Abstract
Objectives
Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) has been used in the differential diagnosis of benign and malignant tumors by visualization of tumor microcirculation and perfusion. However, its diagnostic role in submucosal tumors (SMTs), especially leiomyomas and gastric submucosal tumors (GISTs) was rarely studied. The aim of this study was to analyze the diagnostic role of CEH-EUS for SMTs (<50 mm) and the value of assessing the malignant potential of GISTs.
Materials and Methods
We retrospectively included patients with tumors <50 mm in diameter who underwent preoperative EUS and CEH-EUS examination and had pathologically confirmed as leiomyomas and GISTs. To analyze the imaging features of CEH-EUS with pathological diagnosis as the gold standard and evaluate its diagnostic value.
Results
This study included 10 cases of leiomyomas and 38 cases of GISTs. Under CEH-EUS detection, 86.9% of GISTs showed hyper-enhancement, 89.5% showed diffuse enhancement, 39.5% showed non-enhancing spots, and 97.4% showed obvious capsule enhancement. In contrast, the leiomyoma cases mostly showed hypo-enhancement (50.0%) or non-enhancement (30.0%) (p < 0.05). Then, the value of CEH-EUS in the differential diagnosis of benign and malignant tumors based on blood flow is significantly higher than that of B-EUS. Signal appearance time was significantly faster in the intermediate-high risk GISTs than in the very low-low risk group (5.1 s versus 15.5 s, p < 0.05), and the AUROC values predicted the risk at this time to be 0.903 (0.763–0.975). Heterogeneous perfusion and non-enhancing spots were also more common in the intermediate-high risk group. Univariate and multivariate analysis revealed that intratumoral irregularitie was an independent predictor of moderate to high risk (OR 3.99, 95%CI 1.04–90.95), with sensitivity, specificity and accuracy of 73.33%, 91.30% and 84.21%, respectively.
Conclusions
Through this study, CEH-EUS has a good differential diagnostic ability for leiomyomas and GISTs, and has a high value in predicting the risk of GISTs.
Introduction
Submucosal tumors (SMTs) of the upper digestive tract are often found incidentally during endoscopy and can be classified as benign or malignant tumor depending on the clinical course. Leiomyoma is the most common benign tumor and gastrointestinal stromal tumor (GIST) is the most common malignant tumor. About 10–30% of GISTs are malignant and can be invasive and metastatic [Citation1]. Therefore, early diagnosis, treatment and follow-up of GISTs are particularly important.
Current guidelines recommend surgical treatment for SMTs with a diameter >50 mm, regardless of a definitive histopathological diagnosis [Citation2]. While for SMTs with a diameter <50 mm, follow-up or surgical resection can be chosen according to the risk of malignancy, location and size of the tumor [Citation4]. The European Society of Gastrointestinal Endoscopy (ESGE) recommends endoscopic ultrasonography (EUS) as the best tool to characterize SMTs features (size, location, originating layer, echogenicity, shape), but EUS alone is not able to distinguish among all types of SMTs. EUS is mainly difficult to diagnose hypoechoic lesions originating from the fourth layer. The reported EUS accuracy is 77–89% for GIST diagnosis, 37.5–82.6% for leiomyoma and 45.5–48.0% for small SMTs [Citation3–5].
With the rapid development of ultrasound-guided puncture technology in recent years, Endoscopic ultrasonography tissue acquisition (EUS-TA) is increasingly used for preoperative evaluation, such as EUS-guided fine-needle aspiration (EUS-FNA) and EUS-guided fine-needle biopsy (EUS-FNB). The superiority of the EUS-FNB over the EUS-FNA has been proven [Citation6]. The diagnostic accuracy of EUS-FNB histology is high (83–100%) [Citation7]. However, EUS-TA not only requires a high level of equipment, but also a high level of skill on the part of the operator, which can otherwise lead to a high false negative rate and complications such as bleeding [Citation8,Citation9]. For this reason we would like to have a non-invasive and simple test with less individual variation for diagnosis.
Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) using second-generation ultrasound contrast agents selectively describes microbubble contrast agents, prompting the evolution of CEH-EUS from vascular imaging to images of the perfused tissue. CEH-EUS has emerged as a novel technique to differentiate benign from malignant disease by visualizing microcirculation and vascular homogeneity, and has gained the attention from researchers [Citation10,Citation11]. This study focuses on the ability of CEH-EUS to differentially diagnose leiomyoma and GISTs less than 50 mm in diameter and the value of assessing the risk of malignancy in GISTs.
Material and methods
Study design and patients
This was a single-center, retrospective study. We retrospectively analyzed the case data of patients who visited our hospital for SMTs from August 2019 to March 2022 and underwent EUS and CHE-EUS examination. The patient enrolment process is shown in . The study had been approved by the Clinical Medical Ethics Committee of the Second Hospital of Anhui Medical University(SL-LC2019019).
B-Mode EUS
All patients underwent preoperative B-mode EUS, which was performed by an experienced endoscopist using longiludinal axis endoscopic ultrasonography (LAEUS)(XGF-UCT-180, Olympus Medical Systems Co. Ltd., Tokyo, Japan)in conjunction with the Aloka ProSound Alpha-10 system (Aloka Co. Ltd., Tokyo, Japan). We recorded lesion location, surface mucosa, diameter, the long-to-short ratio (LSR), layer of origin, margins, echogenicity, intra-tumor specific manifestations and Doppler blood flow signal.
CEH-EUS examination and evaluation
CEH-EUS was performed in all patients after B-mode EUS and exclusion of contraindications (such as pregnancy, lactation, severe heart failure, chronic obstructive pulmonary disease, contrast agent allergy, etc.). Focus was placed below the SMTs, contrast specific extended pure harmonic detection (Ex-PHD) mode was engaged, and the mechanical index (MI) was adjusted to 0.15 ∼ 0.35, 2.4 ml contrast agent (SonoVue) was injected into the elbow vein followed by 10 ml normal saline in a pellet manner, and dynamic collection was performed immediately for 120 s.
According to the contrast time, the arterial phase and the venous phase were defined as the arterial phase from 10 s to 30 s after contrast injection and the venous phase after 30 s. Signal appearance time was defined as the time of initial enhancement in the lesion after contrast medium injection. The contrast pattern was divided into hyper-enhancement, iso-enhancement, hypo-enhancement and no-enhancement using normal tissue as a reference. Vessels with continuous thickening visible in the arterial phase were defined as irregular vessels, and spots without enhancement within them were defined as non-enhancing spots. In the venous phase hyper-enhancement and iso-enhancement were defined as diffuse enhancement. The contrast can be divided into homogeneous and heterogeneous perfusion according to reinforcement characteristics. All imaging videos were interpreted by two experienced endoscopists. In the event of disagreement, the opinion of the third physician was used.
Pathological histological diagnosis and malignant potential of GISTs
All tumors were examined by histopathology and immunohistochemistry after surgery. Both leiomyomas and GISTs were composed of spindle cells. Leiomyomas showed diffuse positivity for Desmin and SMA, but negative for CD34 and CD117. GISTs showed positively for CD117 and CD-34. According to the NIH2008 improved version, the GISTs were graded for malignancy according to the location, size and mitotic index [Citation12].
Statistical analysis
Data were analyzed using IBM SPSS version 26.0 for Windows (IBM Corp, Armonk, NY, USA). Categorical variables were expressed as number and percentage (%); continuous variables and means ± standard deviation. The chi-square test or Fisher’s exact test were used for categorical data and the t-test was used for quantitative data between two groups. Values with p < 0.05 were considered statistically significant. The sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the diagnosis of leiomyomas and GISTs, and paired chi-square analysis and consistency tests were performed. We analyzed he receiver operating characteristic curve with the maximum Jorden index to obtain the best cut-off value for predicting the risk of GISTs. Univariate and multivariate logistic regression models were used to estimate independent predictors of GIST risk.
