Abstract
Background
Proactive therapeutic drug monitoring (TDM) is often challenged by long turnaround time when using enzyme-linked immunosorbent assays (ELISAs), especially when analyses are centralised. Point-of-care tests (POCTs) allow rapid assessments, but data on their agreement with existing in-house methodologies are scarce.
Objective
To examine the agreement between a POCT by ProciseDx (San Diego, CA) and the most frequently used in-house ELISA for infliximab (IFX) quantification in Sweden.
Methods
Serum samples were analysed using the in-house ELISA, Karolinska University Hospital, Stockholm, Sweden and a POCT by ProciseDx (San Diego, CA). Agreement was assessed and differences were examined.
Results
Samples from 61 inflammatory bowel disease (IBD) patients were analysed with a median IFX concentration of 7.9 μg/mL (interquartile range (IQR) 5.5–13) for the POCT and 7.9 μg/mL (IQR 5.2–12) for the ELISA (Pearson’s correlation coefficient = 0.95 (95% CI 0.92–0.97, p < .01)). A Passing–Bablok regression yielded an intercept of −0.44 and a slope of 1.09. The Bland–Altman plot showed a systemic bias of −0.77 μg/mL (95% CI −0.18 to −1.4) between the methods. The upper limit of agreement was 3.7 (95% CI 2.7–4.8) (μg/mL), whereas the lower limit agreement was −5.3 (95% CI −6.3 to −4.3) (μg/mL). An excellent reliability was observed, intraclass correlation showed = 0.94 (95% CI 0.89–0.96, p < .0001). When defining IFX concentration as subtherapeutic (<3.0 μg/mL), therapeutic (3.0–7.0 μg/mL) or supratherapeutic (>7.0 μg/mL) drug levels, Kappa statistics showed a substantial agreement (0.79).
Conclusions
The POCT by ProciseDx (San Diego, CA) demonstrated a good agreement with the in-house ELISA, supporting its use for rapid IFX quantification.
Introduction
The introduction of anti-tumour necrosis factor (TNF) agents, such as infliximab (IFX), has changed the treatment landscape and improved outcomes for patients with inflammatory bowel disease (IBD). However, 10–30% of patients do not respond to induction treatment with an anti-TNF agent (primary non-response) and the annual risk of loss of response to IFX per patient year is approximately 10% [Citation1,Citation2]. The precise mechanism for treatment failure is not known, but subtherapeutic drug concentrations and the development of anti-drug antibodies seem to play a critical role [Citation1]. Specifically, low IFX concentrations have been associated with immunogenicity and the formation of anti-drug antibodies [Citation3]. Therapeutic drug monitoring (TDM) is defined as the measurement of drug concentrations and, in patients with low drug concentrations, also anti-drug antibodies. Proactive drug monitoring, i.e., optimising drug dosing regardless of disease activity [Citation4], is increasingly used in IBD care [Citation5]. Studies suggest that proactive TDM could also be beneficial for other immune-mediated disorders, e.g., rheumatoid arthritis and psoriasis [Citation6,Citation7]. The application of proactive TDM is challenged by a considerable lag time between obtaining blood samples and IFX results when using a standard enzyme-linked immunosorbent assay (ELISA) for measurements of IFX concentrations.
In some countries, the measurement of IFX is centralised. In Sweden, most analyses are done using an in-house ELISA at the Department of Clinical Immunology, Karolinska University Hospital, Stockholm, Sweden. Results are obtained after approximately 1–2 weeks, and the method has been described in detail elsewhere [Citation8]. An introduction of point-of-care tests (POCTs) could shorten the turnaround time and overcome logistic obstacles associated with sending blood samples. POCTs such as the ProciseDx (San Diego, CA), using fluorescence resonance energy transfer (FRET), produce results within minutes and allow clinicians to timely individualise the IFX dose based on the concentration. However, the use of different methodologies for IFX measurements raises concerns regarding the agreement between tests since previous studies examining variations across different assays have reported mixed results (Supplementary Table 1). On a national scale, introducing novel assays in combination with the usage of the existing Swedish in-house ELISA could cause uncertainty about comparability and potentially influence clinical decision-making. Therefore, we aimed to compare the agreement between the POCT by ProciseDx for measurements of IFX with the currently used in-house ELISA at Karolinska University Hospital.
