Abstract
Objectives
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death. This study investigated the risk factors, treatment responses and survival outcomes in real-world patients with HCC.
Materials and Methods
This was a large, retrospective cohort study of patients newly diagnosed with HCC at tertiary referral centers in Thailand between 2011 and 2020. Survival time was defined as the time from the date of HCC diagnosis to the date of death or last follow-up.
Results
A total of 1145 patients with a mean age of 61.4 ± 11.7 years were included. Next, 568 (48.7%), 401 (34.4%) and 167 (15.1%) patients were classified as Child-Pugh score A, B and C, respectively. Over half of the patients (59.0%) were diagnosed with noncurative-stage HCC (BCLC B-D). Patients with Child-Pugh A scores were more likely to be diagnosed with curative-stage HCC (BCLC 0-A) than noncurative stage (67.4% vs. 37.2%, p < .001). Patients with curative-stage HCC and Child-Pugh A cirrhosis underwent more liver resections than radiofrequency ablation (RFA) (91.8% vs. 69.7%, p < .001). For BCLC 0-A patients with portal hypertension, RFA was selected more frequently than liver resection (52.1% vs. 28.6%, p < .001). Patients who received RFA monotherapy tended to experience increased median survival times compared to those who underwent resection (55 vs. 36 months; p = .058).
Conclusions
Surveillance programs should be encouraged to detect early-stage HCC, which is suitable for curative treatment improving survival outcomes. RFA may be an appropriate first-line treatment for curative-stage HCC. Sequential multi-modality treatment in the curative stage can achieve favorable 5-year survival.
Introduction
Hepatocellular carcinoma (HCC) is the fourth highest cause of cancer-related mortality worldwide, especially in the Asia–Pacific region [Citation1–3]. In Thailand, HCC is the second leading cause of cancer-related mortality after lung cancer. Males have a significantly higher incidence and progression rate of HCC than females. Cirrhosis is also a significant risk factor for HCC development. Surveillance programs for HCC target high-risk patients with chronic liver disease to detect HCC early and to administer curative therapies. In contrast, previous studies have demonstrated that HCC can be discovered at various stages, even when surveillance measures are used [Citation4]. The Barcelona Clinic Liver Cancer (BCLC) staging system is used to guide therapeutic treatment of patients with HCC [Citation5–7]; however, several factors affect therapy allocation [Citation8,Citation9]. Our 10-year study included patients with HCC from two tertiary referral centers and examined their presenting symptoms, tumor markers, disease stages and treatment methods. Our findings may aid in the selection of suitable therapeutic strategies for patients with HCC.
Materials and methods
Patients and data collection
This retrospective, cohort analysis included patients diagnosed with HCC for the first time at gastrointestinal and liver clinics in tertiary referral hospitals in Thailand between 2011 and 2020. Our HCC surveillance plan comprised measurement of serum alpha-fetoprotein (AFP) and ultrasonography every 3–6 months in older patients with chronic hepatitis B, liver disease with advanced fibrotic stage or cirrhosis. Computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) was performed for high-risk patients with nodules of ≥1 cm. HCC was diagnosed by (i) histological analysis, (ii) typical radiological characteristics of multiphasic CT or dynamic contrast-enhanced MRI in cirrhotic patients, or (iii) typical radiological CT or MRI characteristics with serum AFP concentrations ≥200 IU/L in noncirrhotic patients. International Classification of Diseases-9 registration was used to retrieve information from the medical database. Demographic information, clinical presentation, laboratory values, tumor markers, tumor features (including portal vein invasion or metastasis) and treatment options. HCC was categorized according to the BCLC staging criteria. The Child-Pugh score was used to assess the severity of liver cirrhosis. Most of our selection process was in accordance with the BCLC disease staging guidelines. According to the donor transplantation limitations for very early-stage HCC in our country, most very early-stage HCCs are eligible for radiofrequency ablation (RFA) using percutaneous, laparoscopic or open surgery. Small HCC tumors (single or up to three nodules where each nodule ≤3 cm) with preserved liver function were eligible for RFA or liver resection (LR) depending on the tumor’s position and patient’s comorbidities. Difficult locations, near the hepatic vessels or adjacent extrahepatic organs, were unsuitable for RFA. Patients with a high-risk of comorbid diseases and moderate performance status were less likely to undergo LR. Patients with a solitary tumor of less than 5 cm with maintained liver function and minimal or no portal hypertension were favored for surgical removal with a wedge resection technique. RFA was preferred for Child-Pugh class B patients with tumors less than 3 cm. Conventional transarterial chemoembolization (TACE) was performed for multifocal HCC cases that did not meet the Milan criteria. Under local anesthetic, the femoral artery was catheterized for the TACE. Subsequently, an angiographic examination was conducted to identify all tumor-supplying arteries. In the feeding arteries, an emulsion of lipiodol and a chemotherapeutic agent were delivered. Finally, gelatin sponge particles were injected through the tumor-feeding branch. Survival time was defined as the interval from the date of HCC diagnosis to the date of death or last follow-up.
