Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and the metabolic syndrome is the main risk factor. Alanine aminotransferase (ALT) is widely used to screen for NAFLD, and the aims of this study were to assess the prevalence and risk factors of NAFLD in a general population.
Methods
The study was based on the third population-based Trøndelag Health Study (HUNT3), Norway, performed 2006–2008. In HUNT3, ALT and lipids were analyzed, anthropometric measures done, and comorbidity and risk factors reported. Elevated ALT was used to define NAFLD and participants with other diagnosed liver diseases and excessive alcohol consumption were excluded. Multivariable logistic regression reporting odds ratio (OR) and 95% confidence interval (CI) was used to assess risk factors.
Results
In HUNT3, 2373 (4.7%) of 50,006 participants were diagnosed with NAFLD. The risk increased with obesity (OR 1.73, 95% CI 1.46–2.05) and very increased waist circumference (OR 1.97, 95% CI 1.65–2.35), and the risk increased dose-dependently (p for trend <0.001). Hypertension (OR 1.58, 95% CI 1.42–1.76), diabetes mellitus (OR 1.48, 95% CI 1.30–1.68), high triglycerides (OR 1.55, 95% CI 1.41–1.71), high total cholesterol (OR 1.52, 95% CI 1.29–1.81) and low high-density lipoproteins (OR 1.33, 95% CI 1.21–1.47) also increased the risk of NAFLD. The risk was lower in men (OR 0.71, 95% CI 0.64–0.79) and among current smokers (OR 0.79, 95% CI 0.70–0.89).
Conclusion
NAFLD is a common condition in the general population. NAFLD should be suspected in individuals with abdominal obesity, hypertension, diabetes mellitus and dyslipidemias.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as excessive deposition of triglycerides in the hepatocytes, occurring in the absence of other conditions that may lead to hepatic steatosis, such as alcoholic liver disease, viral hepatitis, autoimmune hepatitis and hemochromatosis [Citation1–4]. Commonly used diagnostic methods include abnormal alanine aminotransferase (ALT) levels, hepatic ultrasound and liver biopsy. Patients with NAFLD may develop nonalcoholic steatohepatitis, a progressive inflammatory liver disease, which may result in cirrhosis [Citation5] and increased risk of hepatocellular carcinoma [Citation6–8].
NAFLD is the most common chronic liver disease in the world [Citation1,Citation9]. The prevalence varies due to different definitions of the disease and depending on the geographical region. A meta-analysis from 2016 estimated a pooled global prevalence of NAFLD of 25.2%, with an average prevalence in Europe of 23.7%, varying between 5% and 44% [Citation10]. However, population-based prevalence data are sparse [Citation11,Citation12].
The association between NAFLD and the metabolic syndrome is well documented, and patients with NAFLD tend to have certain elements of the metabolic syndrome such as obesity, hypertension, diabetes mellitus and dyslipidemias [Citation2,Citation13,Citation14]. Given the rise of obesity across most western countries, the burden of NAFLD will most certainly increase in the near future [Citation11].
The aims of this study were to estimate the prevalence of NAFLD in a general Norwegian population and assess the risk of NAFLD by the elements of the metabolic syndrome.
Methods
Study design
This study was based on the Trøndelag Health Study (HUNT), a population-based study where all inhabitants of Nord-Trøndelag County, Norway, from 20 years of age have been invited [Citation15]. The present study was based on the third survey (HUNT3) performed from 2006 to 2008, including 50,807 participants (54.1% participation rate).
HUNT consists of questionnaires, anthropometric measurements, and biological samples. The questionnaires encompass self-reported diseases, illnesses, major behavioral risk factors and socioeconomic factors. The anthropometric measurements include blood pressure, heart rate, weight, height, and waist- and hip-circumference, performed by trained personnel at local health examination stations established throughout the county during the survey. Nonfasting venous blood samples were also collected and transported daily by courier for storage at HUNT Biobank or analyses. All blood analyses were done at Levanger Hospital, Nord-Trøndelag Hospital Trust.
