Abstract
Objectives
Fecal microbiota transplantation (FMT) is a promising intervention for patients with irritable bowel syndrome (IBS). The present study aimed to identify any differences in FMT response between patients with severe and moderate IBS symptoms.
Materials and method
The study included the 164 patients who participated in our previous study, of which 96 (58.5%) and 68 (41.5%) had severe (S-IBS-S) and moderate (Mo-IBS-S) IBS, respectively. The patients were randomly divided into a placebo group (own feces) and 30-g and 60-g (donor feces) FMT groups. Patients completed three questionnaires that assessed their symptoms and quality of life at baseline and at 2 weeks, 1 month, and 3 months after FMT, and provided fecal samples before and 1 month after FMT. The fecal bacteria were analyzed using the 16S rRNA gene in PCR DNA amplification covering the V3–V9 variable genes.
Results
Response rates of the placebo group did not differ between S-IBS-S and Mo-IBS-S patients at 2 weeks, 1 month and 3 months after FMT. The response rates in the active treatment group were higher in S-IBS-S patients than in Mo-IBS-S patients at each observation time. FMT reduced abdominal symptoms and fatigue and improved the quality of life in patients with both severe and moderate IBS. Patients with S-IBS-S had higher levels of Eubacterium siraeum, and lower levels of Eubacterium rectale than Mo-IBS-S, after FMT.
Conclusion
Patients with S-IBS-S have a higher response rate to FMT and a marked improvement in fatigue and in quality of life compared with those with Mo-IBS-S. The clinical trial registration number is NCT03822299 and is available at www.clinicaltrials.gov.
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder that considerably reduces the quality of life [Citation1,Citation2]. Clinical IBS treatment is directed merely at symptom relief [Citation3]. The gut microbiota and bacterial diversity significantly affect IBS pathophysiology [Citation4]. The outcomes of fecal microbiota transplantation (FMT) in treating IBS differ considerably in different studies from highly beneficial to no effect at all [Citation5]. These mixed results are caused by differences in the selection criteria of the donor and the FMT protocol used as well as the cohort of patients included [Citation5]. These differences explain why pooling all randomized clinical trials showed that FMT is not superior to placebo [Citation6]. However, with careful donor selection and using the appropriate protocol, FMT reduces abdominal symptoms and fatigue and improves the quality of life of IBS patients [Citation5,Citation7].
FMT for IBS has been investigated in seven randomized controlled trials (RCTs) mostly involving patients with moderate-to-severe IBS symptoms [Citation5]. In a recent study with a successful outcome, the patients included were those with refractory IBS patients with severe bloating who had failed to respond to at least three conventional therapies for IBS [Citation8]. This study raised the question as to which category of IBS patients should FMT used as an intervention [Citation8].
The present study aimed to determine the differences between patients with severe IBS symptoms (S-IBS-S) and moderate IBS symptoms (Mo-IBS-S) regarding FMT response, symptom reduction, quality-of-life improvement, dysbiosis and bacteria profile for the same patient cohort included in our previous RCT [Citation9].
Materials and methods
Study design and patient randomization
The study design has been described in detail elsewhere [Citation9]. To summarize, patients completed three questionnaires that assessed their symptoms and quality of life at baseline and at 2 weeks, 1 month and 3 months after FMT. They also provided fecal samples for bacterial analysis both before and 1 month after FMT. The patients were randomly divided at a 1:1:1 ratio into a placebo (30-g own feces) group and 30-g and 60-g (donor feces) FMT groups.[Citation9] The patients who received 30 g or 60 g of donor feces were combined and described as the active treatment group in order to increase the sample size and thereby reduce the probability of type-II statistical errors.
Patients
The present study included 164 patients who had participated in our previous RCT [Citation9]. lists the characteristics of these patients. The details of these patients have previously been described in detail [Citation9]. To summarize, outpatients from Stord Hospital who fulfilled the Rome IV criteria for an IBS diagnosis were included. The inclusion criteria were being older than 18 years and experiencing moderate-to-severe IBS symptoms, as indicated by an IBS Severity Scoring System (IBS-SSS) score of ≥175. The exclusion criteria were pregnancy or planning pregnancy, lactating, the presence of a systemic disease, immune deficiency or having received immune-modulating medication, psychiatric illness, excessive alcohol consumption, or drug abuse. Patients who took probiotics, antibiotics, or IBS medications within 8 weeks before the study were also excluded [Citation9].