Materials and methods
Patients included in this study were previously recruited as part of a prospective cohort of patients treated with biologics at Örebro University Hospital, Örebro, Sweden. To be eligible for inclusion in the cohort, patients had to be aged ≥18 years and have a confirmed diagnosis of IBD, i.e., Crohn’s disease or ulcerative colitis, using internationally accepted criteria [Citation9]. No exclusion criterion was applied in the cohort. After obtaining written informed consent, information on disease characteristics according to the Montreal classification was recorded [Citation10] and body mass index (BMI) was documented. Blood samples were collected before administration of biological therapy and serum aliquots were extracted and stored at −80 °C. Information about demographics, including age, sex, smoking status, concomitant IBD medications and patient-reported outcomes were collected using a study-specific questionnaire. The subscores of patient-reported number of liquid stools per day and abdominal pain (PRO2) derived from the Crohn’s Disease Activity Index were used to characterise clinical disease activity in patients with Crohn’s disease [Citation11]. For patients with ulcerative colitis, the rectal bleeding component from the Mayo Clinic score and the number of bowel movements from the Simple Clinical Colitis Activity Index (SCCAI) were used [Citation12,Citation13]. In the present study, only patients with IFX were included.
POCT for IFX measurements
The POCT developed by ProciseDx (San Diego, CA) has been described in detail previously [Citation14]. In short, the assay is based on FRET. To quantitate IFX levels, a flash of UV light excites a donor fluorophore attached to TNF-alpha and excitation energy is transmitted when it is in the proximity of an acceptor fluorophore. A sandwich assay technique is used; the TNF-alpha labelled fluorophore binds to IFX and, by binding to a separate site of IFX, a Fab anti-IFX with its acceptor fluorophore brings the two fluorophores in close proximity to each other and enables FRET. When the energy is transferred from the donor fluorophore to the acceptor, light emits at specific wavelengths. The light is then filtered through band-pass filters and detected with a silicon multiplier. The intensity of the specific wavelengths is proportional to the amount of donor and acceptor complexes and is reported as μg/mL.
Venous blood samples were collected in serum separator tubes with clot activation. The tubes were then gently inverted five times and left at room temperature for 30–60 min before being centrifuged at 2400 × g for 7 min. Afterwards, serum aliquots were extracted and stored in a −80 °C freezer. In accordance with the manufacturer’s instructions, a high and low control, supplied by ProciseDx, were used prior to analyses. Next, the serum aliquots were thawed at room temperature, and 20 μL of serum was added to the reagent cartridge filled with 1 mL of premade buffer solution. A bead of lyophilised donor and acceptor fluorophores was released into the serum and buffer mix once the cartridge lid was closed. The solution was afterwards mixed by inverting the cartridge five times, and the cartridge was ultimately placed in the apparatus for analysis. All measurements were done within one day using a single operator, and each serum specimen was tested once except for outliers. The total processing time using the POCT was approximately five minutes.