Based on the Personal Identification Number assigned to every Norwegian inhabitant, HUNT data was linked to medical records at Nord-Trøndelag Hospital Trust and St. Olav′s Hospital, covering the vast majority of specialist health care in the HUNT population.
Definition of NAFLD
NAFLD was defined based on ALT measurements in the venous blood samples collected from the HUNT3 participants. ALT was analyzed by reduced nicotinamide adenine dinucleotide methodology (Architect ci8200, Abbot Clinical Chemistry) and the result was checked for outliers as a quality assurance [Citation15]. Elevated ALT was defined as ALT >45 IU/L in women and >70 IU/L in men.
Participants with other possible causes of elevated ALT were identified through linkage with medical records at Nord-Trøndelag Hospital Trust and St. Olav′s Hospital. Based on the International Classification of Diseases (ICD), versions 9 and 10, participants with the following chronic liver disease diagnoses within one year before or after the ALT measurement were defined as not having NAFLD: alcoholic liver disease (ICD-9: 571.0–571.2 and ICD-10: K70), chronic hepatitis B and C infections (ICD-9: 070.0, 070.5, 070.9 and ICD-10: B17.0, B18-24), autoimmune hepatitis (ICD-9: 571.42 and ICD-10: K75.4) and hemochromatosis (ICD-9: 275.0 and ICD-10: E83.1). Participants with the following acute liver disease diagnoses within three months before or after the ALT measurement were also defined as not having NAFLD: acute hepatitis A (ICD-9: 070.1 and ICD-10: B15.0, B15.9), acute hepatitis B (ICD-9: 070.3 and ICD-10: B16.0, B16.1, B16.9), acute hepatitis C (ICD-10: B17.1), acute hepatitis E (ICD-10: B17.2), other acute viral hepatitis (ICD-10: B17.8, B17.9), cytomegalovirus infection (ICD-9: 078.5 and ICD-10: B25.1) and Epstein-Barr virus infection (ICD-9: 075 and ICD-10: B27).
In addition, participants were excluded if they reported excessive alcohol consumption in HUNT3. Alcohol consumption was self-reported and the assessment was based on two questions: (1) ‘About how often in the last 12 months did you drink alcohol?’ with the response alternatives ‘daily’, ‘weekly’, ‘monthly’ and ‘never’; and (2) ‘How often do you drink 5 glasses or more of beer, wine or spirits in one sitting?’ with the response alternatives ‘4–7 times a week’, ‘2–3 times a week’, ‘about once a week’, ‘2–3 times a month’, ‘about once a month’, ‘a few times a year’, ‘not at all the last year’ and ‘never drunk alcohol’. Excessive alcohol consumption was defined as answering ‘daily’ or ‘weekly’ to question (1) or ‘4–7 times a week’, ‘2–3 times a week’ or ‘about once a week’ to question (2).
Risk factors
The available possible risk factors of NAFLD included age, sex, tobacco smoking status, and features of the metabolic syndrome, including body mass index (BMI), waist circumference, blood pressure, diabetes mellitus, serum triglycerides, serum cholesterol, and serum high density lipoproteins (HDL), all collected and assessed at participation in HUNT3.
