Abstract
Background
Drug-induced liver injury (DILI) remains a challenging diagnosis due to an absence of specific biomarkers. DILI due to volatile anaesthetics (VA-DILI) is characterised by trifluoroacetyl and CYP2E1 antibodies, but may not be seen for weeks after injury. Interleukin-4 (IL-4) may be involved in the production of these antibodies and may serve as a clinically useful early biomarker of VA-DILI.
Aim
To prospectively compare serum IL-4 levels between patients who develop VA-DILI and controls following exposure to the volatile anaesthetic.
Methods
A nested case-control study of patients exposed to VA during surgery was conducted. Thirteen DILI cases were identified from the original cohort, and 26 controls were matched according to age, sex and VA agent. Serum samples were collected before and 48-96 h after VA exposure, and analysed for IL-4 using quantitative enzyme-linked immunosorbent assay techniques.
Results
There was a statistically significant difference in serum IL-4 in post-VA samples between DILI cases and controls (control: 0.030 pg/mL, IQR: 0.030 − 0.030 pg/mL vs DILI: 0.044 pg/mL, IQR: 0.030 − 0.061 pg/mL; p = 0.039). A greater proportion of DILI cases had post-VA IL-4 levels above the assay lower limit of detection compared to controls (control: 23% vs DILI: 69%; p = 0.013).
Conclusion
IL-4 is a potential biomarker of DILI. Clinical diagnosis and understanding of DILI disease mechanisms may be improved by further investigation of novel biomarkers, and this IL-4 signal in serum is important as proof of concept for prospective study designs.
Introduction
Drug-induced liver injury (DILI) continues to be a serious issue in drug safety and although rare, remains an important cause of acute liver failure.Citation1,Citation2 DILI due to volatile anaesthetics (VA) was first recognised with halothane, with 25–30% of patients exhibiting abnormal liver biochemistry two to fourteen days post-exposure.Citation3,Citation4 Modern VA, such as sevoflurane and desflurane, are generally considered to be safer; however, both retrospective and prospective studies have found volatile anaesthetic drug-induced liver injury (VA-DILI) may still occur with these drugs.Citation5,Citation6
At present, diagnosis of DILI is primarily based on liver enzyme elevation in conjunction with causality assessment for a culprit agent. This approach is limited by a number of factors however, including the non-specificity of liver biochemistry, and challenging causality assessment in the context of multiple drug exposures. As such, there is a significant clinical need for sensitive and specific DILI biomarkers.Citation7
VA-DILI is unique in that some elements of the mechanism have been identified. Trifluoroacetyl antibodies and cytochrome p450 2E1 antibodies can be seen in the blood a few weeks after a VA-DILI in most cases.Citation8 In addition, there is increasing evidence that interleukin-4 (IL-4) plays a key role in the early stages of VA-DILI. IL-4 promotes B cell isotype switching to subtypes seen in halothane hepatitis, and animal models have demonstrated that high IL-4 levels promote liver injury.Citation8–10 The role of IL-4 in clinical disease and potential as a diagnostic biomarker has yet to be fully explored. The aim of this study was to analyse the IL-4 response in patients who developed DILI following VA exposure compared to those who did not.
Methods
A nested case-control study of adult patients exposed to volatile anaesthetic (VA) during surgery was conducted (). Patients were drawn from a cohort recruited prospectively from a tertiary hospital in Melbourne, Victoria between May 2017 and January 2018. Exclusion criteria for this cohort included surgery involving the upper abdomen, significant intraoperative hypotension, and failure to obtain a follow-up blood sample. From this cohort, patients were also excluded if they had incomplete liver function tests, delayed follow-up, or missing MAC hour data. This study was approved by the Eastern Health Human Research Ethics Committee (ref LR05/2017), and was registered with the Monash University Human Research Ethics Committee (project 28406).
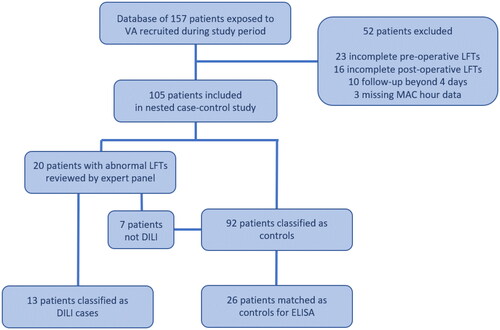
Figure 1. Recruitment, exclusion and matching process. This flowchart outlines the study groups and exclusion criteria. Seven of twenty patients reviewed for possible DILI were determined to not be DILI by expert panel and were subsequently moved into the control group for matching. LFTs: liver function tests.
