Abstract
Background and Aim
Early diagnosis of splanchnic vein thrombosis (SVT) after severe acute pancreatitis (SAP) remains difficult because of its insidious onset. Common serum markers for thrombosis such as D-dimer (D-D) have lost their diagnostic value due to their elevation in non-thrombotic patients with SAP. The aim of this study is to predict SVT after SAP using common serum indicators of thrombosis by establishing a new cut-off value.
Methods
177 SAP patients were included in a retrospective cohort study from September 2019 to September 2021. Patient demographics, dynamic changes of coagulation and fibrinolysis indicators were collected. Univariate analyses and binary logistic regression analyses were applied to assess potential risk factors for the development of SVT in SAP patients. A receiver operating characteristic (ROC) curve was generated to assess the predictive value of independent risk factors. Moreover, clinical complications and outcomes were compared between two groups.
Results
Among 177 SAP patients, 32 (18.1%) developed SVT. The most common cause of SAP was biliary (49.8%), followed by hypertriglyceridemia (21.5%). Multivariate logistic regression analyses showed that D-D (OR, 1.135; 95%CI, 1.043–1.236; p = 0.003) and fibrinogen degradation product (FDP) (OR, 1.037; 95%CI, 1.015–1.060; p = 0.001) were independent risk factors for SVT development in patients with SAP. The area under ROC curve for D-D was 0.891 (p = 0.003, sensitivity= 95.3%, specificity = 74.1%) at a cut-off value of 6.475, and the area under ROC curve for FDP was 0.858 (p = 0.001, sensitivity = 89.4%, specificity = 72.4%) at a cut-off value of 23.155.
Conclusion
D-D and FDP are significant independent risk factors with high predictive value for SVT in patients with SAP.
Introduction
Acute Pancreatitis (AP) is an inflammatory injury caused by the self-digestion of pancreatic tissue for various reasons. The incidence of acute pancreatitis is 110 ∼ 140 cases per 100 000 people worldwide yearly, with an increasing trend [Citation1]. Most AP patients have a mild course, recovering completely within a few days of onset. However, a variable percentage of patients develop severe disease. Complications of severe AP can be broadly classified as systemic or local, which are associated with prolonged hospital duration, significant morbidity, and mortality [Citation1–3]. Splanchnic vein thrombosis (SVT) is a rare complication of acute pancreatitis. It often involves the portal vein (PV), splenic vein (SV), and superior mesenteric vein (SMV), either in combination or separately [Citation4–6]. Until now, the detailed natural history of SVT in AP has remained unknown. The major pathophysiological mechanisms of pancreatitis-related SVT may include (a) direct injury of vascular endothelium due to inflammation and cellular infiltration, (b) systemic activation of hemostasis and hypercoagulable state, (c) compression by pancreatic/peripancreatic collections leading to blood flow stasis [Citation6–9].
The incidence of pancreatitis-induced splanchnic vein thrombosis has been reported varying from 1% to 24% [Citation6,Citation10]. It typically develops within 1–2 weeks of the onset of moderate or severe pancreatitis [Citation2,Citation6–8,Citation10]. The clinical manifestation of SVT varies depending on severity, ranging from mild abdominal discomfort even asymptomatic to hepatic failure or lethal gastrointestinal bleeding [Citation10–13].
In most cases, SVT presents asymptomatic and therefore ignored by clinicians. Clinical findings of SVT usually occurred incidentally in radiological imaging to assess the severity of AP [Citation6]. At present, the main means of diagnosis of SVT post SAP is based on enhanced CT [Citation6,Citation14]. Common serum markers for thrombosis such as D-dimer (D-D) have lost their diagnostic value due to their elevation in non-thrombotic patients with SAP. However, multiple conditions limit the application of enhanced CT in the context of SAP, such as renal impairment, pregnancy, severe underlying conditions, and high medical costs.
Studies have shown that when the homeostasis balance between the coagulation system and the fibrinolytic system is disrupted, it is easy to cause venous thrombosis. Hypercoagulation leads to microvascular thrombosis. It has demonstrated that microvessel changes are significant events in the progression of AP [Citation15,Citation16]. Coagulative disorders are associated with the severity of AP and can be related to multi-organ failure [Citation17]. In this study, we retrospectively analyzed the dynamic changes of coagulation and fibrinolysis parameters and established a new cut-off value using common serum thrombotic indicators to predict SVT in patients with severe pancreatitis.