Table 1. Characteristics of the patients in the placebo and active treated groups.
Donor
The donor used in this study has previously been described in detail [Citation9]. To summarize, he was screened according to the European guidelines for FMT donors [Citation10]. He was a healthy young nonsmoking male, was not taking any medication, and had a normal BMI. He had been born via vaginal delivery, breastfed and had undertaken only a few courses of antibiotics during his life. He regularly exercised and took sport-specific dietary supplements, meaning his diet was richer in protein, fiber, minerals and vitamins than average. Bacterial analysis of his feces revealed normal bacteria diversity (normobiosis) [Citation9].
Collection, preparation and administration of fecal samples
Fecal samples from the donor and patients were immediately frozen and stored at −20 °C until delivery to the laboratory, where they were stored at −80 °C. The FMT process has previously been described in detail [Citation9]. To summarize, fecal samples were thawed for two days at 4 °C, mixed manually (i.e., without using a mechanical device) with 40 ml of sterile saline, and filtered before being administered. The transplant was administered to the distal duodenum via the working channel of a gastroscope [Citation9].
Symptom and quality-of-life assessments
IBS symptoms were assessed using the IBS-SSS questionnaire. This questionnaire contains five questions, each with a maximum score of 100 using a visual analog scale. Total scores between 75 and 175 were considered indicative of mild IBS severity (Mi-IBS-S), 175–300 were Mo-IBS-S, and >300 were S-IBS-S [Citation11]. The response was defined as a decrease in the total IBS-SSS score after FMT of ≥50 points.
Fatigue was assessed using the Fatigue Assessment Scale (FAS) questionnaire [Citation12]. The questionnaire comprises 10 questions with 5-point-scale answers varying from never to always. Five of these questions measured physical fatigue and the other five measured mental fatigue.
Quality of life was measured using the IBS Quality of Life Scale (IBS-QoL) questionnaire [Citation13–15]. This questionnaire contains 34 items concerning the physical and psychological functioning of IBS patients in 8 domains: dysphoria, activity interference, body image, health concerns, food avoidance, social reactions, sexual function and impact on relationships.
Microbiome analysis and dysbiosis index
The fecal bacteria profile and dysbiosis were determined using a method utilizing a pre-determined targets (PDT) approach [Citation16]. The test uses the 16S rRNA gene in PCR DNA amplification covering V3–V9 variable genes, and probe labeling with single nucleotide extension and signal detection using the BioCode 1000 A 128-plex Analyzer (Applied BioCode, Santa Fe Springs, CA, USA) [Citation16]. The 48 bacterial markers used covered different taxonomic levels of >300 bacteria [Citation17]. A dysbiosis index (DI) was measured on a 5-point scale from 1 to 5, where DI values of 1 and 2 indicate normobiosis, while those of 3–5 indicate dysbiosis [Citation16].
Statistical analysis
Differences in response and dysbiosis proportion between S-IBS-S and Mo-IBS-S patients in the placebo and active treatment groups were analyzed using Fisher’s exact test. The nonparametric Kruskal–Wallis’s test with Dunn’s multiple comparisons test as a post-test was used in analyzing the difference between S-IBS-S and Mo-IBS-S patients in their scores for the IBS-SSS, FAS and IBS-QoL. The Mann–Whitney U-test was used to analyze the differences between in fecal bacteria fluorescent signals. A nonparametric Spearman’s test was used for correlation between bacterial fluorescent signals and IBS-SSS total score. These analyses were performed using GraphPad Prism 8 (La Jolla, CA, USA).
Ethics
The West Regional Committee for Medical and Health Research Ethics, Bergen, Norway approved the present study (approval no. 2017/1197/REK vest). All subjects provided both oral and written consent before participation. The study was registered at www.clinicaltrials.gov (NCT03822299) and www.cristin.no (ID657402).
