Abstract
Background and Aims: Epidemiological studies of non-alcoholic fatty liver disease (NAFLD) frequently use the International Classification of Disease (ICD) codes to identify patients. The validity of such ICD codes in a Swedish setting is unknown. Here, we aimed to validate the administrative code for NAFLD in Sweden.
Methods: In total, 150 patients with an ICD-10 code for NAFLD (K76.0) from the Karolinska University Hospital between 1 January 2015 and 3 November 2021 were randomly selected. Patients were classified as true or false positives for NAFLD by medical chart review and the positive predictive value (PPV) for the ICD-10 code corresponding to NAFLD was calculated.
Results: The PPV of the ICD-10 code for NAFLD was 0.82 (95% confidence interval [CI] = 0.76–0.89). After exclusion of patients with diagnostic coding for other liver diseases or alcohol abuse disorder (n = 14), the PPV was improved to 0.91 (95% CI 0.87–0.96). The PPV was higher in patients with coding for NAFLD in combination with obesity (0.95, 95%CI = 0.87–1.00) or type 2 diabetes (0.96, 95%CI = 0.89–1.00). However, in false-positive cases, a high alcohol consumption was common and such patients had somewhat higher Fibrosis-4 scores than true-positive patients (1.9 vs 1.3, p = 0.16)
Conclusions: The ICD-10 code for NAFLD had a high PPV, that was further improved after exclusion of patients with coding for other liver diseases than NAFLD. This approach should be preferred when performing register-based studies to identify patients with NAFLD in Sweden. Still, residual alcohol-related liver disease might risk confound some findings seen in epidemiological studies which needs to be considered.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, with an estimated global prevalence of 25–38% [Citation1–3]. NAFLD is especially common in patients with overweight or obesity, where more than 70% are affected [Citation4]. The prevalence of NAFLD is predicted to increase further in parallel with increasing prevalence of type 2 diabetes (T2D) and obesity [Citation1,Citation2,Citation5].
In Sweden, the National Patient Register (NPR) is frequently used in epidemiological studies to access information on healthcare data from inpatient or outpatient care. The NPR includes information on International Classification of Disease (ICD) coding which can be used to define different exposures and outcomes. The ICD codes allow for identification of large cohorts on a nationwide scale, and many current epidemiological studies of NAFLD rely on the correctness of information in the NPR [Citation6–8]. The Swedish NPR is of high quality and has previously been established to have a high general positive predictive value (PPV) of 85–95% [Citation9]. The accuracy of the Swedish NPR is similar to that of the Danish NPR [Citation10]. However, the accuracy of other NPR and specific ICD coding remain uncertain as few have attempted to investigate this thus far.
Previous studies have validated the ICD code for NAFLD in other countries than Sweden, or other liver-related codes in the Swedish NPR [Citation11–15], but none have studied the validity of the code for NAFLD in a Swedish setting. One study conducted in Australia investigated the validity of the diagnostic coding for NAFLD, and found a positive predictive value (PPV) of 0.91 [Citation12]. However, the accuracy of diagnostic coding for NAFLD in Sweden is unknown. Validation of specific registers is important since coding practices, and with that the accuracy, might differ between countries.
Here, we aimed to validate the accuracy of the ICD-10 code for NAFLD by calculating its PPV compared to information in medical charts. In addition, we aimed to evaluate whether the accuracy could be improved by using ICD code-based algorithms.
Material and methods
Patients
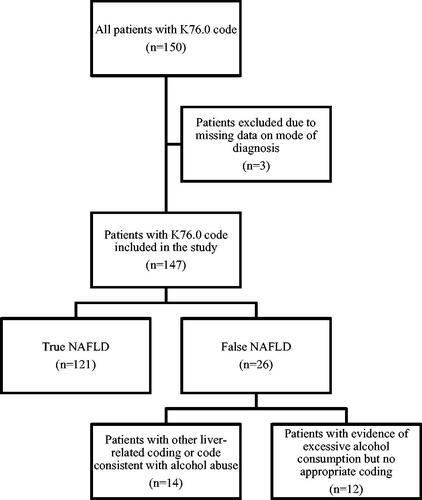
Patients with an ICD-10 code corresponding to NAFLD (K76.0) in inpatient or outpatient specialist care at the Karolinska University Hospital between 1 January 2015 and 3 November 2021 were randomly selected for inclusion. We excluded patients who had received a liver transplant before diagnosis and patients with missing data on mode of diagnosis that left us unable to confirm or dismiss NAFLD upon validation. A flowchart for inclusion and exclusion is provided in .