Results
Clinical characteristics
A total of 48 patients were recruited in this study, including 10 leiomyomas and 38 GISTs. Clinical characteristics of patients were presented in . This study included 25 males and 23 females with an average age of 58.3 years. The average size and LSR of all SMTs were 27.3 mm and 1.3, respectively. There was no significant difference between the two groups (p > 0.05). The most common location of leiomyomas was the esophagus (n = 6, 60%), while GISTs was in the stomach with a statistical difference (p < 0.05). In this study, all GISTs originated in the muscularis propria (n = 38, 100%) and leiomyomas originated in the laminae muscularis mucosae (n = 3, 30%) and muscularis propria (n = 7, 70%), with statistical differences between the two groups (p < 0.05). Both groups presented as hypoechoic tumors with regular margins and intact tumor capsule. Specific manifestations such as calcification and necrosis were common in GISTs, but with no statistical differences from leiomyomas (p > 0.05).
Table 1. Clinical characteristics between leiomyoma and GISTs.
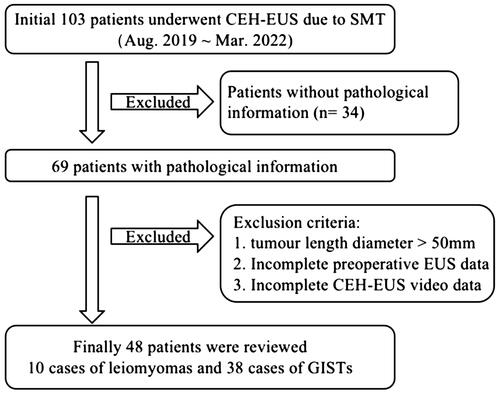
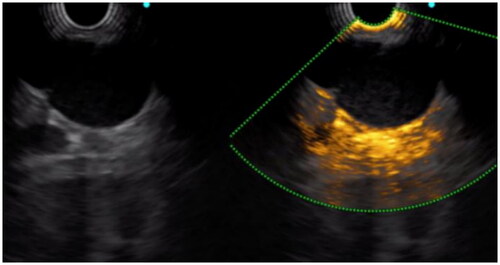
Doppler showed significantly richer blood flow signal in GISTs (60.5%) than in leiomyomas (10%). In the arterial phase of CEH-EUS, 3 of 10 leiomyomas showed no-enhancement, five hypo-enhancement, one iso-enhancement and one hyper-enhancement, while 33 of 38 GISTs showed hyper-enhancement, one iso-enhancement and four hypo-enhancement. In addition, 97.4% (n = 37) and 39.5% (n = 15) of the GISTs showed obvious enhancement of tumor capsule and non-enhancing spots in the tumor, which were significantly more common than leiomyomas (n = 2, 20%) ( and ). In the venous phase, 89.5% (n = 34) of GISTs showed diffuse enhancement, while there were only 20% (n = 2) in leiomyomas. All of the above were statistically significant (p < 0.05).
CEH-EUS differential diagnosis of leiomyomas and GISTs
Using pathological diagnosis as the gold standard, the sensitivity, specificity, accuracy and positive and negative predictive values of applying the blood flow profile to identify leiomyomas and GISTs are shown in . The diagnostic accuracy of CEH-EUS was more than 85%, and the paired Chi-square test and consistency test were significantly higher than Doppler flow, with poor consistency and statistical difference (p < 0.05). Although non-enhancing spots were more common in GISTs, the sensitivity and accuracy of their diagnosis were 39.47% and 52.08%, respectively.
Table 2. Comparison of the diagnostic value of CEH-EUS and consistency tests.