Materials and method
Patients
A total of 177 patients with complete inpatient data and definite diagnoses of SAP patients treated in the Affiliated Hospital of Southwest Medical University from September 2019 to September 2021 were selected. Patients who met the following criteria were excluded: (1) younger than 18 years old; (2) pregnancy or severe immune system disorders; (3) end-stage chronic disease; (4) chronic pancreatitis; (5) incomplete data; (6) taking anticoagulants before admission; (7) cirrhosis or other coagulopathy disorder diseases or other systemic tumors. According to the international guidelines, all the study patients received standard medical treatment, including fluid therapy, pain control, nutritional support, prevention of infectious complications, and intensive care management. The study was approved by the Ethics Board of the Affiliated Hospital of Southwest Medical University.(No.KY2023026)
Diagnosis and definitions
The diagnosis of acute pancreatitis requires two of the following three features: (1) abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (3) characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI) or transabdominal ultrasonography. Severe acute pancreatitis is characterized by local complications accompanied by organ failure lasting more than 48 h. The diagnostic criteria are consistent with the revision of the Atlanta classification [Citation18].
Thrombus was defined as a filling defect within the lumen of the vessel on contrast-enhanced images [Citation14,Citation19]. Pancreatic portal hypertension, also known as left-sided portal hypertension, is diagnosed as isolated gastric varices (with/without esophageal varices) and/or splenomegaly with no signs of liver disease according to CT images or MRI scans [Citation20–22].
Data collection
To investigate the risk factors of SVT, demographic characteristics of patients including age, gender, etiology, smoking, and drinking were collected. Vital signs, serum laboratory indicators, and imaging examinations were collected to calculate the Bedside Index of Severity in Acute Pancreatitis (BISAP), modified Marshall score, and modified Balthazar’s CT score at admission. We also collected dynamic changes of coagulation and fibrinolysis indicators before using anticoagulants, including prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB), fibrin degradation products (FDP) and D-dimer. Moreover, to investigate the effect of SVT on the outcome of SAP, hospital length of stay, systemic and local complications including infected pancreatic necrosis, gastrointestinal hemorrhage, multiple organ dysfunction, and syndrome (MODS) were recorded.
Statistical analysis
Categorical variables were presented as the number of patients (%), and theχ2 test was used for statistical inference. Variable was presented as mean ± standard deviation for the date that was normally distributed and median with interquartile range (IQR) for the date that was not normally distributed for continuous variables. Continuous variables were compared using the t-test or the non-parametric Mann-Whitney U test. Factors potentially associated with the development of SVT clinical manifestations in the univariable analysis were included in the binary regression analysis. We generated ROC to investigate the predictive value of factors of thrombosis and compare differences among the areas under the curve (AUC). Moreover, sensitivity, specificity, and the Youden index (YI) were used in identifying optimal cut-off values. Two-tailed p values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software version 25.0 (SPSS Inc., Chicago, Illinois, USA). Graphs were prepared in GraphPad Prism (version 8.0.2).
Results
Demographic characteristics and serum laboratory indicators
As shown in the flow chart in , a total of 177 patients were included in this study. They were divided into the SVT group and the non-SVT group. Thirty-two (18.08%) patients developed SVT in the study. The demographic characteristics and serum laboratory indicators of patients were presented in . Of the patients, 99 (55.9%) patients were male, and 78 (44.1%) patients were female. The average ages of patients in the SVT and non-SVT groups were 49.28 and 49.99 years, respectively. The most common cause of acute pancreatitis was biliary (49.8%), followed by hyperlipidemia (21.5%). There were no significant differences in demographic characteristics and serum laboratory indicators between the two groups (p > 0.05).
Figure 1. Flow chart of patients with or without SVT secondary to SAP. SAP: severe acute pancreatitis; SVT: splanchnic vein thrombosis.
Table 1. Demographic characteristics and serum laboratory indicators of patients.
Pattern and treatment of splanchnic vein thrombosis
Among the 32 patients with SAP-associated SVT, the portal vein was the most involved vessel (11 cases), followed by SV (6 cases) and SMV (2 cases). Multiple visceral thrombus is commonly observed. Patients with PV and SV were found in 7 cases, followed by PV and SV and SMV in 4 cases, and PV and SMV in 2 cases (). In addition, 1 case with cavernous degeneration of portal vein (CTPV) and 2 cases with venous aneurysm were found in these patients. 27 patients received anticoagulated treatment after diagnosis was confirmed. A therapeutic dose of low molecular-weight heparin was administered during hospitalization.