Statistical analysis
Categorical statistics are expressed as frequencies and percentages. Means and standard deviations are presented for continuous data. Group comparisons were conducted using the chi-square test or Fisher’s exact test for categorical data and the independent t-test for continuous data. The Kaplan–Meier method was used for survival analysis, and the log-rank test was used to evaluate the survival rates of patients receiving various treatment regimens. A Cox proportional hazard model was used to examine prognostic factors and hazard ratios (HRs). Statistical significance was set at p value <.05. The STATA 14.1 software was used for all statistical calculations. The study was performed in accordance with Good Practice Guidelines and the Declaration of Helsinki. This study was approved by the local ethics committee. Patient information was reviewed with the utmost discretion.
Results
This study included 1145 HCC patients with a mean age of 61.4 ± 11.7 years, and 576 (50.7%) were male. Most patients (98.8%) had cirrhosis, categorized as Child-Pugh A (48.7%), Child-Pugh B (34.4%) or Child-Pugh C (15.1%), with a mean Model For End-Stage Liver Disease (MELD) score of 12.68 ± 8.78. Cirrhosis was caused by chronic hepatitis B virus infection (33%), nonalcoholic fatty liver disease (NAFLD) (27.1%), chronic hepatitis C virus infection (HCV) infection (25.8%) and alcohol consumption (17.3%). Of these patients, 461 (39.6%) were asymptomatic. The most prevalent symptoms in patients were stomach pain (32.6%), weight loss (16.8%) and jaundice (16.8%). One-quarter of the patients with HCC reported hepatomegaly, while only 6.2% presented with HCC rupture. Unfortunately, nearly half of the patients (49.5%) did not have measurable AFP. displays the baseline characteristics of all patients and their HCC stage classifications.
Table 1. Baseline characteristics of all patients and by HCC stage.
A total of 470 (41%) patients were classified as having curative-stage BCLC, comprising BCLC-0 (n = 244; 21.31%) and BCLC-A (n = 226; 19.73%). Patients with BCLC-B (n = 417; 36.41%), BCLC-C (n = 102; 8.9%) or BCLC-D (n = 156; 13.62%) were categorized as noncurative. depicts the HCC staging of the patients using the BCLC criteria. The proportion of patients with NAFLD and curative stage HCC was significantly higher than that of patients with noncurative-stage HCC (p = .014). Child-Pugh A patients were more often diagnosed with curative-stage HCC (67.4%) compared with 37.2% with noncurative-stage HCC (p = .001). Using serum AFP levels, less than 20% of the patients were correctly diagnosed with very early or early-stage HCC. Significantly more alcoholic cirrhotic patients were diagnosed with noncurative HCC than with curative HCC (19.7% vs. 13.8%; p = .01), and 10.2% of noncurative HCC patients presented with ruptured HCC compared with 0.4% of curative HCC patients (p = .001). The features of patients with HCC in remission are summarized in by treatment type. A total of 395 patients with very early or early-stage HCC underwent either RFA (47.6%), LR (24.8%), or TACE (27.8%). None of the patients underwent liver transplantation. RFA was performed on a greater number of patients with cirrhosis Child-Pugh B and tumor diameters of 2 cm or smaller than LR (29.3% vs. 7.1%, p = .001 and 63.6% vs. 31.5%, p = .001 respectively). Most RFA techniques were percutaneous, with safe and rapid postoperative recovery. Patients with curative-stage HCC and Child-Pugh A cirrhosis underwent LR, as opposed to RFA (91.8% vs. 69.0%, p = .001). LR included both laparoscopic and open surgical techniques, and one to three segments of wedge resection. After the post-LR procedure, because most patients were within Child-Pugh class A, most patients had no deterioration in liver function.