CEH-EUS assessment of the malignant potential of GISTs
In this study, 38 cases of the GISTs were stratified into 6 very low risk, 19 low risk, 10 intermediate risk, and 3 high risk according to NIH2008 edition. The contrast pattern of the GISTs is shown in . In addition, the GISTs are divided into two groups according to the risk of the GISTs. Group 1 is very low and low risk GISTs, and Group 2 is intermediate and high risk GISTs. The basic characteristics of the two groups are shown in . There was statistical difference in KI-67 between the two groups. Under standard B-mode EUS, surface mucosal ulceration (61.5% versus 12%, p < 0.05) and internal necrosis (61.5% versus 20%, p < 0.05) were more common in group 2, while there were no statistical differences in tumor shape and Doppler blood flow signal (p > 0.05). Among the CEH-EUS manifestations, some of the very low and low risk GISTs showed hypo-enhancement and iso-enhancement, but there was no statistical difference between group 1 and group 2 (p > 0.05). However, the enhancement signal in group 2 was significantly faster than that in group 1 (5.1 s versus 15.5 s, p < 0.05). The AUROC values for predicting the risk of this time was 0.903 (0.763–0.975) (), and when the cut-off value was ≤5, the sensitivity and specificity of the diagnosis were 76.9% (46.2–95.0%) and 92.0% (74.0–99.0%), respectively.
Figure 4. Receiver-operating characteristic curve for the Signal appearance time (Area under the curve [AUC], 0.903).
![Figure 4. Receiver-operating characteristic curve for the Signal appearance time (Area under the curve [AUC], 0.903).](/cms/asset/dca62329-7e17-4da7-a0c0-e2a817d54018/igas_a_2144437_f0004_c.jpg)
Table 3. Arterial phase manifestations of GISTs at different risk levels.
Table 4. Characteristics of very low-low risk GISTs and intermediate-high risk GISTs.
In addition, tumors may exhibit specific manifestations under CEH-EUS. Irregular vessels were more common in group 2 (84.6% versus 16%, p < 0.05), and the diameter of vessels was significantly larger than that in group 1 (2.23 mm versus 1.13 mm, p < 0.05). Heterogeneous perfusion (92.3% versus 16%, p < 0.05) and non-enhancement spots (84.6% versus 16%, p < 0.05) were also significantly higher in group 2 than that in group 1 ( and ). A logistic regression model was used to find independent predictors of malignant potential in . In univariate analysis, irregular vessels, heterogeneous perfusion and non-enhancing spots showed statistical significance. In multivariate analysis, irregular vessel (odds ratio [OR]3.99, 95% confidence interval [CI] 1.04–90.95) was a statistically significant independent predictor. The sensitivity, specificity and accuracy of irregular vessels were 73.33%, 91.30% and 84.21%, respectively.
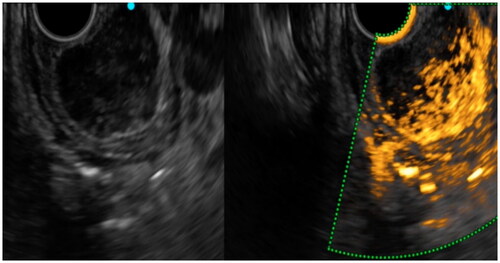
Figure 5. We saw hyper-enhancement within the tumor, but the enhancement is very heterogeneous and non-enhancing spots were visible, which was pathologically confirmed as GIST with a high-risk classification.
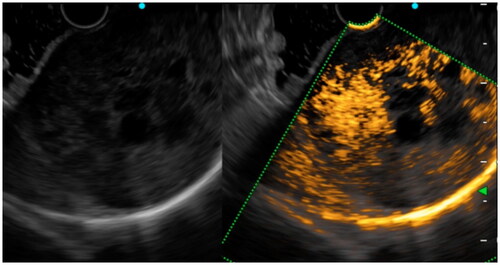
Figure 6. 10 s after contrast injection, we saw a continuous, irregular vessel of approximately 2 mm in diameter in the tumor, which was pathologically confirmed as GIST with an intermediate risk classification.
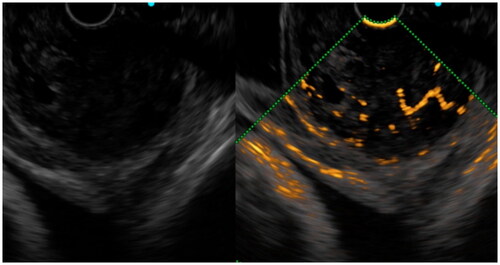
Table 5. Logistic regression model for independent predictors of GISTs.