Tobacco smoking status was assessed based on the participants reported tobacco smoking in HUNT3. The participants were asked ‘Do you smoke?’ with the response alternatives ‘no, I have never smoked’, ‘no, I have stopped smoking’, ‘yes, I occasionally smoke cigarettes’, ‘yes, I occasionally smoke cigars/cigarillos/pipe’, ‘yes, I smoke cigarettes daily’ and ‘yes, I smoke cigars/cigarillos/pipe daily’. BMI, waist circumference and blood pressure were objectively measured by trained personnel at the health examination stations. BMI was calculated as the weight in kilograms squared divided by the height in meters. Both weight and height were measured with the participants wearing light clothes and no shoes. Waist circumference was measured with the participant in a standing position, with the arms hanging relaxed, wearing light clothes, after maximal expiration, and at the level of the navel or midway between the bottom of the ribs and the top of the hip bone, if the latter was largest. Blood pressure was measured three separate times, and the systolic and diastolic pressure was calculated by the rounded arithmetic mean of measurement two and three. If only two measurements were available, the last measurement was used. In addition, the participants were asked ‘Do you take or have you taken medication for high blood pressure?’ with the response alternatives ‘yes’ and ‘no’. Diabetes mellitus was self-reported and based on two questions: (1) ‘Have you ever had or do you have any of the following diseases’ with several diseases listed, including diabetes mellitus; and (2) ‘Has it ever been verified that you had high blood sugar (hyperglycemia)?’ Triglycerides were analyzed by the glycerol phosphate oxidase methodology, serum cholesterol was analyzed by the enzymatic cholesterol esterase methodology, and HDL was analyzed by the accelerator selective detergent methodology.
Statistical analyses
The prevalence of NAFLD was estimated by dividing the number of participants with ALT above the cutoff, excluding the participants with other possible causes of elevated ALT, with the total number of participants in HUNT3 with available ALT measurements.
Multivariable logistic regression, providing odds ratios (ORs) and 95% confidence intervals (CIs), was used to assess the association between NAFLD and the possible risk factors. Age was assessed as a continuous variable. Tobacco smoking status was subdivided into three categories: never, previous and current. In accordance with the World Health Organization’s classification, BMI was subdivided into three categories: under-/normal weight (BMI <25), overweight (BMI 25–30) and obese (BMI >30). The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATPIII) was used to define the cutoffs for the features of the metabolic syndrome (Citation16). According to NCEP ATPIII, waist circumference >88 cm for women and >102 cm for men, is considered a criterion for the metabolic syndrome. However, the European Group for the Study of Insulin Resistance use lower limits of waist circumference, ≥80 cm in women and ≥94 cm in men (Citation16). Thus, in the present study, waist circumference was subdivided into three categories for each sex: normal (<80 cm for women and <94 cm for men), increased (≥80–88 cm for women and ≥94–102 cm for men), and very increased (>88 cm for women and >102 cm for men). According to NCEP ATPIII, the presence of hypertension was defined as systolic blood pressure >130 mmHg, diastolic blood pressure >85 mmHg, or current use of antihypertensive medication. Diabetes mellitus was defined by the self-reported presence or if they reported having experienced a hyperglycemic event. Elevated triglyceride-level was defined as ≥1.7 mmol/L. NCEP ATPIII does not include a definition of elevated total cholesterol and therefore the 75% quartile was used. Total cholesterol was therefore considered as elevated if >6.1 mmol/L. Low levels of HDL were defined as <1.3 mmol/L in women and <1.0 mmol/L in men.
Wald-test for linear trend was performed for the BMI and waist circumference categories to assess if there was an increased risk of NAFLD with increasing BMI and waist circumference.
The statistical analysis was conducted using STATA/MP 16 (College Station, TX, USA).
Ethical approval and informed consent
The present study was approved by the Regional Committee for Medical Research Ethics, South East (2018/2453/REK-sør-øst B). Informed consent was collected at participation in HUNT3 and included approval of future research projects and linkage to health registries and medical records.
Results
The prevalence of NAFLD
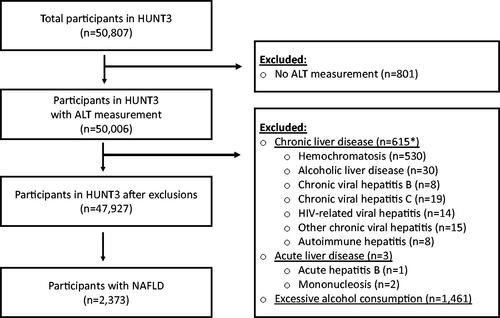
ALT was measured in 50,006 HUNT3 participants and 618 were excluded due to other diagnosed liver diseases and 1461 due to excessive alcohol consumption (). After these exclusions, 2373 participants were defined with NAFLD, corresponding to a prevalence of 4.7%.