Table 2. Pattern of splanchnic vein thrombosis and anticoagulant therapy.
Analysis of coagulation and fibrinolysis markers
Univariate analysis was used to analyze the difference in coagulation and fibrinolysis markers between the SVT or non-SVT groups (). D-dimer and FDP showed a significant difference between the two groups (All, p < 0.001). D-dimer (OR, 1.135; 95%CI, 1.043-1.236; p = 0.003) and FDP (OR, 1.037; 95%CI, 1.015–1.060; p = 0.001) were independent risk factors for SVT in patients with SAP in binary logistic regression model (). We applied the ROC curve to generate an optimal cut-off value for predicting the development of SVT. The area under ROC curve for D-D was 0.891 (p = 0.003), the sensitivity was 95.3%, and the specificity was 74.1% at a cut-off value of 6.475. The area under ROC curve for FDP was 0.858 (p = 0.001), the sensitivity was 89.4%, and the specificity was 72.4% at a cut-off value of 23.155 (, ).
Figure 2. The area under the receiver operating characteristic (ROC) curve for predictive utility of D-Di and FDP for the presence of SVT in SAP are 0.8909 and 0.8578, respectively.
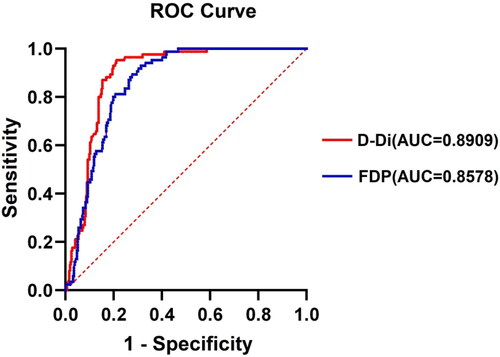
Table 3. The univariate analysis of coagulation and fibrinolysis markers.
Table 4. The binary logistic regression of coagulation and fibrinolysis markers.
Table 5. The predictive value of D-Di and FDP for SVT in SAP patients.
Complications and outcomes
The clinical complications and outcomes were compared between the non-SVT and SVT groups. As shown in , pancreatic pseudocysts were more likely to develop in the SVT group than non-SVT group (21.9% vs 8.9%, p = 0.037). Moreover, the incidence of gastrointestinal bleeding was higher in SVT group than non-SVT group (15.6% vs 4.1%, p = 0.015). Likewise, the incidence of pancreatic portal hypertension (PHH) was higher in SVT group than non-SVT group (43.8% vs 12.4%, p < 0.001). The incidence of pancreatic abscess appeared to be higher in SVT but did not reach a statistical difference (9.4% vs 2.8%, p = 0.082). Of all patients, the median length of hospitalization was 18 days, and there was no significant difference between the two groups. In addition, there were no significant differences in the incidence of pancreatic necrosis, ascites, ACS, ARDS, AKI, SIRS and MODS between the two groups.
Table 6. Clinical Features and Outcome Between SVT and Non-SVT Group.
Discussion
We performed a retrospective study to analyze the risk factors of splanchnic vein thrombosis in patients with severe acute pancreatitis. The incidence of SVT post SAP in our study was 18.08% (32 out of 177). Previously, the incidence of SVT was widely variable ranging from 1% to 24%, depending on detection means and the heterogeneity of studies (mild vs. severe AP, chronic vs. acute pancreatitis) [Citation6,Citation10]. It has been reported that most SVT are diagnosed in the late stage of AP [Citation2,Citation6–8,Citation10], which is consistent with the observation in our study. In addition, our study showed that D-D and FDP were independent risk factors for prediction of developing SVT in patients with SAP. The AUCs of D-D and FDP for predicting SVT in patients with SAP were 0.891 and 0.858, respectively, with statistically significant differences (p < 0.05). The predictive sensitivities of D-D and FDP were 95.3% and 89.4%, respectively. These results suggest that plasma D-D and FDP have high predictive values for predicting SVT in patients with SAP. Comparison of clinical complications showed the incidence of pancreatic pseudocysts, gastrointestinal bleeding and PHH was higher in SVT group than non-SVT group. Comprehensive treatment is needed to reduce the complications and improve the disease outcomes.