In-house ELISA for IFX measurements
Infliximab concentrations were analysed by an in-house developed ELISA described previously and used in clinical routine [Citation8]. Microtiter plates coated with 100 ng/mL recombinant human TNF-alpha (R&D Systems, Minneapolis, MN) in 0.05 M sodium carbonate buffer for 2 h shaking at room temperature and thereafter incubated overnight at +4 °C. The coating buffer was aspirated and the plates were subsequently incubated with phosphate-buffered saline (PBS) containing 5% sucrose (Sigma, St. Louis, MO) and 1% bovine serum albumin (Sigma, St. Louis, MO), for 2 h at room temperature. After aspiration of the buffer, the plates were dried at room temperature overnight and thereafter stored in heat sealed foil bags with 1 g desiccant at +4 °C. After wash three times in PBS plus 0.05% Tween 20, standard dilutions (0.40–100 ng/mL) of IFX (Schering Plough, Kenilworth, NJ) were added together with defined IFX-spiked sera as well as patient samples in duplicates, diluted 1/500 in blocking buffer (PBS containing 0.05% Tween 20 and 1% bovine serum albumin). Samples were incubated for 1 h at room temperature followed by washing three times. An alkaline phosphatase-conjugated goat anti-human IgG (Fc-specific) (Sigma, St. Louis, MO) diluted 1/10,000 in blocking buffer was thereafter added. After further incubation for an hour at room temperature, the plates were washed three times and substrate (p-nitrophenyl-phosphate, 5 mg/mL in 1 M diethanolamine with 0.5 mM Mg, pH 9.8) was added. Finally, colour development was monitored after 30 min at 405 nm.
Blood samples for the ELISA were obtained simultaneously with the samples for the POCT and handled similarly except for being centrifugated at 2000 × g for 10 min and stored at +4 °C for up to seven days before being sent to Karolinska University Hospital for IFX measurements as part of clinical routine.
Statistical analysis
Descriptive statistics were calculated, and categorical variables were described as absolute numbers (n) and relative frequencies (%). Numerical values were presented as mean or median with standard deviation or interquartile range (IQR) as appropriate depending on visual inspection of the data. A Bland–Altman plot was used to visualise the agreement between the POCT and the in-house ELISA and the intraclass correlation (ICC) two-way mixed single-measures test for absolute agreements was calculated. We interpreted the ICC as follows: <0.5 ‘poor reliability’, 0.5–0.75 ‘moderate reliability’, 0.75–0.9 ‘good reliability’ and >0.90 ‘excellent reliability’ [Citation15]. Pearson’s correlation coefficient was calculated to assess correlation, and since none of the methods are gold standard, Passing–Bablok regression was used. The POCT has a reportable range of 1.7–77 μg/mL, and the reported range of the comparator ELISA was set to <0.5 and 50 μg/mL [Citation8]. For each method, values below the lower limit of detection were substituted with the lower limit of detection/√2 [Citation16]. Agreement using weighted kappa statistics was based on predefined categories of subtherapeutic (<3.0 μg/mL), therapeutic (3.0–7.0 μg/mL) and supratherapeutic (>7.0 μg/mL) drug levels [Citation17]. The interpretation was made as proposed by Landis and Koch [Citation18]. Statistical analysis was done using R (version 4.1.1, R Core Team, Auckland, New Zealand) with ggplot2 (v.3.4.2), mcr (v.1.3.2) and irr (v.0.84.1). All tests were two-tailed, and p values <.05 were considered statistically significant.
Ethical consideration
This study was approved by the Swedish Ethical Review Authority (2021-00920 and 2022-03757-02).
Results
Study population
Demographics and clinical characteristics of the studied population are shown in . In total, 61 patients with IBD (Crohn’s disease, n = 35; ulcerative colitis, n = 26) were included. The mean BMI of the patients was 26 kg/m2, and 36 individuals (59%) were classified as overweight, based on the definition of a BMI >25 kg/m2.
Table 1. Demographics and clinical characteristics of patients with inflammatory bowel disease receiving infliximab treatment (n = 61).