Results
Response to FMT
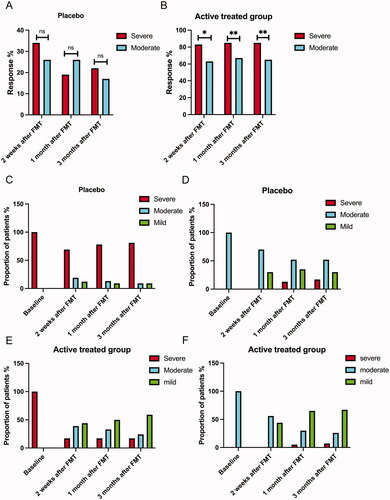
The response rates of the placebo group did not significantly differ between S-IBS-S and Mo-IBS-S patients at 2 weeks, 1 month and 3 months after FMT. The response rates in the active treatment group were significantly higher in S-IBS-S patients than in Mo-IBS-S patients at each observation time (). In the placebo group, a small proportion of the S-IBS-S patients converted to Mo-IBS-S or Mi-IBS-S at 2 weeks, 1 month and 3 months after FMT. Similarly, a small proportion of patients with Mo-IBS-S converted to S-IBS-S or Mi-IBS-S (). Conversely, a large proportion of either S-IBS-S or Mo-IBS-S patients experienced lower degrees of symptom severity at 2 weeks, 1 month and 3 months after FMT ().
Figure 1. Fecal microbiota transplantation (FMT) response rates in patients with severe and moderate irritable bowel syndrome (IBS) symptoms at 2 weeks, 1 month and 3 months after FMT in the placebo (A) and active treatment (B) groups. Changes in IBS symptom severity over time in the placebo (C, D) and active treatment group (E, F). ns, not significant; *adjusted p < .05; **adjusted p < .01.
Symptoms and quality of life
The total IBS-SSS scores of the active treatment group decreased significantly after FMT at each observation interval, whereas those of the placebo group did not. The placebo and active treatment groups had higher total IBS-SSS scores among the S-IBS-S than the Mo-IBS-S patients at each observation interval (). However, the difference in the total IBS-SSS score between S-IBS-S and Mo-IBS-S was smaller in the active treatment group at 2 weeks, 1 month and 3 months after FMT (). Notably, this suggests that the means of the total IBS-SSS scores of the S-IBS-S in the active treatment group were in the region of Mo-IBS-S after FMT at all of the observation intervals. Similarly, the means of the total IBS-SSS scores for Mo-IBS-S patients in the active treatment group were lower than those for Mi-IBS-S patients (, ).
Figure 2. Total scores of IBS-SSS (A, B), of FAS (C, D), of IBS-QoL (E, F). at baseline and at 2 weeks, 1 month and 3 months after FMT. In (A) and (B), values above and below the red line indicate severe and moderate IBS symptoms, respectively. Values above and below the green line indicate moderate and mild IBS symptoms, respectively. ns, not significant; ***adjusted p < .001; ****adjusted p < .0001.
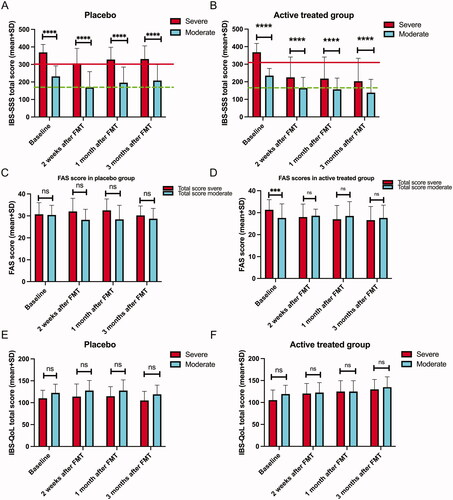
Table 2. IBS-SSS total scores and scores for the four items of the scale in patients with severe and moderate IBS symptoms in placebo and active-treated groups at different intervals following FMT.
The total FAS scores in both S-IBS-S and Mo-IBS patients decreased significantly compared to the baseline in the active treatment group at 2 weeks, 1 month and 3 months after FMT (, ).
Figure 3. The Dysbiosis index (DI) of patients with severe and moderate IBS symptoms at the baseline and 1 month after FMT in the placebo (A) and active treatment (B) groups.Comparison between DIs in patients with severe and moderate IBS symptoms at the baseline and 1 month after FMT in the placebo (C) and active treatment (D) groups. The bacteria whose fluorescence signals differed between patients with severe and moderate IBS symptoms belonging to the active treatment group at the baseline (E) and 1 month after FMT (F). ns, not significant; *adjusted p < .05; **adjusted p <.01; ****adjusted p < .0001.