Discussion
Leiomyomas and GISTs are the most common SMTs of the upper digestive tract, and the diagnosis depends on histopathology and immunohistochemistry. Leiomyomas usually show positive SMA and Desmin, while GISTs show positive CD117, CD34 and DOG1 [Citation13]. Although EUS is still the preferred test for SMTs, previous studies have shown that the diagnostic accuracy of EUS findings varied widely, with particular difficulty in distinguishing hypoechoic lesions from the fourth layer [Citation14]. In this study, leiomyomas and GISTs tended to present as hypoechoic lesions with smooth surface mucosa, regular margins and predominantly intraluminal growth, where location was of some value in the differential diagnosis, but with a sensitivity of 60%. In studies correlating CT to GISTs necrosis can suggest GISTs rather than benign SMTs, whereas several studies have shown no statistically significant difference in calcification between GIST and benign SMTs while the differential diagnosis of necrosis versus calcification is deficient due to EUS imaging features that are less sensitive to CT [Citation6,Citation15]. Due to EUS imaging characteristics, sensitivity to necrosis and calcification was inadequate for CT, and no statistically significant difference was seen between necrosis and calcification in GISTs and benign SMTs.
Many studies have found that the density and the distribution of blood vessels in tumors has important predictive value for the biological behavior of tumors, and is an important method to aid in the diagnosis of benign and malignant properties [Citation16–18]. Generally, Doppler only images large or faster flowing vessels. Recently, more and more studies have been conducted on CEH-EUS. CEH-EUS combines ultrasonic harmonic imaging with microbubble contrast agents. Since the harmonic signals from microbubbles is obviously greater than that from tissues, it not only enables the imaging of microscopic and slow-flowing vessels, but also enables real-time scanning with high temporal resolution [Citation17]. Kannengiesser et al. [Citation19] found that the GISTs showed hyper-enhancement and benign tumors such as leiomyoma showed hypo-enhancement with a diagnostic sensitivity and specificity of 100%. Lefort et al. [Citation20] noted that CEH-EUS improved the diagnosis of leiomyomas compared to EUS alone. Ignee A showed that CE-EUS and CEUS show hyperenhancement and avascular areas in a high percentage of GIST but not in leiomyoma [Citation21]. A mate analysis covering a total of 354 SMTs showed that CEH-EUS had a sensitivity of 0.87 (95% CI 0.82 − 0.91) and specificity of 0.82 (95% CI 0.74–0.89) for differentiating GIST from benign SMTs [Citation22]. In our study, 80% of leiomyomas showed hypo-enhancement or no-enhancement, only one case with diameter > 40 mm presented hyper-enhancement, 86.9% of GISTs showed hyper-enhancement, and non-hyper-enhancement was found to be in the very low-low risk group by risk classification. The sensitivity, specificity and accuracy of this diagnosis were 86.84%, 90% and 87.5%, respectively. However, 1 case (10%) of leiomyomas and 23 cases (60.5%) of GISTs showed abundant blood flow under CDFI. The accuracy of CDFI in determining benign and malignant was only 66.67%. It was significantly higher than that EUS alone. In addition, GISTs tended to have diffuse-enhancement, and the tumor capsule was significantly enhanced. The sensitivity and accuracy of identifying GISTs and leiomyomas by flow characteristics were over 85%, which were higher than those of CDFI (p < 0.05).
The European Society for Medical Oncology recommend surgical resection when a SMT is immunohistologically diagnosed as a GIST, even when smaller than 20 mm [Citation23]. But the biological behavior of the part of the small GISTs located in the stomach is more benign. Some studies show that only 1.9% of patients with very low risk in the small GISTs showed disease progression during the follow-up [Citation24,Citation25]. Evaluating malignancy risk of the GISTs often requires a combination of the size, location and mitotic rate. In addition, tumor rupture is considered. However, to obtain the mitotic rate of a tumor often requires surgical excision [Citation26]. There is not enough evidence in the literature that SMTs require EUS-FNA/FNB to obtain tissue [Citation27]. All patients included in this study who underwent surgery did so partly because EUS or CEH-EUS suggested the possibility of GISTs and partly because patients chose to have surgery on their own. In terms of post-operative pathological findings, it seemed that follow-up was more likely to reduce the harm to some patients than surgery. So pre-operative assessment of the risk in GISTs is essential for the treatment of therapeutic options.