Method comparison
Using the POCT, only one outlier was identified by visual inspection. This sample was tested in duplicate and reported with mean. Overall, the median IFX concentration was 7.9 μg/mL (IQR 5.5–13) for the POCT and 7.9 μg/mL (IQR 5.2–12) for the in-house ELISA. There was a correlation of 0.95 (95% CI 0.92–0.97, p < .01) between the two methods, and a Passing–Bablok regression yielded an intercept of −0.44 and a slope of 1.09 (). A Bland–Altman plot was used to visualise potential differences in reported IFX concentration between the in-house ELISA and the POCT. The bias between the ELISA and the POCT was −0.77 μg/mL (95% CI −0.18 to −1.4). The upper limit of agreement (LOA) was 3.7 (95% CI 2.7–4.8), and the lower LOA was −5.3 (95% CI −6.3 to −4.3) (). Calculation of the ICC showed excellent reliability between the methods, 0.94 (95% CI 0.89–0.96, p < .0001).
Figure 1. Passing–Bablok regression (blue line) with 95% confidence interval (light blue) on infliximab concentrations between the point-of-care test (POCT) based on fluorescence resonance energy transfer (FRET) and an in-house enzyme-linked immunosorbent assay (ELISA). Identity line in black.
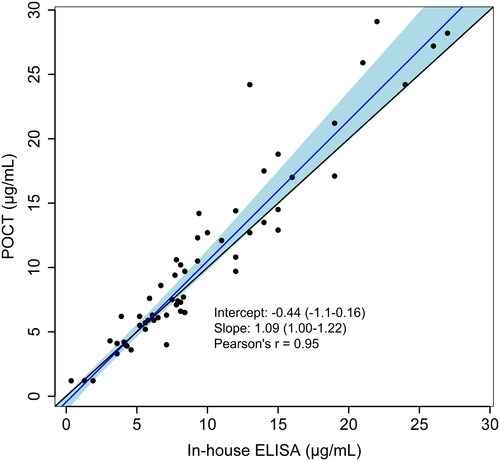
Figure 2. Bland–Altman plot on infliximab concentration between conventional in-house ELISA and point-of-care test device with bias (red line) and limits of agreement (dashed red lines).
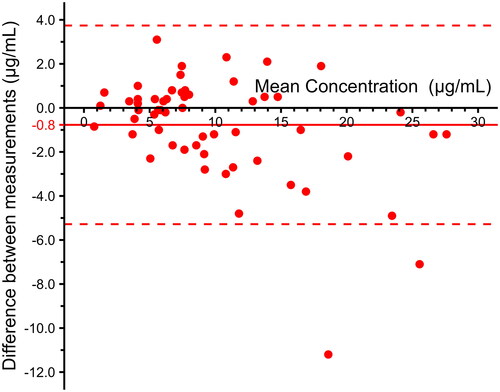
Using Kappa statistics, the results showed a substantial agreement (0.79) between the two methods when measuring the accuracy of specifying IFX concentration as subtherapeutic (<3.0 μg/mL), therapeutic (3.0–7.0 μg/mL) or supratherapeutic (>7.0 μg/mL).
A sensitivity analysis of all samples where at least one of the methods resulted in an IFX concentration of 10 μg/mL or less was performed (Supplementary Figure 1). Compared to the overall analysis, a lower systemic bias (0.3 μg/mL) and narrower limits of agreement (–3.2 to 2.6 μg/mL) were observed.
Discussion
By measuring IFX serum levels in blood samples from IBD patients with ongoing IFX treatment, we demonstrate a reasonably good agreement between one of the currently used methodologies in Sweden, i.e., the in-house ELISA at Karolinska University Hospital, and the POCT by ProciseDx (San Diego, CA). Measurements with the POCT yielded slightly higher concentrations of IFX with a systemic bias of −0.77 μg/mL and limits of agreement indicating pronounced differences in some samples. However, less apparent differences were observed when restricting the comparison to a lower spectrum of IFX concentrations, i.e., <10 μg/mL. Collectively, these results support the introduction of POCTs for IFX measurements and that these assays could be applicable when using proactive TDM in Sweden. Mixed use of the two methodologies is expected to have limited clinical implications for patients with IFX concentrations in the therapeutic range. Still, it may give rise to inconsistent results for patients with high concentrations.