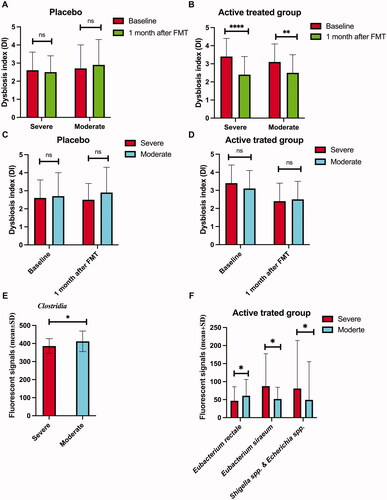
Table 3. FAS total scores and scores for physical fatigue and mental fatigue in patients with severe and moderate IBS symptoms in placebo and active-treated groups at baseline, 2 weeks, 1 month and 3 months after FMT.
The total IBS-QoL scores increased in both S-IBS-S and Mo-IBS-S patients in the active treatment group 2 weeks after FMT (). The total IBS-QoL scores increased in S-IBS-S patients, but not in Mo-IBS-S patients in the active treatment group 1 month after FMT. The total IBS-QoL scores increased in both S-IBS-S and Mo-IBS-S patients at 3 months after FMT (). There were no changes in the total IBS-QoL scores in the placebo group at any observation interval. Mo-IBS-S patients had higher total IBS-QoL scores than S-IBS-S patients at all observation intervals in the placebo group (). In the actively treated group, the total IBS-QoL scores were higher in Mo-IBS-S patients than in S-IBS-S patients at the baseline, but there were no differences after FMT at any observation interval ().
Figure 4. Correlation between IBS-SSS total score, and Eubacterium rectale (A), Eubacterium siraeum (B) and Shigella spp. (C).
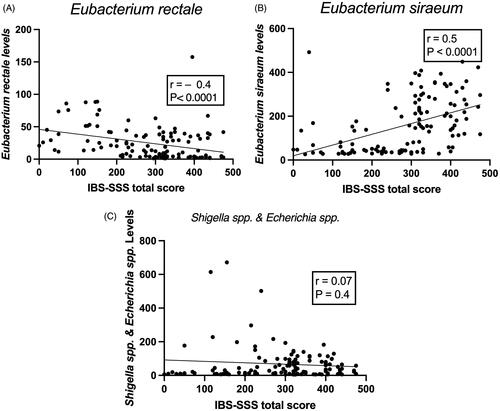
Table 4. IBS-QoL total scores and scores in the eight domains of the scale in placebo and active treated groups.
Bacterial analysis
DIs in the active treatment group in both S-IBS-S and Mo-IBS-S patients decreased 1 month after FMT, whereas they did not in the placebo group (). DIs did not differ between patients with S-IBS-S and Mo-IBS-S at either the baseline or 1 month after FMT in both the placebo and active treatment groups (). The fluorescence signals for all investigated bacteria did not differ between S-IBS-S and Mo-IBS-S patients in the placebo group at either the baseline or 1 month after FMT. In the active treatment group, Mo-IBS-S patients had higher fluorescence signals of Clostridia species than did S-IBS-S patients at the baseline. The fluorescence signals of Eubacterium siraeum, and Shigella spp., were significantly higher in S-IBS-S than Mo-IBS-S patients, and that of was lower in S-IBS-S than Mo-IBS-S patients 1 month after FMT (). In Mo-IBS-S the fluorescence signals of Eubacterium rectale, E. siraeum and Shigella spp. at 1 month after FMT did not differ from those at the baseline (). The fluorescence signals of E. siraeum significantly decreased 1 month after FMT, but not those of E. rectale, and Shigella spp. (). Whereas E. rectale correlated negatively with IBS-SSS total scores, E. siraeum, correlated positively with IBS-SSS total scores. Shigella spp. did not correlate with IBS-SSS total scores ().
Table 5. The fluorescence signals of Eubacterium rectale, Eubacterium siraeum and Shigella spp. at baseline and 1 month after FMT in IBS patients with moderate IBS symptoms (Mo-IBS-S) and patients with severe IBS Symptoms (S-IBS-S).
Discussion
FMT as a treatment for IBS patients was investigated in seven randomized controlled trials (RCTs) [Citation8,Citation9,Citation18–22]. four of which showed a positive effect [Citation8,Citation9,Citation18,Citation22] while the other three showed no effect [Citation19–21]. It is challenging to compare these RCTs as they differed considerably in the criteria used to select the donors and patients, in the dose of the fecal transplant used and in the FMT protocols [Citation5].