Baseline demographic data including age, sex, body mass index (BMI), self-reported ethnicity, alcohol intake, comorbidities, allergies, concomitant medications, and surgery type were collected at the time of consent. The anaesthetic report was reviewed post-operatively to identify the VA agent used, intraoperative medications and any intraoperative complications. The degree of VA exposure was recorded in minimum alveolar concentration (MAC) hour, calculated by multiplying the MAC value of the agent by the number of hours the patient was exposed.Citation5,Citation11 Blood samples for liver function tests and serum samples were collected immediately prior to surgery and 2–4 days post-operatively.
Patients with alanine-aminotransferase (ALT) more than twice their baseline were reviewed separately by an expert panel of three hepatologists to determine causality. The expert panel combined patient risk factors and past history, time of drug exposure and resolution of DILI, clinical status and liver biochemistry to determine the likelihood of DILI. Each case was scored as definite (>95% likelihood), highly likely (75–95%), probable (50–74%), possible (25–49%), unlikely (<25%) or insufficient data to assess, according to the causality assessment model outlined by the Drug-Induced Liver Injury Network (DILIN).Citation12 Any case determined to have a likelihood of more than 50% was classified as a positive DILI case, with consensus from all three hepatologists required. Any discrepancy between reviewers resulted in panel discussion to achieve consensus.
Cases were matched with controls in a 2:1 ratio according to age, gender and VA agent using a Greedy Nearest-Neighbour Algorithm. This algorithm is a standard algorithm that minimises the distance between cases and controls, where distance is a function of the differences in matching variables, but without a global cost function.Citation13
Serum samples were analysed in duplicate using commercial Human IL-4 Quantikine High-sensitivity Kits (R&D Systems, cat# HS400), which employed a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique. The lower limit of detection (LLOD) for this assay is reported as 0.03 pg/mL. This value was assigned to any sample with undetectable IL-4 for the purposes of statistical analysis (excluding comparison of detectable IL-4). Each step was performed according to the manufacturer’s instructions. Microplates were analysed using a BioTek Synergy HT microplate reader with BioTek Gen5 V2.01.13 software.
Parametric continuous data were reported as mean ± standard deviation, and compared using the unpaired t-test. Non-parametric continuous data were reported as median with interquartile range, and compared using the Mann-Whitney U test. Categorical variables were expressed as frequencies and percentages and compared using Fisher’s exact test. Non-parametric paired data were analysed using Wilcoxon signed-rank test. The area under the receiver operating characteristic curve (ROC) was used to estimate the ability of IL-4 levels to discriminate DILI compared to non-DILI. A p-value of ≤0.05 was considered statistically significant. All statistical analysis was conducted using Stata/IC 16.1 (StataCorp LP, Texas, USA, 2020) and the statistical programming language R (RStudio, PBC, Boston, MA, USA, 2022).
Results
During the study period between May 2017 to January 2018, 157 patients exposed to VA were recruited. A total of 52 patients were excluded from analysis: 23 had incomplete pre-operative LFTs, 16 had incomplete post-operative LFTs, ten did not have follow-up serum from within the four-day specified follow-up period, and three had missing MAC hour data. Twenty of the 105 patients had an ALT increase of more than twice baseline. Thirteen of these were diagnosed with probable DILI following expert panel review, and 26 controls were subsequently matched. Of the seven patients deemed to not be DILI, one was determined to be ischaemic hepatitis, and the remaining six to be an ALT change of unclear significance (). One DILI patient was found to have insufficient pre-VA serum for biomarker analysis, and was consequently only included in post-VA analyses.
The clinical characteristics of the study participants are listed in . There were 24 males and 15 females, with a median age of 63 years. DILI patients were more likely to have undergone a urological procedure compared to controls, with laparoscopic partial nephrectomy being the most common procedure type. Patients undergoing urological procedures received a greater MAC hour of VA exposure compared to other procedures (4.48 vs 3.50; p = 0.046) (Supplementary Table 1). There were no statistically significant differences of patient characteristics seen between groups.
Table 1. Patient characteristics of the nested case-control group undergoing ELISA analysis.