In the late 90s, IFX was introduced for treating Crohn’s disease and, subsequently, for ulcerative colitis [Citation19,Citation20], and the drug is still one of the most commonly used biological agents in IBD. Significant interindividual variations in serum drug concentrations have been observed in patients on IFX treatment [Citation17,Citation21,Citation22], and low IFX concentrations have been reported to correlate with unfavourable treatment outcomes [Citation23]. Therefore, fixed dosing of IFX by weight has been increasingly replaced by TDM in many healthcare systems. As a result, several assays for the measurement of IFX have been developed. However, comparisons of different assays have reported pronounced inter-assay differences, with ICC coefficients ranging between 0.59 and 0.98 and mean differences up to 3.44 μg/mL (Supplementary Table 1) [Citation24]. These inter-assay differences may cause dissimilarities in disease management, and parallel use of different assays may yield contradictive results. Amongst Swedish gastroenterologists, assessment of IFX trough levels during disease relapse is a well-established practice [Citation5], and most analyses are performed at Karolinska Hospital, Stockholm, Sweden, using an in-house ELISA. Even though the assay has been rigorously developed according to guidelines from the Clinical and Laboratory Standards Institute (CLSI) [Citation8], comparative agreement analyses with commercially available tests have not been published.
Recently, results from a Norwegian randomised controlled trial demonstrated that proactive measurements of IFX concentrations and personalised dose adaptation to a target concentration during maintenance therapy improved treatment outcomes in patients with ulcerative colitis and potentially Crohn’s disease [Citation6]. These novel findings support the implementation of proactive dosing of IFX. However, the centralised analysis of IFX concentrations in Sweden hampers timely proactive management, as it usually takes approximately 1–2 weeks to get the results when using the currently available in-house ELISA. Advances in various technologies have led to the development of POCTs, where information about drug concentration is rapidly retrieved. Amongst these tests, the CE-marked device from ProciseDx (San Diego, CA) is recognised for its fast turnaround and ability to analyse both serum and capillary blood [Citation14,Citation25]. As compared to the currently used in-house ELISA in Sweden, measurements with this POCT yielded higher concentrations of IFX with a systemic bias of −0.77 μg/mL. The observed low degree of systemic bias is in accordance with a Portuguese study where a systemic bias of −1.3 μg/mL was observed when comparing the Quantum Blue® POCT with another in-house ELISA [Citation26]. However, the absence of spiked samples with known concentrations of IFX in our analyses and in the Portuguese study limits our ability to make definitive conclusions about the accuracy of these POCTs compared to the two in-house ELISAs. Given that the POCT by ProciseDx (San Diego, CA) but none of the two ELISAs have been calibrated against WHO standards [Citation14,Citation27], the results obtained by the POCT in our study could potentially be more precise. Disagreement between assays is probably most influential if concentrations within or below the therapeutic window differ. A previous study comparing IFX levels measured with an ELISA and a POCT showed relatively good agreement for concentrations <3.0 μg/mL but an overestimation of concentrations >3.0 μg/mL by the POCT [Citation28]. In this study, we observed low systemic bias and narrow LOA between the in-house ELISA and the POCT by ProciseDx for measurements in the range 0–10 μg/mL and kappa values reflecting substantial agreement when concentrations were categorised as subtherapeutic, therapeutic and above the therapeutic window. Even though these findings are reassuring for IBD patients on IFX treatment, introducing POCT-based IFX dosing may be cumbersome for patients requiring higher trough levels. Specifically, patients with fistulising Crohn’s disease seem to benefit from trough levels >10 μg/mL [Citation29] and transitioning from the currently used ELISA to POCT-based measurements must be done with caution in these patients.