Characteristics
The characteristics of the cases with NAFLD (n = 2373) and the remaining HUNT3-participants (reference population, n = 45,554) are presented in . The mean age was the same in both groups, 52.1 and 53.5 years in cases and the reference population, respectively. The proportion of women was higher among cases (63.3%) than among the reference population (55.2%). The mean ALT was 80.1 IU/L among cases and 25.6 IU/L among the reference population. There were fewer current tobacco smokers among cases (20.3%) than among the reference population (23.6%). The mean BMI was higher in cases (29.6) than in the reference population (27.0). In addition, a larger proportion of cases (44.3%) than the reference population (21.8%) were obese (BMI ≥30). The mean waist circumference was also higher in cases (99.6 cm) than in the reference population (93.2 cm), and a larger proportion of cases (65.8%) than the reference population (40.5%) had very increased waist circumference (>88 cm for women and >102 cm for men). The proportion of participants with hypertension was higher among cases (65.7%) than among the reference population (54.2%), and the proportion of participants with diabetes mellitus was higher among cases (14.7%) than among the reference population (8.2%). The mean triglyceride level was higher among cases (2.1 mmol/L) than among the reference population (1.6 mmol/L), and a larger proportion of cases (54.1%) than the reference population (36.2%) had elevated triglycerides. There were no differences in the mean cholesterol or HDL levels among cases and the reference population, but a greater proportion of cases (7.4%) than the reference population (4.1%) were defined with elevated cholesterol and a greater proportion of cases (38.9%) than the reference population (23.2%) were defined with lowered HDL.
Table 1. Characteristics of cases with NAFLD and the reference population.
Risk factors of NAFLD
The risk of NAFLD for each risk factor is presented in . Age in years was inversely, but weakly associated with NAFLD (adjusted OR 0.98, 95% CI 0.98–0.98). Men had reduced risk of NAFLD, compared to women (adjusted OR 0.71, 95% CI 0.64–0.79). Current tobacco smoking was associated with reduced risk of NAFLD (adjusted OR 0.79, 95% CI 0.70–0.89). Obesity (BMI ≥30) was associated with increased risk of NAFLD (adjusted OR 1.73, 95% CI 1.46–2.05), and the risk of NAFLD increased with increasing BMI category (p for trend <0.001). The risk of NAFLD was also increased with increasing waist circumference (adjusted OR 1.97, 95% CI 1.65–2.35 for very increased waist circumference, p for trend <0.001). Hypertension (adjusted OR 1.58, 95% CI 1.42–1.76), diabetes mellitus (adjusted OR 1.48, 95% CI 1.30–1.68), elevated triglycerides (adjusted OR 1.55, 95% CI 1.41–1.71), elevated cholesterol (adjusted OR 1.52, 95% CI 1.29–1.81) and lowered HDL (adjusted OR 1.33, 95% CI 1.21–1.47) increased the risk of NAFLD. These associations were similar in analyses done separately for each sex (data not shown).
Table 2. The risk of NAFLD.
Discussion
In this population-based study from Norway, NAFLD was a common condition. The risk of NAFLD was higher in women, in individuals with obesity, especially abdominal obesity and in individuals with hypertension, diabetes mellitus and dyslipidemias.
The United States National Health and Nutrition Examination Surveys in 1988–1994 and 1999–2002, used elevated ALT levels (>43 U/L) to define NAFLD, and found an increase in the prevalence of NAFLD over time in the population from 3% to 7% after excluding participants with excessive alcohol consumption or known hepatitis B, C, iron overload or diabetes [Citation7,Citation17,Citation18]. In a more recent study from the United States, the prevalence of NAFLD based on two or more elevated ALT values (≥40 IU/mL) increased 2.8-fold from 6.3% to 17.6% between 2003 and 2011, with an annual incidence of NAFLD in the population ranging from 2% to 3% [Citation3]. A meta-analysis from 2016, that searched Medline from 1989 to 2015, found a pooled NAFLD prevalence of 13% in Europe among patients diagnosed by blood testing, including elevated liver enzymes and fatty liver index [Citation10]. A study from 2015 found higher prevalence of NAFLD in Europe (28%) compared to East-Asia (19%) and the Middle-East (13%) [Citation12]. The increasing prevalence is likely to continue due to a steady incidence overall, combined with a rising incidence in the younger population. Currently, NAFLD is the most common liver abnormality among children aged 2–19 years. An autopsy study, found a prevalence of hepatic steatosis of 38% among obese children [Citation19].
