a higher score of sedation side effects (97.78 ± 1.45 vs. 95.72 ± 2.02, p < .001) and recovery procedure (96.92 ± 2.44 vs. 92.57 ± 3.84, p < .001). The moderate sedation group was also superior to the deep sedation group in sedation time (11.90 ± 2.04 min vs. 13.21 ± 2.75 min), recovery time (25.40 ± 3.77 min vs. 28.00 ± 4.85 min), and expenses before medicare reimbursement (433.04 ± 0.00 Yuan vs. 789.85 ± 0.21 Yuan), with all p < .001. In females, the moderate sedation group also showed a higher total score of PSSI and CSSI, less sedation time, recovery time, and expenses before medicare reimbursement, with p < .001. Similar results were found in females (all p < .001) (). shows the comparison of effectiveness and cost between the two groups in patients with different BMIs. In patients with BMI < 18.5 kg/m2, compared to deep sedation, moderate sedation had advantage in CSSI score (98.84 ± 0.68 vs. 97.12 ± 0.57), recovery time (24.33 ± 4.44 min vs. 29.20 ± 2.90 min), and expenses (433.04 ± 0.00 Yuan vs. 789.88 ± 0.00 Yuan), with all p < .05. In the 18.5 kg/m2 ≤ BMI < 24 kg/m2 group, patients accepting moderate sedation displayed higher PSSI and CSSI scores, shorter sedation time and recovery time, and fewer expenses, with p-value less than .001. We found similar results in patients with BMI ≥ 24 kg/m2.
Table 2. The comparison of efficiency and cost between moderate sedation group and deep sedation group.
Table 3. The comparison of efficiency and cost between the two groups based on gender.
Table 4. The comparison of efficiency and cost between the two groups based on BMI.
Comparison of safety between the two groups
displays the adverse reactions and other indicators in moderate sedation and deep sedation groups. The moderate sedation group had a lower occurrence rate of hypoxaemia (0.36% vs. 3.27%, p = .010) and hypotension (17.44% vs. 44.00%, p < .001). Before EGD, HR was lower (p = .004) and SaO2 was higher (p = .011) in the moderate sedation group. Patients accepting moderate sedation also have higher DBP, SBP, MAP and SaO2 during EGD, and higher SaO2 after EGD (all p < .05).
Table 5. The comparison of safety between moderate sedation group and deep sedation group.
Discussion
To improve patients’ tolerance to EGD, sedation and anesthesia techniques are introduced into routine examination [Citation3]. Our study was to compare the effectiveness, cost, and safety of moderate sedation and deep sedation in the Chinese population. The results showed that moderate sedation was superior to deep sedation in PSSI, CSSI, sedation time, recovery time, and expenses. The similar results were found in male, female, patients with BMI < 18.5 kg/m2, 18.5 kg/m2 ≤ BMI < 24 kg/m2, and BMI ≥ 24 kg/m2. In addition, moderate sedation showed less occurrence of an adverse reaction than deep sedation.
Sedation is a drug-induced suppression of consciousness level, and the routine use of sedation is increasing in endoscopic examinations all over the world [Citation19]. The clinical objectives of endoscopic sedation are to maximize the comfort of patients and minimize the risk of adverse events, thereby improving the examination outcome [Citation2]. The endoscopists usually relieve patients’ anxiety, discomfort, and pain by routinely using moderate sedation and deep sedation [Citation3]. Evidence showed that proper sedation can increase patients’ satisfaction after EGD [Citation20]. In our study, the moderate sedation group has given a higher total score of PSSI and CSSI, indicating that patients and clinicians were more satisfied with the effectiveness of moderate sedation. A study performed in Anhui, a province of China, also showed that moderate sedation provided high satisfaction [Citation1]. In addition, we found that the recovery time in the moderate sedation group was faster than in the deep sedation group, which was similar to studies of VanNatta et al. [Citation6]. It is known that adverse events were associated with the sedation level, and deep sedation was more often related to adverse cardiovascular and respiratory events than moderate sedation [Citation11]. In the study of McQuaid et al., moderate sedation not only increased physician and patient satisfaction but also lowered the risk of serious adverse events [Citation13]. Lee et al. also support patients with moderate sedation reduced suppression in cardiovascular and respiratory function when undergoing EGD [Citation21]. Similarly, our results displayed patients in the moderate sedation group had a less occurrence of adverse reactions.
Moderate sedation refers to that patients can respond to verbal orders with or without the requirement for light tactile stimulation [Citation22]. Deep sedation is defined that patients will elicit a response with repeated or painful stimulation [Citation22]; these people have a risk of entering into general anesthesia, making them unconscious and possibly unable to protect their airways. Considering these additional risks, deep sedation needs to be monitored by additional personnel and equipment, which increases the cost [Citation23]. In addition, propofol is a hypnotic agent and is commonly used in deep sedation with or without opioids [Citation24]. Although propofol can immediately induce anaesthesia and is easy to use to maintain an appropriate sedation level, evidence has shown that propofol sedation is costly compared to the potential benefits [Citation8,Citation25,Citation26]. In China, moderate sedation was performed by a nurse specially trained in sedation under the supervision of an endoscopic physician specially trained in sedation, and deep sedation was performed by an anesthesiologist [Citation27]. A meta-analysis demonstrated that the sedation performed by trained nurses was more economical than by anesthesiologists [Citation28]. In our study, expenses of moderate sedation were largely less than that of deep sedation, indicating that moderate sedation superior to deep sedation in cost.