Several studies have revealed the potential relationship between systemic hypercoagulable or prothrombotic state with clinical outcomes in AP [Citation6,Citation7,Citation10]. In the pathogenesis of thrombosis, D-D and FDP, as functional markers of the fibrinolytic system, played fundamental role in the process of venous thrombosis, which can be greatly elevated during thrombosis [Citation23]. D-D is a specific degradation product of cross-linked fibrin monomers hydrolyzed by plasmin proteins, and its elevation reflects secondary endogenous fibrinolysis, indicating a hypercoagulable state in the body. In addition, D-D, a marker of activated coagulation and fibrinolysis, is elevated in patients with venous thromboembolism (VTE). It has high sensitivity and negative predictive value. Negative or low D-D is often applied to rule out the presence of acute VTE [Citation24]. D-D is mostly used as an effective diagnostic tool to rule out deep vein thrombosis (DVT) and pulmonary embolism, and it has been reported to have great predictive power in the early phase of AP [Citation25–27]. In our study, D-D was an independent risk factor for patients with SAP, which showed a good AUC of 0.891 at a cut-off value of 6.475. This result indicates that D-D lower than 6.475 may be a good predictor for excluding SVT in SAP. FDP is a degradation product of fibrinogen in response to plasmin proteins, and the rise of FDP level reflects the fibrinolytic activity enhancement in the body [Citation28]. It has been reported that Increased level of FDP can predict the occurrence of venous thrombus embolism in postoperative patients with breast cancer [Citation23]. Increases in plasma FDP were closely related to the occurrence of thrombosis in the patients after laparoscopic surgery [Citation29]. Our study showed a high predictive value of plasma FDP for SVT in patients with SAP at a cut-off value of 23.155.
SAP-associated SVT may generate sinistral portal hypertension, leading to gastrointestinal and intra-abdominal hemorrhage, ascites, and splenomegaly [Citation7–10]. Our study also showed significant differences of incidence in pancreatic pseudocysts, pancreatic portal hypertension, and gastrointestinal bleeding between SVT and non-SVT patients. Several studies have shown that persistent pancreatic inflammation and external compression from pancreatic necrosis or pseudocysts lead to stenosis, thrombosis, or occlusion of the splenic vein [Citation6–8,Citation21]. The incidence of sinistral portal hypertension in patients with acute pancreatitis was 3.3% [Citation22,Citation30], which was slightly higher than in our study, with incidence of 1.4% (32 out of 2230). In pancreatic portal hypertension, collateral branches to the fundus of stomach are the main passageway for blood circulation, which is the basis of isolated gastric varices. In our study, 11 out of 177 SAP patients (6.2%) developed gastrointestinal bleeding during hospitalization, of which 2 were due to rupture of gastric varices in SVT group. These complications induced by SVT post SAP highlight the importance of thrombus management and early detection to avoid severe consequences. D-D and FDP may serve as monitoring means for thrombosis, recanalization of thrombosed splanchnic veins and management of thrombus in SAP patients.
The pathophysiology of AP is intricate and still not fully known, but the role of coagulopathy in AP progression is evident. Specifically, damaged pancreatic acinar cells trigger the activation of digestive enzymes, resulting in local vascular epithelial cell injury, tissue factor exposure, platelet activation, and coagulation cascade activation. Severe inflammation can lead to systemic activation of hemostasis, increasing the risk of SVT and multiple organ failure [Citation31]. Clinically, therapeutic anticoagulation (AC) is used to prevent the progression of thrombosis, recanalize the vein and avoid further complications following SVT [Citation32]. However, some researchers have taken the opposite view, suggesting that therapeutic AC may increase the risk of hemorrhage in patients with SVT [Citation3,Citation33,Citation34]. Inversely, other studies indicated no statistically significant increase in hemorrhagic complications or overall mortality, nor did they find fatal hemorrhage during anticoagulation [Citation33]. To date, there is no consensus on the routine use of AC for the treatment of SVTS secondary to acute pancreatitis. In addition, due to the heterogeneity of studies, the effect of AC on the recanalization of SVT has not been determined. Anis, F.S. et al. reported a statistically significant increase in the rate of recanalization with therapeutic AC, and there was no increase in hemorrhagic complications or mortality [Citation33]. However, Junare PR et al. noted that they did not observe a statistical difference in SVT outcomes (including recanalization and mortality) between