The current study has several strengths but also some weaknesses. We analysed samples from a real-world cohort of well-characterised patients with a low reported symptom burden during maintenance IFX therapy, supporting that the results can be generalised to other cohorts of patients on IFX maintenance treatment. The ELISA-based analyses of IFX were performed in clinical routine, and the person who did the POCT measurements was blinded to the results until comparisons were performed. The major limitation of our study lies in the analysis of serum and not capillary blood with the POCT. The measurement of IFX in capillary blood holds the potential to streamline the implementation of this method in clinical practice. In contrast, our utilisation of serum samples may not translate to the ease and efficiency that capillary blood analysis could offer. Other limitations include the non-identical handling of serum samples and that we did not examine potential batch effects, as all the analyses with the POCT were done within one day and using the same batch. However, an intra-assay coefficient of variation of 2.7% and an inter-assay coefficient of variation of <2% for the POCT have previously been reported [Citation14]. Also, we did not measure external factors, e.g., humidity and temperature, that could potentially interfere with the analyses.
Conclusions
In summary, we demonstrate a good agreement between the currently used methodology for measuring IFX concentrations in Sweden, i.e., the in-house ELISA at the Department of Clinical Immunology, Karolinska Hospital, Stockholm, Sweden, and the POCT by ProciseDx (San Diego, CA). Our data support the use of this POCT as an alternative option for analysing IFX concentrations in clinical practice, particularly in IBD patients with sub-therapeutic or therapeutic IFX levels. Introducing POCTs for measuring IFX could potentially facilitate the implementation of proactive TDM.
Author contributions
Guarantor: JH had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design, acquisition of data, drafting of the manuscript, and statistical analysis: JT and JH. Interpretation of data and critical revision of the manuscript for important intellectual content: all authors.
Ethical approval
This study was approved by the Swedish Ethical Review Authority (registration number 2021-00920 and 2022-03757-02) on 2022-07-11.
| Abbreviations | ||
| TDM | = | therapeutic drug monitoring |
| ELISA | = | enzyme-linked immunosorbent assay |
| POCT | = | point-of-care test |
| IFX | = | infliximab |
| IBD | = | inflammatory bowel disease |
| IQR | = | interquartile range |
| CI | = | confidence interval |
| TNF | = | tumour necrosis factor |
| FRET | = | fluorescence resonance energy transfer |
| BMI | = | body mass index |
| SCCAI | = | Simple Clinical Colitis Activity Index |
| PBS | = | phosphate-buffered saline |
| ICC | = | intraclass correlation |
| LOA | = | limits of agreement |
| SD | = | standard deviation |
| CLSI | = | Clinical and Laboratory Standards Institute |
| WHO | = | World Health Organisation |
Supplemental Material
Download Zip (370.2 KB)Acknowledgements
Rui Rodrigues, MD, Clinical Immunology and Transfusion Medicine, Karolinska University Hospital is acknowledged for providing support regarding method description of in-house IFX analysis.
Disclosure statement
JT has nothing to declare. OG has nothing to declare. DB received lecture fee/advisory board from BMS, Janssen, Pharmacosmos, Takeda, Pfizer and Sandoz. CE received grant support/lecture fee/advisory board from Takeda, Janssen Cilag, Pfizer and AbbVie. IV has served as a speaker for Takeda. ME: received honoraria for lectures and consultancy from AbbVie, Merck (MSD), Takeda, Ferring, Orion Pharma, Otsuka, Tillotts, ITH, Novartis, Pfizer, Celltrion, Galapagos, Bristol Myers Squibb and Janssen, and received research funding from AbbVie, Tillotts and MSD. JH has served as a speaker, consultant or advisory board member for: AbbVie, Aqilion, BMS, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Ferring, Galapagos, Gilead, Index Pharma, Janssen, Lincs, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and received grant support from Janssen, MSD and Takeda.
Data availability statement
No additional data are available due to the Swedish data protection regulation.
Additional information
Funding
References
- Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi: 10.1038/ctg.2015.63.
- Savelkoul EHJ, Thomas PWA, Derikx LAAP, et al. Systematic review and meta-analysis: loss of response and need for dose escalation of infliximab and adalimumab in ulcerative colitis. Inflamm Bowel Dis. 2023;29(10):1633–1647. doi: 10.1093/ibd/izac200.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353. doi: 10.1016/S2468-1253(19)30012-3.