A meta-analysis found that men had higher prevalence of NAFLD compared to women in Europe, which is expected as men in general have more abdominal obesity [Citation12]. However, the present study showed that women had higher risk of NAFLD. NAFLD is closely associated with the metabolic syndrome and the individual components of the metabolic syndrome and this was also confirmed in the present study. The burden of obesity and its related comorbidities are lower in Norway compared to the United States, so it was expected that the prevalence of NAFLD would be lower in Norway. However, a study from 2015 demonstrated that the burden of NAFLD is correlated with the nation’s economic status [Citation12]. As Norway is one of the wealthiest countries in the world, it would be expected high prevalence of NAFLD in Norway. In the United States Veterans Administration population, patients who met the NAFLD criteria (defined as at least two ALT values ≥40 IU/mL ≥6 months apart and no evidence of hepatitis B, hepatitis C or excessive alcohol use) were older, more likely to be white, obese, and with higher prevalence of diabetes mellitus and hypercholesterolemia [Citation2]. These findings are in agreement with the results from the present study, except that older age was associated with a slightly reduced risk.
The gold standard for diagnosing NAFLD is a liver biopsy, but this is seldom used to establish the diagnosis, as it is resource-demanding, invasive and associated with complications [Citation20]. Thus, defining NAFLD on the basis of elevated ALT, which is a noninvasive test performed on one single blood sample, shorten the time to diagnosis and reduces the costs and the risk of complications [Citation21]. A weakness of using elevated ALT to define NAFLD is the diurnal variation of ALT levels. ALT has highest levels in the afternoon, with up to 45% difference compared to the measurements done in the morning [Citation22]. In addition, day-to-day variability may reach 10%–30%. ALT-levels are also affected by meal intake and can both increase and decrease depending of age and sex [Citation22]. To minimize these sources of error, repeated ALT measurement should be done and the presence of steatosis confirmed by ultrasound or other imaging methods, including MRI, which is the most sensitive noninvasive method of detecting steatosis [Citation23]. Moreover, NAFLD can also exist without an elevated ALT value [Citation13] and other, undetected liver disease may be the cause of the elevated ALT value.
Strengths of this study included the population-based study design based on a large unselected general population with a wide age range, which reduced selection bias and improved validity. Nord-Trøndelag County is quite similar to the rest of Norway, with a homogenous and stable population, but lacks a big city [Citation15]. The large sample size and the available assessment of multiple possible risk factors increased the precision and reduced confounding of the estimates. BMI, waist circumference, blood pressure and lipids were objectively measured by standardized procedures, which reduced bias. However, as this was an observational study, residual confounding cannot be ruled out and causality cannot be claimed. Other weaknesses included the risk of misclassification of NAFLD as the study was based on only one blood sample and lacked confirmation of liver steatosis by hepatic ultrasound or liver biopsy. ALT could be normal in a majority of persons with NAFLD and the prevalence reported in the present study might therefore be an underestimation. However, misclassification due to other possible causes of elevated ALT was reduced by excluding individuals with other liver disease diagnoses and that reporting excessive alcohol consumption. However, the degree and duration of ALT elevation depend on several factors, including the specific diagnosis and treatment. In the present study, participants who developed a chronic liver disease, other than NAFLD, within one year before or after ALT measurement were excluded since their ALT value might be secondary to the liver disease. On the other hand, a chronic liver disease does not have to affect the ALT value on a long-term basis. To avoid misclassification with acute liver disease, participants with an acute liver disease were excluded if the ALT measurement was within three months before or