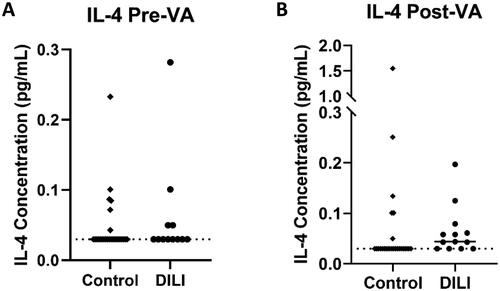
There was no statistically significant difference in the proportion of pre-VA samples with detectable IL-4 (assay lower limit of detection LLOD: 0.03 pg/mL) in DILI compared to control (33% vs 23%, p = 0.69) (). The proportion of post-VA samples that exceeded assay lower limit of detection was greater in DILI compared to control (69% vs 23%, p = 0.013) (). Median pre-VA exposure IL-4 concentration in DILI cases was not significantly different to controls (0.03 pg/mL vs 0.03 pg/mL, p = 0.50). Median post-VA exposure IL-4 concentration in DILI cases was higher compared to controls (0.044 pg/mL vs 0.030 pg/mL, p = 0.039) (). Paired analysis for IL-4 change between pre-VA to post-VA was not significant in DILI or controls (p = 0.17 and p = 0.82 respectively). Median fold change in DILI cases compared to controls was 1.33 vs 1.00 (p = 0.19). The area under the ROC curve (AUC) for post-VA IL-4 was 0.68 [95% CI:0.52 − 0.72; p = 0.031] (Supplementary Figure 1).
Figure 2. Pre-VA and Post-VA IL-4 concentration in control and DILI. (A) Median pre-VA IL-4 concentration was not significantly different between DILI and control and post-VA samples (0.03pg/mL vs 0.03pg/mL, p = 0.50). (B) Median post-VA IL-4 was higher in DILI compared to control (0.044pg/mL vs 0.03pg/mL, p = 0.039).
One of the seven non-DILI patients with ALT change requiring panel review was matched to the control group. This patient had a 2.59 fold ALT change, but was deemed to be likely ischaemic hepatitis following panel review. Their serum IL-4 decreased from 0.042 pg/mL to undetectable levels following surgery.
Discussion
This study’s primary strength is its novel prospective design, comparing an individual case’s own pre-exposure and post-exposure serum to a drug as a control for biomarker research in DILI. Additionally, this is one of the few studies to quantify serum IL-4 in DILI patients compared to healthy, drug exposed controls where limited clinical evidence is available.
Diagnostic criteria used for DILI, such as those outlined by Aithal et al. typically require significant elevations of ALT and ALP in relation to the upper limit of the normal range.Citation15 However these criteria have limitations, including the reliance on normal values that are usually based on results from healthy young controls that do not reflect real world patient groups. These biochemical values would be better replaced by multiples of a patient’s own baseline for the purposes of investigating DILI.Citation16 Consequently, this study employed the criteria of twice baseline ALT as the cut-off for determining cases that required expert panel review. Importantly, while this threshold has been applied in this experimental context, this approach may not be fully applicable to clinical contexts, where unnecessary drug cessation may occur. Indeed, mild elevations in ALT may resolve spontaneously without progression to serious liver injury in many cases.Citation17 Nonetheless, identification of mild cases of DILI with the use of more inclusive criteria is important, as these carry similar latencies and biochemical profiles to more severe episodes.Citation18 This is particularly valuable in the context of VA-DILI, as repeated exposure with mild liver enzyme elevation may eventually result in severe acute or chronic liver injury.Citation19
These results further support an immune-mediated mechanism involving IL-4 and Th2 responses in VA-DILI as suggested in animal models. High intrahepatic IL-4 levels seem to mediate direct hepatocellular injury whilst simultaneously suppressing anti-inflammatory responses during halothane induced DILI in mice.Citation10 Additionally, elevated blood IL-4 levels have also been observed in a number of murine models of DILI due to a variety of other agents, suggesting the importance of IL-4 may not be limited to VA related liver injury only.Citation20–22 Indeed, variant alleles associated with increased IL-4 transcription combined with decreased IL-10 activity were observed to be more common in patients taking diclofenac who had suffered DILI compared to those who did not.Citation23 Based on these results, further evaluation of IL-4 in other forms of DILI would be useful to potentially identify similar disease mechanisms involving antibody responses to drug-hapten proteins. Moreover, how IL-4 levels in DILI differ from other liver injury such as viral and metabolic injury, still needs to be determined. Although one control with ischaemic hepatitis showed a decrease in IL-4 in this study, further work to assess IL-4 in non-DILI patients with ALT change and other liver injury is required.