Figure 1. Hepatocellular carcinoma staging by BCLC criteria. BCLC: Barcelona Clinic Liver Cancer; HCC: hepatocellular carcinoma.
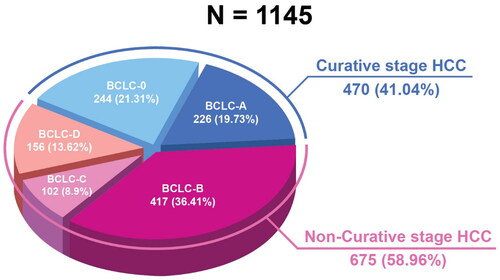
Table 2. Characteristics of curative-stage HCC by treatment modalities.
The presence of portal hypertension is an additional crucial criterion for assessing therapeutic options. Patients in the curative-stage of HCC with signs of portal hypertension were more likely to undergo RFA than LR (52.1% vs. 28.0%, p = .001). Copayment was requested for the RFA needles according to the hospital protocol. Cost had a significant impact on the treatment decisions for individual patients. Consequently, 27.6% of patients with very early and early-stage HCC received TACE as initial therapy. After receiving curative treatment, CT scans were administered every three months for the first year and every four to six months thereafter. As shown in , 71 patients who previously underwent RFA had recurrent HCC and subsequently received alternative treatment modalities. The majority of RFA patients received one to two bouts of therapy, whereas TACE patients received an average of two sequential cycles of chemoembolization.
Table 3. Survival rates and median survival time of curative-stage HCC patients according to treatment modalities.
demonstrates that people with curative-stage HCC (BCLC stages 0 and A) who received treatment had a considerably longer median survival time than patients with BCLC stages B, C and D (40 months [95% confidence interval (CI): 33.20–46.80] vs. 6 months [95%CI: 5.50–6.99]). As shown in and , patients who received RFA monotherapy tended to have a longer median survival time than those who underwent LR (55 months [95%CI: 45.35–64.65] vs. 36 months [95%CI: 23.89–48.11], p = .058). Both the sequential RFA with LR and sequential RFA with LR followed by TACE groups had good three-year survival rates. With sequential therapy, most patients had good follow-up adherence, with a single intrahepatic recurrence one year after the first RFA treatment. The recurrent lesion was both de novo and adjacent to the primary tumor. For HCCs smaller than 3 cm, patients treated with TACE but not LR had significantly higher mortality (HR = 1.98, 95%CI: 1.4–2.81, p = .001) compared to those treated with RFA ().
Figure 2. Survival analysis of curative-stage HCC (BCLC 0-A) compared with noncurative-stage HCC (BCLC B-D). BCLC: Barcelona Clinic Liver Cancer; HCC: hepatocellular carcinoma.
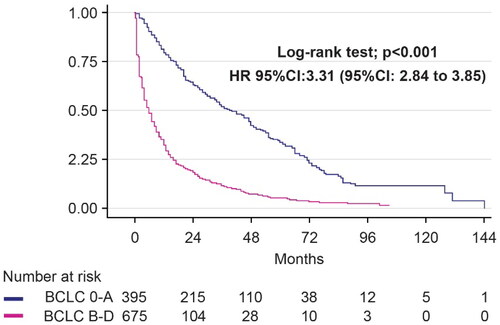
Figure 3. Survival analysis of curative-stage HCC by monotherapy with RFA and resection. HCC: hepatocellular carcinoma; RFA: radiofrequency ablation.
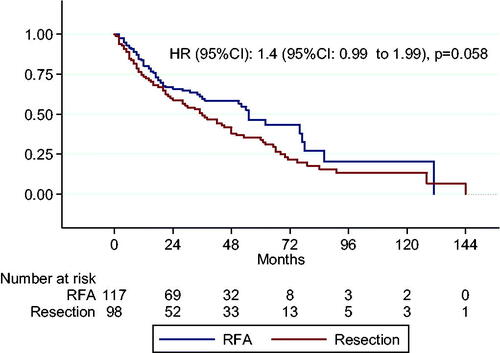
Table 4. Real-world treatment of HCC which tumor size <3 cm.