after they got the acute liver disease diagnosis. However, this probably did not affect the outcome, since only three participants were excluded due to an acute liver disease diagnosis. To avoid misclassification with alcoholic liver disease, participants with excessive alcohol consumption were excluded. None of the available alcohol variables included grams of alcohol, but were based on the self-reported frequency and units of alcohol consumption, and may thus be misunderstood and typically the consumption is underreported. The HUNT study did not include fasting glucose and the participants were labeled as diabetics if they reported to be previously diagnosed with diabetes or had experienced a hyperglycemic event. This resulted in ∼8.5% of the participants being labeled as diabetics. Compared to the national Norwegian prevalence of diabetes at 4.7%, this might have overestimated the prevalence of diabetes in the HUNT population and misclassified some participants [Citation24]. The definition of hypertension, based on the NCEP ATP III criteria of the metabolic syndrome, uses lower cutoff values than defined by the European Society of Cardiology and the European Society of Hypertension 2018 guidelines. By using the NCEP ATP III criteria, the presence of hypertension might have been overestimated. Alcohol consumption, tobacco smoking, hypertension and diabetes mellitus were self-reported, which also could introduce bias.
In conclusion, NAFLD is a common condition in the general Norwegian population. NAFLD should be suspected in individuals with abdominal obesity, hypertension, diabetes mellitus and dyslipidemias.
| Abbreviations | ||
| ALT | = | alanine aminotransferase |
| BMI | = | body mass index |
| CI | = | confidence interval |
| HDL | = | high density lipoproteins |
| HUNT | = | Trøndelag Health Study |
| ICD | = | International Classification of Diseases |
| NAFLD | = | nonalcoholic fatty liver disease |
| NCEP ATPIII | = | National Cholesterol Education Program Adult Treatment Panel III |
| OR | = | odds ratio |
Acknowledgments
The Trøndelag Health Study (HUNT) is collaboration between HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330–9337.
- Husain N, Blais P, Kramer J, et al. Nonalcoholic fatty liver disease (NAFLD) in the veterans administration population: development and validation of an algorithm for NAFLD using automated data. Aliment Pharmacol Ther. 2014;40(8):949–954.
- Kanwal F, Kramer JR, Duan Z, et al. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301–308.e1-2.
- Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276.
- Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): is it really a serious condition? Hepatology 2012;56(4):1580–1584.
- Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51(5):1820–1832.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285.
- Farrell GC. The liver and the waistline: Fifty years of growth. J Gastroenterol Hepatol. 2009;24 Suppl 3:S105–S118.
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
- Pimpin L, Cortez-Pinto H, Negro F, EASL HEPAHEALTH Steering Committee, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735.
- Zhu J-Z, Dai Y-N, Wang Y-M, et al. Prevalence of nonalcoholic fatty liver disease and economy. Dig Dis Sci. 2015;60(11):3194–3202.
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64.
- Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42(4):968–977.
- Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48.
- Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124(1):71–79.
- Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101(1):76–82.
- Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357.
- Kim WR, Flamm SL, Di Bisceglie AM, Public Policy Committee of the American Association for the Study of Liver Disease, et al. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370.
- Engelmann G. Biomarkers in focus: Alanine aminotransferase. In: Patel VB, Preedy VR, editors. Biomarkers in liver disease. Dordrecht: Springer Netherlands; 2017. p. 267–280.
- Isabela Andronescu C, Roxana Purcarea M, Aurel Babes P. The role of noninvasive tests and liver biopsy in the diagnosis of nonalcoholic fatty liver disease. J Med Life. 2018;11(3):243–246.
- Mørch Stene LC, Gulseth , Hanne L. Diabetes i Norge FHI; [updated 2017 June 2; cited 2020 June 22]. Available from: https://www.fhi.no/nettpub/hin/ikke-smittsomme/diabetes/.