the AC and non-AC groups [Citation34]. On the other hand, limited literature so far have suggested that spontaneous recanalization of SVT occurs in up to 30% of cases, especially in splenic vein thrombosis [Citation3,Citation33,Citation35]. Therefore, in patients with pancreatitis who have isolated splenic vein thrombosis without complications of portal hypertension or mesenteric vein involvement, treatment for pancreatitis alone may be continued without initiation of antithrombotic therapy, but the dynamic change of the thrombosis should be closely monitored [Citation35]. SVT can be classified as acute, subacute, and chronic. Acute SVT involves thrombosis of one or more splanchnic veins without radiologic evidence of a portal cavernoma or collateral portosystemic circulation. Therefore, treatment in these patients is aimed at preventing complications such as intestinal ischaemia or infarction, and at recanalizing the affected vessels to reduce the risk of splanchnic hypertension and hemorrhage [Citation36]. Current evidence suggests that the recanalization rate of acute extrahepatic portal vein thrombosis after AC therapy may be as high as 40%–50% [Citation36]. Hall TC et al. reported antithrombotic therapy should be actively given for patients with pancreatitis who have SVT extending to the mesenteric vein and with clinical presentation of intestinal ischemia. Since acute SVT may lead to severe complications, acute symptomatic SVT secondary to pancreatitis should be actively treated with AC, and early initiation of AC therapy could increase the recanalization rate of SVT in such patients [Citation35,Citation37]. In our study, the majority of SVT patients (27 out of 32) were administered with therapeutic AC. Treatment-related complications such as gastrointestinal bleeding developed in only 2 patients. All patients had well outcomes and no patients died. Although the clinical relevance of SVT is still undetermined, AC therapy aims to prevent further thrombus propagation and vessel recanalization. More clinical exploration is needed to maintain coagulation balance in patients with pancreatitis. Indeed, further prospective studies are also required to evaluate the long-term impact of AC on patients with SVT.
The present study has several limitations. First, our study was a single center with a relatively small sample size, which might have affected the results. Second, complete follow-up data were missing in this study, it is unavailable to observe the long-term effects of AC treatment on patients. Third, as a retrospective study, the findings should be interpreted cautiously and validated in prospective studies.
Conclusion
In conclusion, we suggest that D-D and FDP are independent significant risk factors with high predictive value for splanchnic vein thrombosis in patients with severe acute pancreatitis. A multicenter, large-sample, and well-designed prospective study is warranted in the future.
Ethical approval
This study was approved by the Ethics Board of the Affiliated Hospital of Southwest Medical University (KY2023026).
Written informed consent was obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Authors’ Contribution
Jie Zheng: Collection and assembly of data, Data curation, Formal analysis, Writing - original draft.
Ming Han and Jie Chen: Collection data.
Ming ming Deng: Conceptualization.
Gang Luo: Conceptualization, Supervision, Review & editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets analyzed during our study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390.
- Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12(5):854–862.
- Junare PR, Udgirkar S, Nair S, et al. Splanchnic venous thrombosis in acute pancreatitis: does anticoagulation affect outcome? Gastroenterology Res. 2020;13(1):25–31.
- Mallick IH, Winslet MC. Vascular complications of pancreatitis. JOP. 2004; 5(5):328–337.
- Mendelson RM, Anderson J, Marshall M, et al. Vascular complications of pancreatitis. ANZ J Surg. 2005;75(12):1073–1079.
- Nadkarni NA, Khanna S, Vege SS. Splanchnic venous thrombosis and pancreatitis. Pancreas. 2013; 42(6):924–931.
- Zelez HJ, Sahay SJ, Samadi B, et al. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB . 2011;13(12):860–864.
- Toqué L, Hamy A, Hamel JF, et al. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: a 6-year single-center experience. J Dig Dis. 2015;16(12):734–740.
- Zhou J, Ke L, Yang D, et al. Predicting the clinical manifestations in necrotizing acute pancreatitis patients with splanchnic vein thrombosis. Pancreatology. 2016;16(6):973–978.
- Zhou J, Ke L, Tong Z, et al. Risk factors and outcome of splanchnic venous thrombosis in patients with necrotizing acute pancreatitis. Thromb Res. 2015;135(1):68–72.
- Ding L, Deng F, Yu C, et al. Portosplenomesenteric vein thrombosis in patients with early-stage severe acute pancreatitis. World J Gastroenterol. 2018;24(35):4054–4060.