Evidence has shown that the effectiveness of sedation was associated with gender and BMI [Citation29]. Our study showed both males and females had higher satisfaction with moderate sedation. Patients in each BMI group presented a higher satisfaction degree of moderate sedation, except that the CSSI score in patients with BMI < 18.5 kg/m2 was not significantly different between the two groups. This might be explained by that the sample size in BMI < 18.5 kg/m2 group was small, which could not support us in obtaining significant results.
Our study compares the effectiveness, safety, and cost of moderate sedation and deep sedation, which has not been reported in the Chinese population. In addition, the PSM method is used to overcome the bias caused by any difference in patients’ baseline information. However, some limitations should be concerned. First, this is a quasi-experimental study in that we cannot conduct random allocation due to economic reasons (expenses for the two sedation levels are different). Second, this is a single-center study. Although confounder biases are eliminated by PSM, a multicenter study has a higher level of evidence. Third, the number of patients with BMI < 18.5 kg/m2 is relatively small, and a larger sample size should be included in the future.
Conclusions
In conclusion, our study showed that moderate sedation was superior to deep sedation in terms of effectiveness, cost, and safety, indicating that moderate sedation could be widely used in clinical practice and endoscopists should increase the popularity rate of moderate sedation in China. In the future, a multicenter trial with a larger sample size should be performed to further study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wu Y, Zhang Y, Hu X, et al. A comparison of propofol vs. dexmedetomidine for sedation, haemodynamic control and satisfaction, during esophagogastroduodenoscopy under conscious sedation. J Clin Pharm Ther. 2015;40(4):419–425.
- Cohen LB, Delegge MH, Aisenberg J, et al. AGA institute review of endoscopic sedation. Gastroenterology. 2007;133(2):675–701.
- Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19(4):463–481.
- Liu H, Waxman DA, Main R, et al. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003-2009. JAMA. 2012;307(11):1178–1184.
- Wu X, Li Q, Li Y. New advances in sedation/anesthesia in the diagnosis and treatment of gastrointestinal endoscopy. Int J Anesth Resus. 2020;41:71–75.
- VanNatta ME, Rex DK. Propofol alone titrated to deep sedation versus propofol in combination with opioids and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol. 2006;101(10):2209–2217.
- Crepeau T, Poincloux L, Bonny C, et al. Significance of patient-controlled sedation during colonoscopy. Results from a prospective randomized controlled study. Gastroenterol Clin Biol. 2005;29(11):1090–1096.
- Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137(4):1229–1237.
- Inadomi JM. Editorial: endoscopic sedation: who, which, when? Am J Gastroenterol. 2017;112(2):303–305.
- Hassan C, Rex DK, Cooper GS, et al. Endoscopist-directed propofol administration versus anesthesiologist assistance for colorectal cancer screening: a cost-effectiveness analysis. Endoscopy. 2012;44(5):456–464.
- Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5(11):527–533.
- Early DS, Lightdale JR, Vargo JJ, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2018;87(2):327–337.
- McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67(6):910–923.
- Waring JP, Baron TH, Hirota WK, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58(3):317–322.
- Wang L, Du X, Dong JZ, et al. Body mass index and all-cause mortality in patients with atrial fibrillation: insights from the China atrial fibrillation registry study. Clin Res Cardiol. 2019;108(12):1371–1380.
- Kasuya Y, Govinda R, Rauch S, et al. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesthesia Analgesia. 2009;109(6):1811–1815.
- Xi C, Sun S, Pan C, et al. Different effects of propofol and dexmedetomidine sedation on electroencephalogram patterns: wakefulness, moderate sedation, deep sedation and recovery. PLoS One. 2018;13(6):e0199120.
- Vargo J, Howard K, Petrillo J, et al. Development and validation of the patient and clinician sedation satisfaction index for colonoscopy and upper endoscopy. Clin Gastroenterol Hepatol. 2009;7(2):156–162.
- Igea F, Casellas JA, González-Huix F, et al. Sedation for gastrointestinal endoscopy. Clin Pract Guidelines Soc Española Endosc Digest. 2014;106:195–211.
- Teh JL, Shabbir A, Yuen S, et al. Recent advances in diagnostic upper endoscopy. World J Gastroenterol. 2020;26(4):433–447.
- Lee BS, Ryu J, Lee SH, et al. Midazolam with meperidine and dexmedetomidine vs. midazolam with meperidine for sedation during ERCP: prospective, randomized, double-blinded trial. Endoscopy. 2014;46(04):291–298.
- Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017.
- Froehlich F, Harris JK, Wietlisbach V, et al. Current sedation and monitoring practice for colonoscopy: an international observational study (EPAGE). Endoscopy. 2006;38(5):461–469.
- Chen Z, Liu L, Tu J, et al. Improvement of atropine on esophagogastric junction observation during sedative esophagogastroduodenoscopy. PLoS One. 2017;12(6):e0179490.
- Kim EH, Park JC, Shin SK, et al. Effect of the midazolam added with propofol-based sedation in esophagogastroduodenoscopy: a randomized trial. J Gastroenterol Hepatol. 2018;33(4):894–899.
- Kılıc E, Demiriz B, Isıkay N, et al. Alfentanil versus ketamine combined with propofol for sedation during upper gastrointestinal system endoscopy in morbidly obese patients. Saudi Med J. 2016;37(11):1191–1195.
- Cang J, Deng X, Feng Y, et al. Expert consensus on sedation/anesthesia for digestive endoscopy in China. Int J Anesth Resus. 2014;35:769–777.
- Qadeer MA, Vargo JJ, Khandwala F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the. Am Gastroenterol Assoc. 2005;3(11):1049–1056.
- Salahuddin M, Salamo O, Karanth S, et al. Safety and incidence of complications associated with bronchoscopy in an obese population. Clin Respir J. 2021;15(6):670–675.