The validity of the ICD-10 code K76.0 was investigated upon medical chart review by a single physician (HÅ) and the first occurrence of ICD-10 coding for NAFLD was considered as the baseline. Data from the medical charts were used as gold standard. The diagnosis was considered valid if it met the current criteria for NAFLD [Citation16,Citation17], defined as positive evidence upon pathology, radiology, or if the diagnosis was made by a consultant in hepatology.
For patients with a diagnosis based on pathology, the presence of steatosis in >5% of hepatocytes was required [Citation18]. Histological findings indicative of non-alcoholic steatohepatitis (NASH) (i.e. steatosis with lobular inflammation and hepatocellular ballooning) [Citation18] were also accepted, but not required, for the ICD-10 code to be considered valid. The NASH ICD-10 code (K75.8) is used infrequently in Swedish healthcare, and we did not intend to evaluate its specific validity in the current study.
In addition, a diagnosis through different radiological methods (vibration-controlled transient elastography with available controlled attenuation parameter data, ultrasound, computer tomography [CT] or magnetic resonance imaging [MRI]) was accepted. A controlled attenuation parameter value >236 dB/m was accepted as a cut-off for the presence of steatosis [Citation19,Citation20]. For diagnosis through ultrasound, signs of increased echogenicity (where the echogenicity of the liver parenchyma was higher than the kidney and spleen) were required [Citation21]. For a diagnosis through CT, signs of hypoattenuation of the liver or steatosis were required. Lastly, for diagnosis through MRI, we required at least 5% of steatosis [Citation19]. Radiological and histological data were extracted from radiology and pathology reports, and we did not perform an independent review of the images or slides.
We evaluated the presence of coexisting liver diseases to identify patients with ‘pure’ NAFLD. These included 1) alcohol-related liver disease, defined as either a history of overconsumption of alcohol (≥30 g/day in men and ≥20 g/day in women), phosphatidylethanol levels ≥0.3 μmol/L, if a physician documented a high alcohol consumption without note of the quantity, or a diagnostic code for alcohol use disorders (ICD-10: F10.0–F10.7W) or alcohol-related liver disease (ICD-10: K70.0–K70.9); 2) signs of hepatitis B or hepatitis C defined as either presence of hepatitis B surface antigen, hepatitis C RNA or hepatitis C antibodies on testing, or a diagnostic code for viral hepatitis (ICD-10: B18); 3) signs of autoimmune liver disorders (autoimmune hepatitis [K75.4], primary biliary cholangitis [K74.3], or primary sclerosing cholangitis [K83.0A]), requiring that an ICD-10 diagnosis was made by a specialist in hepatology using standard criteria; or 4) an ICD-10 code for any other competing chronic liver disease (hemochromatosis [E83.1] Wilson’s disease [E83.0B], alpha-1-antitrypsin deficiency [E88.0A], porphyria [E80], or other rare liver-related diagnoses). We required that none of these criteria were met before, at, or within six months after the NAFLD diagnosis, to define ‘pure ‘NAFLD.
Parameters
For each patient, data were collected on age, sex, body mass index (BMI), alanine transaminase (ALT), aspartate transaminase (AST), and platelet count at the first date of a registered K76.0 code. For the laboratory parameters, data was considered valid if they were analysed within three months before or after the ICD-10 diagnosis date, and not recorded during emergency care. This data was then used to calculate the Fibrosis-4 score, a validated non-invasive scoring system that assesses the degree of fibrosis with a summary score that is used to exclude advanced fibrosis, for patients where possible [Citation22,Citation23].
We investigated the accuracy of coding for T2D and obesity in patients with a diagnosis of NAFLD. Here, ICD-10 codes for T2D (E11) and obesity (E65-66) were registered if made before or at the date of the NAFLD diagnosis. Obesity was defined as having a BMI ≥30 kg/m2 in patients of at least 18 years of age and the appropriate iso-BMI for patients younger than 18 years of age. T2D was defined by laboratory values (two separate measurements of fasting glucose ≥7.0 mmol/L, two separate measurements for HbA1c ≥48 mmol/mol, an oral glucose tolerance test ≥11.1 mmol/l or a combination of any of the previously mentioned parameters), or a prescription for any antidiabetic medication (oral or injections). Patients with coding for type 1 diabetes (T1D) were excluded from the analysis.