There are some safety concerns when using FMT as an IBS treatment. These concerns emerged when two patients had serious adverse events in the form of infectious complications (with one fatality) after FMT for indications other than IBS [Citation23,Citation24]. However, long-term follow-ups of patients receiving FMT because of a Clostridium difficile infection concluded despite the development of a number of new conditions post-FMT, no clustering of diseases was associated with FMT [Citation25]. Furthermore, recently published studies showed that FMT is safe when the international rigorous guidelines for selecting and testing the donor were applied [Citation26–28]. IBS has been considered a benign gastrointestinal condition that is not life-threatening and using FMT as an intervention for this condition is not justified due to possible complications [Citation29–31]. The adverse events of FMT in IBS patients are mild and self-limited, in the form of mild abdominal pain, diarrhea, or constipation [Citation5]. To minimize risks from FMT for IBS, it has been proposed that donor screening should include testing for extended-spectrum-beta-lactase-producing Escherichia coli and SARS-CoV-2, and FMT for IBS patients should be restricted to those without systemic disease, immune deficiency, immune-modulating medication treatment, or severe illness [Citation31]. A recently published RCT involving refractory IBS patients with a successful outcome indicated that not all patients will benefit from FMT treatment and that the success of FMT is dependent on the IBS subset [Citation8].
The present study showed that FMT reduces abdominal symptoms and fatigue and improves the quality of life in both S-IBS-S and Mo-IBS-S patients. FMT is therefore beneficial for both groups of IBS patients, but its effects differed between these two groups. The response rates were higher in S-IBS-S patients than in Mo-IBS-S patients at all observation intervals. Moreover, Fatigue that was higher in S-IBS-S than that of Mo-IBS-S patients at baseline decreased to the same level of MO-IBS-S after FMT. Furthermore, the quality of life of S-IBS-S patients, which was lower than that of Mo-IBS-S patients, increased to the same level as that of Mo-IBS-S patients after the FMT intervention.
S-IBS-S patients had lower levels of E. rectale, and higher levels of E. siraeum than Mo-IBS-S patients at 1 month after FMT. It is worthy of note that the levels of E. siraeum decreased in S-IBS-S patients 1 month after FMT. Eubacterium rectale and E. siraeum are both anaerobic rod-shaped, Gram-positive bacteria belonging to the Eubacteriaceae family. Eubacterium rectale produces butyrate and E. siraeum produces acetic acid during carbohydrate fermentation [Citation32,Citation33]. Butyrate is a modulator of colonic hypersensitivity, and butyrate intake decreases abdominal pain in IBS patients [Citation34–36]. Butyrate is also used as an energy source for epithelial cells in the colon. Furthermore, butyrate decreases intestinal cell permeability and intestinal motility, and modulates the immune response [Citation37,Citation38]. Recent research has shown that butyric acid levels are inversely correlated with the total scores for both the IBS-SSS and FAS [Citation39]. On the other hand, acetic acid has been indicated to induce visceral hypersensitivity in rodents [Citation40]. It is therefore tempting to speculate that the lower levels of E. rectale and the higher levels of E. siraeum in S-IBS-S patients after FMT with the subsequent decrease of butyrate and increase in acetic acid production explains why the total IBS-SSS score was higher in S-IBS-S patients than in Mo-IBS-S patients in the present study. In support of this assumption are the present findings that E. rectale correlated negatively with IBS-SSS total score, and E. siraeum correlated positively with IBS-SSS total score.
The strengths of this study are that it included a relatively large cohort of IBS patients, comprising three IBS subtypes and used a single well-defined donor. Thus, allowing a subgroup analysis. limitations of this study are that it did not include the fourth IBS subtype, IBS-U, predominated by females and it only investigated some of the intestinal bacterial contents.
In conclusion, FMT reduced abdominal symptoms and fatigue and improved the quality of life in both S-IBS-S and Mo-IBS-S patients. However, S-IBS-S patients have a higher response rate to FMT and a marked improvement in fatigue and in quality of life compared with Mo-IBS-S patients. Although FMT seems to be more beneficial for S-IBS-S patients, FMT trials should also include Mo-IBS-S patients.
Author contributions
M.E.S. designed the study, obtained the funding, administered the study, recruited the patients, performed FMT, collected, analyzed and interpreted the data, and drafted the manuscript. T.M., T.H. and J.G.H. contributed to the design of the study, to data analysis and critically revised the manuscript for important intellectual content.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80.
- El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21(25):7621–7636.
- El-Salhy M, Gilja OH, Hatlebakk JG. Overlapping of irritable bowel syndrome with erosive esophagitis and the performance of Rome criteria in diagnosing IBS in a clinical setting. Mol Med Rep. 2019;20:787–794.