Previous studies had identified irregular borders, ulcers, internal heterogeneity and hyperechoic areas in B-mode EUS as high-risk factors for GISTs [Citation28]. In our study, we found statistical differences in surface mucosal ulceration and the internal necrotic areas, but tumor irregular borders, internal heterogeneity and hyperechoic areas between the two groups. The occurrence of necrosis in the tumor in the GISTs suggests a high-risk possibility. CEH-EUS can visualize the microvascularization of SMTs and improves their characterization. Necrotic tissue often does not have a blood supply and may be non-enhancing spots in CEH-EUS. The sensitivity of non-enhancing spots to predict risk in previous studies was 63.6% [Citation29]. In our study, the sensitivity of non-enhancing spots was 84.61%. Previous studies have shown that heterogeneous perfusion can favor malignant tumors [Citation16]. Yamashita et al. [Citation16] found that the vascular density of the intermediate-high risk GISTs was significantly higher than that of the low risk GISTs, and that histologically verified large vessels lacking elastic fibers (diameter > 500 μm) were only found in the intermediate-high risk GISTs. It had been shown in CEH-EUS that irregular vessels were the major factor in identifying and predicting the risk of GISTs [Citation18,Citation30]. On univariate analysis, heterogeneous perfusion and irregular vessels were risk factors for GIST, and irregular vessels were independent risk factors after multifactorial analysis. The diagnostic sensitivity, specificity and accuracy of irregular vessels were 73.33%, 91.30% and 84.21%, respectively. By combining the characteristics of heterogeneous vascular density distribution (heterogeneous perfusion) and irregular vessels, CEH-EUS can improve the evaluation of malignant potential of GISTs before surgery and provide a more reliable basis for clinicians to select surgical methods or follow-up time.
Previous studies have proved that enhancement degree such as light, moderate and obvious intensities of the GISTs in enhanced CT cannot predict the malignant risk of the GISTs [Citation31]. Hyper-enhancement cannot predict the risk of malignancy in GISTs. However, CEH-EUS has the advantage of real-time dynamic observation compared with enhanced CT. Therefore, in addition to qualitative diagnosis, quantitative diagnosis can also be used. In the study by Iwasa Y et al. [Citation32], the time to peak of malignant lesions was significantly shorter than those of benign lesions. In the study of Lee HS et al. [Citation33], the high-risk malignancy of GIST showed significantly higher values for time to peak, wash-in rate. CEH-EUS with perfusion analysis revealed that GISTs had more blood flow of abnormal angiogenesis within the tumors. In the present study, two investigators recorded the time of appearance of the enhanced signal in the video with unknown video-related tumor pathology findings, and we found that the signal appearance time was significantly shorter in the intermediate-high risk group than in the very low or low risk group, with an AUROC value of 0.903 (0.763–0.975). The enhanced signal appearance time provides a simple method for the quantitative determination of GISTs, but it has a limitation in that the analysis depends on the operator subjectively.
There were some limitations in this study. First, the number of patients included in this study was small, only patients undergoing surgery in our hospital were included, and some patients were not included due to short follow-up time. Second, this study was a single-center retrospective study, and a multi-center prospective study is needed to obtain more accurate data.
In conclusion, our study shows that the CEH-EUS has unique diagnostic advantages. Leiomyomas tend to show hypo-enhancement or no-enhancement, while GISTs tend to show hyper-enhancement. CEH-EUS improves the diagnosis of leiomyomas and GISTs through different enhancement degree. Irregular vessel is an independent predictor of intermediate-high risk GISTs and CEH-EUS may provide a valid, safe and reliable method for preoperative malignancy risk assessment of GISTs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112(3):608–615.
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19(1):3–14.
- He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc. 2016;30(10):4206–4213.
- Kim SY, Shim KN, Lee JH, et al. Comparison of the diagnostic ability of endoscopic ultrasonography and abdominopelvic computed tomography in the diagnosis of gastric subepithelial tumors. Clin Endosc. 2019;52(6):565–573.