Statistical analysis
We calculated PPVs with corresponding 95% confidence intervals (CI). All patients were either classified as true positives (i.e., having the ICD-10 code for NAFLD and evidence of NAFLD upon chart review) or false positives (i.e., having the ICD-10 code for NAFLD but no evidence of NAFLD upon chart review). We could not calculate other test metrics such as negative predictive values (NPV) since we did not have data on patients with negative test characteristics (i.e., absence of both the ICD-code for NAFLD and no signs of NAFLD on chart review). For obesity and T2D, we further calculated the sensitivity, specificity, PPV and NPV of the respective ICD-10 code, in patients with NAFLD. p < 0.05 (2-sided) was considered statistically significant. Mann-Whitney U-test, Chi-squared test and Student’s t-test was used as appropriate. All statistical analyses were performed in IBM SPSS 29.0, (SPSS Inc., Chicago, IL)
Results
Participants
In total, 150 patients with an ICD-10 code of K76.0 were selected for inclusion. Three patients were excluded from the analysis after having only received the ICD-10 code as part of a telephone consultation, with no data available to determine NAFLD status. A total of 147 patients were therefore included in the final analysis ().
In total, 117 patients (80%) received their first ICD-10 code for NAFLD at a gastroenterology unit. An additional 15 patients (10%) were diagnosed by a paediatrician, and 15 patients (10%) were diagnosed at other departments at the Karolinska University Hospital.
Patient characteristics are shown in . Most patients were male (60%), and the median age was 55 years. In total, 16 patients were younger than 18 years old at the time of receiving the K76.0 code.
Table 1. Patient characteristics stratified on NAFLD status upon medical chart review.
Medical chart review and validation of the ICD-10 code for NAFLD
In total, 121 patients were found to have NAFLD upon medical chart review and were considered ‘true positives’. Of the 26 patients considered ‘false positives’, fourteen patients had ICD-coding for other liver diseases or alcohol abuse disorder: seven with alcohol-related liver disease, one patient with hemochromatosis, one patient with hepatitis C, one with primary sclerosing cholangitis, one with PMM2-congenital disorder of glycolisation and three with alcohol abuse disorder. The remaining patients (n = 12) considered ‘false positives’ all had a history of overconsumption of alcohol on chart review but did not have an ICD-10 based diagnosis of this. These 12 patients had somewhat higher FIB-4 values compared to those with ‘true’ NAFLD, although this was not statistically significant (median 1.9 vs 1.3, p = 0.16).
Most patients were diagnosed upon pathology (n = 47) or radiology (n = 96). Four patients had not undergone any radiology or pathology as part of their diagnosis but were instead diagnosed by a consultant in hepatology. In total, three of these patients were correctly diagnosed and the remaining patient had a history of overconsumption of alcohol.
The PPV for the ICD-10 code K76.0 in the entire cohort was 0.82 (95% CI 0.76–0.89) (), which increased to 0.91 (95% CI 0.87–0.96) after exclusion of patients with coding for other liver diseases or alcohol use disorders. The PPV of the NAFLD code in patients with coding for T2D or obesity was 0.96 (95% CI 0.89–1.00) and 0.95 (95% CI 0.90–1.00), respectively after exclusion for other liver diseases or alcohol use disorders.
Table 2. Positive predictive values for the ICD-10 code K76.0 and different associated subgroups.
For patients who had received their first NAFLD code at a gastroenterology or hepatology department (n = 117), 22 patients were considered false positives. In total, 14 of these patients had received a code for other liver diseases or alcohol abuse prior to or within six months after the first instance of NAFLD coding. After excluding these 14 patients, the PPV for a diagnosis of NAFLD made at a gastroenterology department was 0.92 (95% CI 0.87–0.97) ().
Data on BMI was available for 143 patients (97%) and a total of 69 patients had an ICD-10 code for obesity (E65–66). After exclusion of other liver-and alcohol related coding, 83 patients were found to have a BMI indicative of obesity and 65 patients had an ICD-10 code for obesity (E65–66). Of these, 56 patients were found to be true positive and nine patients were considered false positive for obesity upon medical chart review. A total of 27 patients had BMI indicative of obesity but no corresponding ICD-10 code for this. After the exclusion of other liver- and alcohol-related coding, the sensitivity, specificity, PPV and NPV of the code for obesity were: 0.67 (56/83); 0.82 (37/45); 0.86 (56/65) and 0.58 (37/64), respectively.