- Nawacki Ł, Matykiewicz J, Stochmal E, et al. Splanchnic vein thrombosis in acute pancreatitis and its consequences. Clin Appl Thromb Hemost. 2021;27:10760296211010260.
- Pandey V, Patil M, Patel R, et al. Prevalence of splenic vein thrombosis and risk of gastrointestinal bleeding in chronic pancreatitis patients attending a tertiary hospital in Western India. J Family Med Prim Care. 2019;8(3):818–822.
- Mortelé KJ, Mergo PJ, Taylor HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52(1):67–72.
- Yang N, Hao J, Zhang D. Antithrombin III and D-dimer levels as indicators of disease severity in patients with hyperlipidaemic or biliary acute pancreatitis. J Int Med Res. 2017;45(1):147–158.
- Ou ZB, Miao CM, Ye MX, et al. Investigation for role of tissue factor and blood coagulation system in severe acute pancreatitis and associated liver injury. Biomed Pharmacother. 2017;85:380–388.
- Liu C, Zhou X, Ling L, et al. Prediction of mortality and organ failure based on coagulation and fibrinolysis markers in patients with acute pancreatitis: a retrospective study. Medicine. 2019;98(21):e15648.
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111
- Cai DM, Luo Y, Li YZ, et al. Assessing splenic vein complications in patients with acute pancreatitis using color doppler ultrasound and contrast enhanced ultrasound. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(5):850–853.
- Köklü S, Coban S, Yüksel O, et al. Left-sided portal hypertension. Dig Dis Sci. 2007 May;52(5):1141–1149.
- Xie CL, Wu CQ, Chen Y, et al. Sinistral portal hypertension in acute pancreatitis: a magnetic resonance imaging study. Pancreas. 2019;48(2):187–192.
- Kul M, Haliloğlu NÜ, Hürsoy N, et al. Sinistral portal hypertension: computed tomography imaging findings and clinical appearance-a descriptive case series. Can Assoc Radiol J. 2018;69(4):417–421.
- Pang M, Zhao F, Yu P, et al. The significance of coagulation and fibrinolysis-related parameters in predicting postoperative venous thrombosis in patients with breast cancer. Gland Surg. 2021;10(4):1439–1446.
- Tritschler T, Kraaijpoel N, Le Gal G, et al. Venous thromboembolism: advances in diagnosis and treatment [published correction appears in JAMA. 2018 dec 18;320(23):2486]. JAMA. 2018;320(15):1583–1594.
- Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(5):957–970.
- Lucassen W, Geersing GJ, Erkens PM, et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011;155(7):448–460.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. 2020;41(4):543–603.
- Bai Y, Shi M, Yang X, et al. The value of FDP/FIB and D-dimer/FIB ratios in predicting high-risk APL-related thrombosis. Leuk Res. 2019;79:34–37.
- Yang C, Zhu L. Coagulation and deep vein flow changes following laparoscopic total extraperitoneal inguinal hernia repair: a single-center, prospective cohort study. Surg Endosc. 2019;33(12):4057–4065.
- Li H, Yang Z, Tian F. Clinical characteristics and risk factors for sinistral portal hypertension associated with moderate and severe acute pancreatitis: a seven year single center retrospective study. Med Sci Monit. 2019;25:5969–5976.
- Kröner PT, Wallace MB, Raimondo M, et al. Systemic anticoagulation is associated with decreased mortality and morbidity in acute pancreatitis. Pancreatology. 2021;21(8):1428–1433.
- Tozlu M, Kayar Y, İnce AT, et al. Low molecular weight heparin treatment of acute moderate and severe pancreatitis: a randomized, controlled,open-label study. Turk J Gastroenterol. 2019;30(1):81–87.
- Anis FS, Adiamah A, Lobo DN, et al. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):446–454.
- Sissingh NJ, Groen JV, Koole D, et al. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a systematic review and meta-analysis. Pancreatology. 2022;22(2):235–243.
- Pancreas Study Group, Chinese Society of Gastroenterology, Chinese Medical Association Practice guidance for diagnosis and treatment of pancreatitis-related splanchnic vein thrombosis (shenyang, 2020). J Dig Dis. 2021;22(1):2–8.
- Hall TC, Garcea G, Metcalfe M, et al. Management of acute non-cirrhotic and non-malignant portal vein thrombosis: a systematic review. World J Surg. 2011;35(11):2510–2520.
- Norton W, Lazaraviciute G, Ramsay G, et al. Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2020;19(2):116–121.