Discussion
This was a large, retrospective, multicenter study that included patients with HCC treated at Thailand’s tertiary referral facilities over the past decade. Our study demonstrated a higher frequency of HCC in patients with NAFLD-related cirrhosis than prior studies in the Asia–Pacific region [Citation10,Citation11]. Due to underdiagnosis and inadequate surveillance for HCC progression, more than half of NAFLD-related HCCs with or without cirrhosis were detected at an advanced stage in prior studies [Citation12,Citation13]. Nearly half of the cirrhotic patients with NAFLD in this study were diagnosed with HCC BCLC stage 0-A, indicating early diagnosis of NAFLD and good adherence to HCC surveillance, particularly in patients with metabolic syndrome and advanced liver fibrosis.
Our study demonstrated that AFP levels alone could detect less than one-fifth of early-stage HCCs in patients. The addition of a dynamic increase in AFP over time or the establishment of AFP-adjusted algorithms based on specific etiology and other biochemical tests can improve identification of early-stage HCC [Citation4]. The current meta-analysis indicated that surveillance for HCC using serum AFP and transabdominal hepatic ultrasonography boosted the early HCC detection sensitivity from 45 to 63 percent [Citation14]. Our HCC surveillance approach could detect 40% of early-stage HCC, an increase over the previous study’s detection rate of 25% [Citation15]. In addition, more than half of all patients with HCC were diagnosed with Child-Pugh class A cirrhosis. Thus, the patients were eligible for appropriate curative care. As indicated by Taiwan’s and Japan’s high HCC diagnosis rates, surveillance programs for at-risk Thai patients should be encouraged to identify the disease at an earlier stage [Citation16].
The limitation of living donor transplantation was one reason why none of our patients received transplantation, which resulted in RFA treatment being chosen about twice as often as LR for HCC patients with BCLC stages 0 and A and favorable RFA survival. However, approximately one-third of our patients with HCC with Child-Pugh score B disease and more than half of those with portal hypertension underwent RFA. In addition, for tumors ≥3 cm, LR was performed more often than RFA and the larger tumor diameter was associated with reduced survival. Recent randomized clinical studies and meta-analyses reported that RFA appears to be superior to LR with fewer complications, shorter hospital stays and greater in-hospital cost-effectiveness [Citation17–20]. In the real-world, RFA is preferable to LR for patients with HCC with moderate liver function, irrespective of portal hypertension. In patients with Child-Pugh A and B HCC, RFA was regarded as the primary therapeutic modality for HCCs smaller than 2 cm and an alternative treatment option for HCCs larger than 3 cm, providing survival outcomes equivalent to those of LR [Citation21].
Compared with RFA, individuals who underwent LR had a significantly shorter median survival time, which was shorter than that reported in the global data. Portal hypertension or high preoperative MELD scores may explain the reduced median survival time in surgically treated individuals. In patients with cirrhosis and HCC, significant portal hypertension is one of the most important predictors of catastrophic outcomes after extensive LR. Approximately 30% of our LR-eligible patients had portal hypertension, which was characterized by a platelet count < 100,000/mm3. Utilizing of MELD score to stratify the severity of end-stage liver disease could assist in determining whether resectable HCC should be treated with liver transplantation or resection. With an MELD score of 10, resectable HCC should be treated with liver transplantation [Citation22]. Intriguingly, the MELD score of our patients with HCC who underwent LR was 11.4. In addition, individuals who underwent LR had considerably higher AFP levels (> 400 ng/mL) than those who underwent RFA. A previous study [Citation23] found that high AFP levels were associated with poor survival prognosis.
Although HCC was at a curable stage, TACE was acceptable for unfavorable tumor sites, such as dome, subcapsular and approximated vessels or bile duct lesions. TACE is an alternate therapy option for early-stage HCC patients with a solitary nodule who are ineligible for resection or RFA, with a positive three-year survival rate ranging from 64.8% to 80% [Citation24–26]. TACE was administered to almost one-quarter of our HCC BCLC stage 0-A patients, mainly for patients with unfavorable tumor positions, and only few patients underwent TACE because their insurance would not cover RFA treatment. Unfortunately, post-TACE patients with stage 0-A BCLC disease had worse three-year survival outcomes than post-RFA patients. Regression analysis indicates that individuals with HCC and tumors sized less than 3 cm who underwent TACE may have a 1.98-fold higher mortality rate than those who underwent RFA. The median survival period for patients with stage 0-A BCLC was less than 4 years. Owing to the influence of the 10-year enrollment period on the evolution of RFA or resection techniques, the five-year survival rate for most treatment modalities was less than 50%. Some patients did not strictly follow the BCLC therapy guidelines, and approximately 16% of them decided against receiving a particular treatment recommended by the BCLC guidelines.