Data on T2D status was available for 118 patients (80%) and a total of 47 patients had an ICD-10 code for T2D. After exclusion for other liver- and alcohol-related coding (n = 1) as well as coding for T1D (n = 6), 40 patients had an ICD-10 code for T2D. Of these, 38 patients were true positive and two patients were false positive for T2D upon medical chart review. In addition, two patients without a code for T2D had been described antidiabetic medication and were considered false negatives of T2D upon medical chart review. After exclusion of other liver- and alcohol-related coding, the sensitivity, specificity, PPV and NPV of the code for T2D were: 0.95 (38/40); 0.98 (98/100); 0.95 (38/40) and 0.98 (98/100), respectively.
Discussion
Here, we found that the ICD-10 code for NAFLD had high accuracy. The positive predictive value was further improved after the exclusion of patients with coding for other liver diseases or alcohol-related diseases, which is the current practice in contemporary epidemiological studies based on ICD codes. Thus, the current study supports that this method to define NAFLD is accurate and can be used in epidemiological research. However, some caution should be made since some patients with coding for NAFLD had a high alcohol consumption but no corresponding diagnosis for this, making it difficult to exclude such patients at the time of diagnosis using diagnostic coding in register-based studies. This suggests that some patients with NAFLD in register-based research are misclassified. It is unknown to what extent such a misclassified population drives clinically relevant events, such as progression to cirrhosis. Here, such patients had somewhat higher FIB-4 values, suggestive of a more severe disease. Therefore, further censoring patients with coding for alcohol-related diagnoses made after baseline or using a broader set of codes to identify alcohol abuse before baseline are likely good approaches [Citation24].
It is common to use different algorithms to define exposure or outcomes in epidemiological studies. Such algorithms need to be validated to assure good accuracy [Citation25,Citation26]. Philip et al. previously validated an algorithm using a hierarchical system based on ICD-9 data to define etiologies of liver disease, including NAFLD [Citation26]. In general, Philip et al. found a high PPV for different liver etiologies, but a slightly lower for NAFLD (0.77). In their study, patients with NAFLD were grouped together with patients with cryptogenic liver disease which could explain the difference seen in PPV in the respective studies (Here: 0.91 vs Philip et al.: 0.77). We suggest that the algorithm used to define ‘pure’ NAFLD in the current study is superior when studying NAFLD as motivated by the improved PPV found using this method (0.82 vs 0.91).
Additionally, we investigated the PPV of the code for NAFLD specifically in patients with obesity or T2D. We found that the PPV for NAFLD was high in both subgroups (0.95 and 0.96, respectively) after exclusion of patients with coding for other liver diseases or alcohol-related diagnoses. It has previously been established that patients with T2D and obesity frequently have NAFLD [Citation5,Citation27] and here we found that the code for NAFLD is highly accurate in such patients. These subgroups were however small which should be considered when interpreting the data since using this approach would result in a reduced sensitivity for the NAFLD diagnosis.
The current study additionally assessed the validity of the ICD-10 codes for obesity and T2D in patients with coding for NAFLD. Both the obesity and T2D coding performed better in the current cohort compared to previous validations in non-NAFLD populations [Citation12,Citation28–31]. The T2D coding presented with high sensitivity and PPV for T2D, whereas the obesity coding was slightly less accurate with a PPV of 0.86, and a NPV of 0.58. T2D and obesity are frequently associated with NAFLD, and the current study supports that coding for these conditions in a NAFLD population is highly accurate. However, the current study found that obesity remains undercoded for and we encourage medical professionals to use these codes when appropriate to ensure their future applicability in research.
Our results are in accordance with a previous validation of NAFLD and NASH ICD-10 codes in Australia by Hayward et al. [Citation12]. The setting was similar to ours, as they identified patients primarily diagnosed in a tertiary care centre. Hayward et al. identified a total of 57 patients with either a NAFLD or NASH code and found a PPV of 0.91 [Citation12]. However, most of these patients had a diagnostic code for NASH, which could affect the comparability with our study as the diagnosis of NASH requires a liver biopsy and thus more thorough differential diagnosis.
Bengtsson et al. previously validated liver-specific ICD-10 coding in a Swedish cohort based on data supplied from 72 different healthcare providers. In this study, the PPV was in general above 0.90 in patients with ICD-10 codes for alcohol-related cirrhosis (K70.3), cirrhosis without specified aetiology (K74.6), oesophageal varices (I85.0, I85.9) and hepatocellular carcinoma (C22.0) [Citation13], which is in agreement with other validation studies of these codes [Citation11]. Together, these studies suggest that most liver-specific coding have high accuracy. Our study supports the high quality of Swedish national registers and coding practices. However, further research is warranted to elucidate to what extent the small proportion of misclassified patients with higher alcohol consumption, but with no formal diagnosis of this, drives clinical events.