This study was not powered to characterise VA-DILI risk factors; however, a few baseline characteristics did seem to be associated with the likelihood of DILI. The first was a higher baseline ALT, probably reflecting that mild metabolic associated fatty liver disease (MAFLD) was more common in those with DILI. This is consistent with other literature which has suggested an increased risk of VA-DILI associated with MAFLD, due to an existing inflammatory state as well as higher hepatic CYP2E1 activity.Citation24,Citation25 Alcohol, a putative risk factor for VA-DILI due to CYP2E1 induction, also showed a trend towards higher average consumption in VA-DILI cases.Citation26 These observations were not statistically significant and will need to be examined in a larger cohort to be confirmed.
VA-DILI occurred more frequently after urological procedures compared to other surgical procedures. One possible explanation is the greater median MAC hour of exposure observed in these procedures compared to others. Higher doses of medications are a known risk factor for DILI, and higher MAC hour calculations were observed in the urological patients.Citation27 VA-DILI was particularly associated with laparoscopic partial nephrectomy.
The absolute difference in IL-4 concentration detected in this study was small, and it is possible that peak IL-4 levels were missed in this study. The follow-up time of 2-4 days post-operatively was chosen to coincide with when peak ALT is usually expected to occur in VA-DILI.Citation5,Citation6,Citation28 Serial measurements of serum IL-4 in the early phases of VA-DILI has only been conducted in the mouse model of halothane-induced VA-DILI, and identified the peak at 18 h, before declining by 24 h, despite ALT levels being at their highest at 24 h after exposure.Citation29 This may suggest that peak serum IL-4 levels had already occurred by the time follow-up samples were collected in this study.
This study was limited by the small sample size, which was partly due to the prospective nature of recruitment and the low incidence of VA-DILI. Additionally, assay limitations in sensitivity likely prevented quantification of IL-4 changes occurring below 0.03 pg/mL. As undetectable samples were assigned a value of 0.03 pg/mL for statistical analysis, it is likely that these results underestimate the true rise in IL-4 in VA-DILI patients. This study was also limited by its lack of validation experiments to ascertain clinical validity. Comparison with trifluoroacetyl and CYP2E1 antibodies would be useful in this study of VA-DILI but were not available. Finally, this study did not compare IL-4 serum changes between VA-DILI with other forms of liver disease to determine its specificity as a DILI biomarker. This remains a key area of further investigation following the results of this study.
In conclusion, this prospective study of pre and post VA exposure is consistent with serum IL-4 being a serological marker of liver injury that may help to understand the pathogenesis of DILI. Future studies comparing this to other liver injuries, and to clinical outcomes will help to determine its clinical value.
| Abbreviations | ||
| ALF | = | Acute liver failure |
| ALP | = | Alkaline phosphatase |
| ALT | = | Alanine aminotransferase |
| AST | = | Aspartate aminotransferase |
| BMI | = | Body mass index |
| CAM | = | Causality assessment method |
| CYP450 | = | Cytochrome P450 |
| DILI | = | Drug-induced liver injury |
| ELISA | = | Enzyme-linked immunosorbent assay |
| GGT | = | Gamma-glutamyl transferase |
| IL-4 | = | Interleukin 4 |
| LFT | = | Liver function test |
| LLOD | = | Lower limit of detection |
| MAC | = | Mean alveolar concentration |
| MAFLD | = | Metabolic-associated fatty liver disease |
| ROC | = | Receiver operating characteristic |
| TFA | = | Trifluoroacetyl |
| VA | = | Volatile anaesthetic |
| VA-DILI | = | Volatile anaesthetic drug-induced liver injury |
Supplemental Material
Download MS Word (159.9 KB)Acknowledgements
We are grateful for the support, input and helpful discussions from Professor Dolores Njoku, Washington University, St Louis, USA, in the preparation of this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–2076. doi: 10.1002/hep.23937.
- Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–2534. doi: 10.1056/NEJMra1208937.
- Neuberger JM. Halothane and hepatitis. Incidence, predisposing factors and exposure guidelines. Drug Saf. 1990;5(1):28–38. doi: 10.2165/00002018-199005010-00004.