We demonstrated a trend in favor of RFA over LR for patients with BCLC 0-A HCC. In patients who were eligible for RFA and had strong follow-up adherence, recurrent tumors treated with sequential resection followed by TACE resulted in excellent survival for patients with BCLC 0-A HCC. Although a minority of patients in our study underwent sequential therapy at the curative stage in a real-world setting, our data yielded a remarkable outcome.
The limitations of this study are as follows. First, due to retrospective real-world practice, we cannot perform propensity matching for tumor size, BCLC staging and liver status between RFA and LR, so we cannot conclude that RFA is superior to LR. Second, of the 102 patients with BCLC-C, approximately one-third were classified as Child-Pugh A, indicating that they should receive systemic therapy according to the guidelines. Most patients were unable to receive systemic chemotherapy owing to constraints in the present national health care policy. During the study period, a minority of advanced HCC patients with Child-Pugh A were eligible for systemic therapy, whereas nearly all of them received targeted therapy. We only had a small number of patients treated with immune checkpoint inhibitors. This is one reason why we could not draw any conclusions on the efficacy of targeted or immune checkpoint inhibitor treatment for advanced HCC. In conclusion, good adherence to HCC surveillance programs can help detect HCC at an early stage, which is amenable to curative treatment and improves survival rates. RFA should be encouraged for very early and early-stage HCC with compensated cirrhosis and portal hypertension. In clinical practice, sequential multimodality treatment during the curative phase can result in an improved five-year survival rate.
| Abbreviations | ||
| HCC | = | hepatocellular carcinoma |
| BCLC | = | Barcelona Clinic Liver Cancer |
| AFP | = | alfa-fetoprotein |
| CT | = | computed tomography |
| MRI | = | magnetic resonance imaging |
| HBV | = | hepatitis B virus; |
| NAFLD | = | nonalcoholic fatty liver disease |
| HCV | = | hepatitis C virus |
| RFA | = | radiofrequency ablation |
| TACE | = | transarterial chemoembolization. |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1–61.
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1.
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
- Grgurevic I, Bozin T, Mikus M, et al. Hepatocellular carcinoma in non-alcoholic fatty liver disease: from epidemiology to diagnostic approach. Cancers (Basel). 2021;13(22):5844.
- Wong SW, Ting YW, Chan WK. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma and its implications. JGH Open. 2018;2(5):235–241.
- Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63(3):827–838.
- Anastasopoulos NT, Lianos GD, Tatsi V, et al. Clinical heterogeneity in patients with non-alcoholic fatty liver disease-associated hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2020;14(11):1025–1033.
- Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1.
- Chonprasertsuk S, Vilaichone RK. Epidemiology and treatment of hepatocellular carcinoma in Thailand. Jpn J Clin Oncol. 2017;47(4):294–297.
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166.
- Wang Q, Tang M, Zhang S. Comparison of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the milan criteria: a meta-analysis. ANZ J Surg. 2021;91(7-8):E432–e438.
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104(13):1775–1784.
- Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol. 2019;25(4):354–359.
- Jia Z, Zhang H, Li N. Evaluation of clinical outcomes of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the milan criteria: a systematic review and meta-analysis of recent randomized controlled trials. J Gastroenterol Hepatol. 2021;36(7):1769–1777.
- Shiina S, Sato K, Tateishi R, et al. Percutaneous ablation for hepatocellular carcinoma: comparison of various ablation techniques and surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147.
- Vitale A, Huo TL, Cucchetti A, et al. Survival benefit of liver transplantation versus resection for hepatocellular carcinoma: impact of MELD score. Ann Surg Oncol. 2015;22(6):1901–1907.
- Chan MY, She WH, Dai WC, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy– an analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52.
- Bargellini I, Sacco R, Bozzi E, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81(6):1173–1178.
- Baek MY, Yoo JJ, Jeong SW, et al. Clinical outcomes of patients with a single hepatocellular carcinoma less than 5 cm treated with transarterial chemoembolization. Korean J Intern Med. 2019;34(6):1223–1232.
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36.