Strengths and limitations
This is to the best of our knowledge the largest study of the PPV of the ICD-10 K76.0 code to date, including more than twice the number of patients than previous work [Citation12]. Few studies have investigated the validity of the administrative coding for NAFLD on its own. The current study included calculations of PPV for both the ICD-10 code K76.0 on its own and after exclusions of competing liver diseases, which is a major strength as it allowed us to compare the two methods in the same patient population. Additionally, the patients included in this study were randomly selected.
The most important limitation to consider when interpreting the results was that the cohort in the current study was identified from patients that received an ICD-10 code for NAFLD at the Karolinska University Hospital. It is possible that the general coding accuracy is better at a university hospital and that the results in the current study overestimate the PPV of the K76.0 code in unselected populations. Most patients received the ICD-10 code for NAFLD at a gastroenterology unit (80%) which could further affect the PPV. In the current study, we found better coding accuracy in the patient group that had received their NAFLD coding at a gastroenterology unit compared to other hospital departments. However, the patients in the ‘other hospital department’ group were few, which should be considered when interpreting this difference.
We were unable to perform a repeated reading of radiological and histological data in the current study as it was beyond the scope to validate separate diagnostic techniques. Many radiological imaging techniques are user-dependent, and we are unable to exclude some degree of misclassification in the current study. However, all patients included in the current study were diagnosed at a university hospital with experts in gastroradiology and gastropathology so the accuracy of diagnosis is expected to be high. This might differ between different sites with varying expertise, but the sensitivity and specificity of radiological imaging of NAFLD have previously been described as high in patients with moderate to severe steatosis and slightly less in mild steatosis [Citation32].
Furthermore, most patients were diagnosed using radiology or histology, but a few patients (n = 4) were diagnosed by a consultant in hepatology based on other clinical information. While we cannot guarantee uniformity in the diagnostic process among all hepatologists, it is unlikely that misclassification of these patients would have affected the results given the fact that they were few.
Medical chart review was only performed by a single physician (HÅ) which is a limitation. However, all unclear cases were discussed with a senior consultant in hepatology (HH) to ensure high quality of the validation process.
Using administrative coding in the Swedish NPR in NAFLD epidemiological studies allow us to study disease on a nationwide scale. However, NAFLD has previously been found to be undercoded for [Citation33], meaning that many patients with NAFLD are not identified using the ICD-10 codes. It is possible that patients with an ICD-10 code for NAFLD could represent a population that are at higher risk of liver-related events which is important to consider when interpreting epidemiological data based on ICD-10 coding. We were unable to investigate the prevalence of NAFLD in patients without an ICD-10 coding (i.e., false negatives) as this was outside the scope of the current study. Further investigation on different methods to identify patients with NAFLD is warranted to improve and optimise cohort design in NAFLD epidemiology.
Conclusion
The ICD-10 code for NAFLD has a high PPV that was further improved after the exclusion of patients with coding for other liver diseases and alcohol use disorder and we suggest this as the preferred practice when conducting register-based research of NAFLD in Sweden. However, some patients with high alcohol consumption remained incorrectly diagnosed with NAFLD and further studies are warranted to elucidate to what extent these patients affect the results in epidemiological studies.
Author’s contributions
Study conception and design: All; Acquisition of data: HÅ; Statistical analysis: HÅ; Analysis and interpretation of data: All; Drafting of the manuscript: HÅ; Critical revision: All; Guarantor of the article: HH; All authors approved the final version of the article, including the authorship list. Writing assistance: None.
Ethical approval
This study was approved by the Regional Ethics Committee in Stockholm (dnr 2016/177231/2 and 2018/450-32). The committee determined that informed consent was not required.
| Abbreviations | ||
| BMI | = | Body mass index |
| CT | = | Computer tomography |
| ICD | = | International classification of disease |
| MRI | = | Magnetic resonance imaging |
| NPR | = | National patient register |
| NPV | = | Negative predictive value |
| NAFLD | = | Non-alcoholic fatty liver disease |
| NASH | = | Non-alcoholic steatohepatitis |
| PPV | = | Positive predictive value |
| T1D | = | Type 1 diabetes |
| T2D | = | Type 2 diabetes |
Acknowledgements
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes: HEPATOLOGY, vol. 64, no. X 2016. Hepatology. 2016; 64(1):73–84.