- El-Salhy M, Hatlebakk JG, Hausken T. Diet in irritable bowel syndrome (IBS): interaction with gut microbiota and gut hormones. Nutrients. 2019;11(8):1824.
- El-Salhy M, Hausken T, Hatlebakk JG. Current status of fecal microbiota transplantation for irritable bowel syndrome. Neurogastroenterol Motil. 2021;33(11):e14157.
- Xu D, Chen VL, Steiner CA, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(7):1043–1050.
- El-Salhy M, Kristoffersen AB, Valeur J, et al. Long-term effects of fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2022;34(1):e14200.
- Holvoet T, Joossens M, Vázquez-Castellanos JF, et al. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. 2021;160(1):145–157.
- El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69(5):859–867.
- Cammarota G, Ianiro G, Tilg H, European FMT Working Group, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Tther. 1997;11(2):395–402.
- Hendriks C, Drent M, Elfferich M, et al. The fatigue assessment scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018;24(5):495–503.
- Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterology. 2000;95(4):999–1007.
- Wong RK, Drossman DA. Quality of life measures in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2010;4(3):277–284.
- Arslan G, Lind R, Olafsson S, et al. Quality of life in patients with subjective food hypersensitivity: applicability of the 10-item short form of the Nepean dyspepsia index. Dig Dis Sci. 2004;49(4):680–687.
- Casén C, Vebø HC, Sekelja M, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42(1):71–83.
- Enck P, Mazurak N. Dysbiosis in functional bowel disorders. Ann Nutr Metab. 2018;72(4):296–306.
- Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3(1):17–24.
- Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67(12):2107–2115.
- Aroniadis OC, Brandt LJ, Oneto C, et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4(9):675–685.
- Holster S, Lindqvist CM, Repsilber D, et al. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome: a randomized controlled study. Clin Transl Gastroenterol. 2019;10(4):e00034.
- Lahtinen P, Jalanka J, Hartikainen A, et al. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51(12):1321–1331.
- DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050.
- Blaser MJ. Fecal microbiota transplantation for dysbiosis - predictable risks. N Engl J Med. 2019;381(21):2064–2066.
- Perler BK, Chen B, Phelps E, et al. Long-term efficacy and safety of fecal microbiota transplantation for treatment of recurrent Clostridioides difficile infection. J Clin Gastroenterol. 2020;54(8):701–706.
- Ooijevaar RE, van Nood E, Goorhuis A, et al. Ten-year follow-up of patients treated with fecal microbiota transplantation for recurrent Clostridioides difficile infection from a randomized controlled trial and review of the literature. Microorganisms. 2021;9(3):548.
- Merrick B, Allen L, Masirah MZN, et al. Regulation, risk and safety of faecal microbiota transplant. Infect Prev Pract. 2020;2(3):100069.
- Cold F, Svensson CK, Petersen AM, et al. Long-term safety following faecal microbiota transplantation as a treatment for recurrent Clostridioides difficile infection compared with patients treated with a fixed bacterial mixture: results from a retrospective cohort study. Cells. 2022;11(3):435.
- Barbara G, Ianiro G. Faecal microbial transplantation in IBS: ready for prime time? Gut. 2020;69(5):795–796.
- Camilleri M. FMT in IBS: a call for caution. Gut. 2021;70(2):431.
- El-Salhy M. FMT in IBS: how cautious should we be? Gut. 2021;70(3):626.1–628.
- Karcher N, Pasolli E, Asnicar F, et al. Analysis of 1321 Eubacterium rectale genomes from metagenomes uncovers complex phylogeographic population structure and subspecies functional adaptations. Genome Biol. 2020;21(1):138.
- Zou Y, Liang N, Zhang X, et al. Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet Res. 2021;17(1):216.
- Zhang J, Song L, Wang Y, et al. Beneficial effect of butyrate-producing lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34(8):1368–1376.
- Long X, Li M, Li LX, et al. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol Motil. 2018;30(4):e13227.
- Banasiewicz T, Krokowicz L, Stojcev Z, et al. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013;15(2):204–209.
- El-Salhy M, Hatlebakk JG, Hausken T. Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS). Neuropeptides. 2020;79:101973.
- Hamer HM, Jonkers D, Venema K, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119.
- El-Salhy M, Valeur J, Hausken T, et al. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33(2):e13983.
- Winston J, Shenoy M, Medley D, et al. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132(2):615–627.