- Ye H, Nelson LJ, Gomez Del Moral M, et al. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol. 2018;24(13):1373–1385. doi: 10.3748/wjg.v24.i13.1373.
- Lin J, Moore D, Hockey B, et al. Drug-induced hepatotoxicity: incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int. 2014;34(4):576–582. doi: 10.1111/liv.12278.
- Bishop B, Hannah N, Doyle A, et al. A prospective study of the incidence of drug-induced liver injury by the modern volatile anaesthetics sevoflurane and desflurane. Aliment Pharmacol Ther. 2019;49(7):940–951. doi: 10.1111/apt.15168.
- Atallah E, Freixo C, Alvarez-Alvarez I, et al. Biomarkers of idiosyncratic drug-induced liver injury (Dili) - a systematic review. Expert Opin Drug Metab Toxicol. 2021;17(11):1327–1343. doi: 10.1080/17425255.2021.1999410.
- Njoku DB, Mellerson JL, Talor MV, et al. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin Vaccine Immunol. 2006;13(2):258–265. doi: 10.1128/CVI.13.2.258-265.2006.
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0.
- Njoku DB, Li Z, Washington ND, et al. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury. Eur J Immunol. 2009;39(6):1652–1663. doi: 10.1002/eji.200838135.
- Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68(5):512–522. doi: 10.1111/anae.12168.
- Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-induced liver injury network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005.
- Bania RK, Halder A. R-Ensembler: a greedy rough set based ensemble attribute selection algorithm with kNN imputation for classification of medical data. Comput Methods Programs Biomed. 2020;184:105122. doi: 10.1016/j.cmpb.2019.105122.
- Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34(2):134–144. doi: 10.1055/s-0034-1375955.
- Aithal G, Watkins P, Andrade R, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–815. doi: 10.1038/clpt.2011.58.
- M’Kada H, Munteanu M, Perazzo H, et al. What are the best reference values for a normal serum alanine transaminase activity (ALT)? impact on the presumed prevalence of drug induced liver injury (Dili). Regul Toxicol Pharmacol. 2011;60(3):290–295. doi: 10.1016/j.yrtph.2011.04.002.
- Jiang F, Yan H, Liang L, et al. Incidence and risk factors of anti-tuberculosis drug induced liver injury (Dili): large cohort study involving 4652 Chinese adult tuberculosis patients. Liver Int. 2021;41(7):1565–1575. doi: 10.1111/liv.14896.
- Regev A, Seeff LB, Merz M, et al. Causality assessment for suspected Dili during clinical phases of drug development. Drug Saf. 2014;37 Suppl 1(Suppl 1):S47–S56. doi: 10.1007/s40264-014-0185-4.
- Nicoll A, Moore D, Njoku D, et al. Repeated exposure to modern volatile anaesthetics may cause chronic hepatitis as well as acute liver injury. BMJ Case Rep. 2012;2012:bcr2012006543. doi: 10.1136/bcr-2012-006543.
- Higuchi S, Kobayashi M, Yano A, et al. Involvement of Th2 cytokines in the mouse model of flutamide-induced acute liver injury. J Appl Toxicol. 2012;32(10):815–822. doi: 10.1002/jat.1706.
- Kobayashi M, Higuchi S, Ide M, et al. Th2 cytokine-mediated methimazole-induced acute liver injury in mice. J Appl Toxicol. 2012;32(10):823–833. doi: 10.1002/jat.2731.
- Higuchi S, Kobayashi M, Yoshikawa Y, et al. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicol Lett. 2011;200(3):139–145. doi: 10.1016/j.toxlet.2010.11.006.
- Aithal GP, Ramsay L, Daly AK, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39(5):1430–1440. doi: 10.1002/hep.20205.
- Massart J, Begriche K, Moreau C, et al. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017;3(Suppl 1):212–232.
- Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12(1):60. doi: 10.1186/s13098-020-00570-y.
- Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28(6):802–811. doi: 10.1159/000324289.
- Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146(4):914–928. doi: 10.1053/j.gastro.2013.12.032.
- Jang Y, Kim I. Severe hepatotoxicity after sevoflurane anesthesia in a child with mild renal dysfunction. Paediatr Anaesth. 2005;15(12):1140–1144. doi: 10.1111/j.1460-9592.2005.01648.x.
- Proctor WR, Chakraborty M, Fullerton AM, et al. Thymic stromal lymphopoietin and interleukin-4 mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2014;60(5):1741–1752. doi: 10.1002/hep.27169.