- Younossi ZM. Non-alcoholic fatty liver disease – A global public health perspective. J Hepatol. 2019;70(3):531–544.
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022; 7(9):851–861.
- Quek J, Chan KE, Wong ZY, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023; 8(1):20–30.
- Li L, Liu DW, Yan HY, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies: obesity and non-alcoholic fatty liver disease. Obes Rev. 2016; 17(6):510–519.
- Bengtsson B, Widman L, Wahlin S, et al. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: a swedish nationwide population-based cohort study. United European Gastroenterol J. 2022; 10(5):465–476.
- Shang Y, Hagström H. Statins are underused in women with NAFLD After cardiovascular events compared with matched control subjects. Clin Gastroenterol Hepatol. 2022; S1542–3565(22):00295–00296.
- Shang Y, Nasr P, Widman L, et al. Risk of cardiovascular disease and loss in life expectancy in NAFLD. Hepatology. 2022; 76(5):1495–1505.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011; 11(1):450.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. CLEP. 2015;7:449–490.
- Hayward KL, Johnson AL, Mckillen BJ, et al. ICD-10-AM codes for cirrhosis and related complications: key performance considerations for population and healthcare studies. BMJ Open Gastroenterol. 2020; 7(1):e000485.
- Hayward KL, Johnson AL, Horsfall LU, et al. Detecting non-alcoholic fatty liver disease and risk factors in health databases: accuracy and limitations of the ICD-10-AM. BMJ Open Gastroenterol. 2021; 8(1):e000572.
- Bengtsson B, Askling J, Ludvigsson JF, et al. Validity of administrative codes associated with cirrhosis in Sweden. Scand J Gastroenterol. 2020; 55(10):1205–1210.
- Corey KE, Kartoun U, Zheng H, et al. Development and validation of an algorithm to identify nonalcoholic fatty liver disease in the electronic medical record. Dig Dis Sci. 2016;61(3):913–919.
- Forns J, Cainzos‐Achirica M, Hellfritzsch M, et al. Validity of ICD‐9 and ICD‐10 codes used to identify acute liver injury: a study in three european data sources. Pharmacoepidemiol Drug Saf. 2019; 28(7):965–975.
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004.
- Ando Y, Jou JH. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis. 2021;17(1):23–28.
- Kleiner DE, Brunt EM, Van Natta M, , et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41(6):1313–1321.
- Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637.e7.
- Sirli R, Sporea I. Controlled attenuation parameter for quantification of steatosis: which cut-offs to use?. Can J Gastroenterol Hepatol 2021;:1–7.
- Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–1653.
- Sterling RK, Lissen E, Clumeck N,et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317–1325.
- McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010; 59(9):1265–1269.
- Hagström H, Adams LA, Allen AM, et al. Administrative coding in electronic health care record‐based research of NAFLD: an expert panel consensus statement. Hepatology. 2021;74(1):474–482.
- Danford CJ, Lee JY, Strohbehn IA, et al. Development of an algorithm to identify cases of nonalcoholic steatohepatitis cirrhosis in the electronic health record. Dig Dis Sci. 2021;66(5):1452–1460.
- Philip G, Djerboua M, Carlone D, et al. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLOS One. 2020; 15(2):e0229218.
- Zhou Q, Wang Y, Wang J, et al. Prevalence and risk factor analysis for the nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Medicine. 2021; 100(10):e24940.
- Ammann EM, Kalsekar I, Yoo A, et al. Assessment of obesity prevalence and validity of obesity diagnoses coded in claims data for selected surgical populations: a retrospective, observational study. Medicine. 2019; 98(29):e16438.
- Martin BJ, Chen G, Graham M, et al. Coding of obesity in administrative hospital discharge abstract data: accuracy and impact for future research studies. BMC Health Serv Res. 2014; 14:70.
- Khokhar B, Jette N, Metcalfe A, et al. Systematic review of validated case definitions for diabetes in ICD-9-coded and ICD-10-coded data in adult populations. BMJ Open. 2016; 6(8):e009952.
- Nedkoff L, Knuiman M, Hung J, et al. Concordance between administrative health data and medical records for diabetes status in coronary heart disease patients: a retrospective linked data study. BMC Med Res Methodol. 2013; 13(1):121.
- Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol. 2017;23(4):290–301.
- Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018; 16(1):130.