- Deprez PH, Moons LMG, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2022;54(4):412–429.
- Chen Z, Yang J, Sun J, et al. Gastric gastrointestinal stromal tumours (2–5 cm): correlation of CT features with malignancy and differential diagnosis. Eur J Radiol. 2020;123:108783.
- Kim GH, Ahn JY, Gong CS, et al. Efficacy of endoscopic Ultrasound-Guided Fine-Needle biopsy in gastric subepithelial tumors located in the cardia. Dig Dis Sci. 2020;65(2):583–590.
- Moon JS. Role of endoscopic ultrasonography in guiding treatment plans for upper gastrointestinal subepithelial tumors. Clin Endosc. 2016;49(3):220–225.
- Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European society of gastrointestinal endoscopy (ESGE) clinical Guideline - Updated January 2017. Endoscopy. 2017;49(7):695–714.
- Alvarez-Sanchez MV, Napoleon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20:15549–15563.
- Tamura T, Kitano M. Contrast enhanced endoscopic ultrasound imaging for gastrointestinal subepithelial tumors. Clin Endosc. 2019;52(4):306–313.
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29(4):281–293.
- Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57(2):259–270.
- Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71(4):722–727.
- Huh CW, Jung DH, Kim JS, et al. CT versus endoscopic ultrasound for differentiating small (2–5 cm) gastrointestinal stromal tumors from leiomyomas. AJR Am J Roentgenol. 2019;213(3):586–591.
- Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound. 2015;43(2):89–97.
- Kitano M, Yamashita Y. New imaging techniques for endoscopic ultrasonography: contrast-Enhanced endoscopic ultrasonography. Gastrointest Endosc Clin N Am. 2017;27(4):569–583.
- Chhoda A, Jain D, Surabhi VR, et al. Contrast enhanced harmonic endoscopic ultrasound: a novel approach for diagnosis and management of gastrointestinal stromal tumors. Clin Endosc. 2018;51(3):215–221.
- Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47(12):1515–1520.
- Lefort C, Gupta V, Lisotti A, et al. Diagnosis of gastric submucosal tumors and estimation of malignant risk of GIST by endoscopic ultrasound. Comparison between B mode and contrast-harmonic mode. Dig Liver Dis. 2021;53(11):1486–1491.
- Ignee A, Jenssen C, Hocke M, et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017;6(1):55–60.
- Yang YT, Shen N, Ao F, et al. Diagnostic value of contrast-enhanced harmonic endoscopic ultrasonography in predicting the malignancy potential of submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2020;34(9):3754–3765.
- Casali PG, Blay JY, Abecassis N, ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: [email protected], et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33.
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81–89.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83.
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274.
- Akahoshi K, Oya M, Koga T, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014;23(4):405–412.
- Benson AB, Venook AP, Al-Hawary MM, et al. Anal carcinoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):852–871.
- Park HY, Jeon SW, Lee HS, et al. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc Ultrasound. 2016;5(6):384–389.
- Cho IR, Park JC, Roh YH, et al. Noninvasive prediction model for diagnosing gastrointestinal stromal tumors using contrast-enhanced harmonic endoscopic ultrasound. Dig Liver Dis. 2019;51(7):985–992.
- Peng G, Huang B, Yang X, et al. Preoperative CT feature of incomplete overlying enhancing mucosa as a high-risk predictor in gastrointestinal stromal tumors of the stomach. Eur Radiol. 2021;31(5):3276–3285.
- Iwasa Y, Iwashita T, Ichikawa H, et al. Efficacy of Contrast-Enhanced harmonic endoscopic ultrasound for pancreatic solid tumors with a combination of qualitative and quantitative analyses: a prospective pilot study. Dig Dis Sci. 2022;67(3):1054–1064.
- Lee HS, Cho CM, Kwon YH, et al. Predicting malignancy risk in gastrointestinal subepithelial tumors with contrast-enhanced harmonic endoscopic ultrasonography using perfusion analysis software. Gut Liver. 2019;13(2):161–168.