- Papamichael K, Afif W, Drobne D, et al. Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol Hepatol. 2022;7(2):171–185. doi: 10.1016/S2468-1253(21)00223-5.
- Bjørlykke KH, Jahnsen J, Brynskov J, et al. Therapeutic drug monitoring in inflammatory bowel disease: implementation, utilization, and barriers in clinical practice in Scandinavia. Scand J Gastroenterol. 2023;58(1):25–33. doi: 10.1080/00365521.2022.2108684.
- Syversen SW, Jørgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases. JAMA. 2021;326(23):2375–2384. doi: 10.1001/jama.2021.21316.
- Pfeiffer-Jensen M, Liao D, Tarp U, et al. Reduced prescription of TNF-inhibitors in chronic arthritis based on therapeutic drug monitoring: a randomized controlled trial. Scand J Rheumatol. 2023;52(5):468–480. doi: 10.1080/03009742.2022.2121081.
- Marits P, Landucci L, Sundin U, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohns Colitis. 2014;8(8):881–889. doi: 10.1016/j.crohns.2014.01.009.
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164. doi: 10.1093/ecco-jcc/jjy113.
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl. A):5A–36A. doi: 10.1155/2005/269076.
- Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41(1):77–86. doi: 10.1111/apt.13001.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603.
- Walmsley RS, Ayres RCS, Pounder RE, et al. A Simple Clinical Colitis Activity Index. Gut. 1998;43(1):29–32. doi: 10.1136/gut.43.1.29.
- Ong E, Huang R, Kirkland R, et al. Therapeutic drug monitoring: performance of a FRET-based point-of-care immunoassay for the quantitation of infliximab and adalimumab in blood; 2020. Available from: doi: 10.26434/chemrxiv.13277321.v2.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012.
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. doi: 10.1080/1047322X.1990.10389587.
- Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–1329.e3. doi: 10.1053/j.gastro.2015.02.031.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310.
- Remicade ema.europa.eu: European Medicines Agency; 2022 [updated 2022 Sep 26; cited 2022 Nov 27]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/remicade
- Kornbluth A. Infliximab approved for use in Crohn’s disease: a report on the FDA GI Advisory Committee Conference. Inflamm Bowel Dis. 1998;4(4):328–329. doi: 10.1097/00054725-199811000-00014.
- Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57(8):929–942. doi: 10.1007/s40262-017-0627-0.
- Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211–1228. doi: 10.1007/s00228-009-0718-4.
- Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655–1668.e3. doi: 10.1016/j.cgh.2019.03.037.
- Vande Casteele N. Assays for measurement of TNF antagonists in practice. Frontline Gastroenterol. 2017;8(4):236–242. doi: 10.1136/flgastro-2016-100692.
- Volkers A, Löwenberg M, Braad M, et al. Validation study of novel point-of-care tests for infliximab, adalimumab and C-reactive protein in capillary blood and calprotectin in faeces in an ambulatory inflammatory bowel disease care setting. Diagnostics. 2023;13(10):1712. doi: 10.3390/diagnostics13101712.
- Afonso J, De Sousa HT, Rosa I, et al. Therapeutic drug monitoring of CT-P13: a comparison of four different immunoassays. Therap Adv Gastroenterol. 2017;10(9):661–671. doi: 10.1177/1756283X17722915.
- WHO International Standard 1st International Standard for Infliximab. World Health Organization; 2019 [cited 2023 May 16]. Available from: https://www.nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=16/170
- Nasser Y, Labetoulle R, Harzallah I, et al. Comparison of point-of-care and classical immunoassays for the monitoring infliximab and antibodies against infliximab in IBD. Dig Dis Sci. 2018;63(10):2714–2721. doi: 10.1007/s10620-018-5144-y.
- Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45(7):933–940. doi: 10.1111/apt.13970.
