Abstract
Objectives
It is thought that esophageal hypersensitivity in combination with an impaired mucosal barrier function contributes to PPI-resistant reflux symptoms. Ziverel, a bioadhesive agent that coats the esophageal wall, was shown to have a positive effect on reflux symptoms. However, the mechanisms of action are unclear. We aimed to assess the effect of Ziverel on esophageal sensitivity to acid and mucosal barrier function.
Methods
We performed a double-blind randomized placebo-controlled crossover trial in PPI-refractory patients with reflux symptoms. Patients were assigned (1:1) to 14 days of Ziverel followed by 14 days of placebo or opposite treatment order. The effect was evaluated using acid perfusion tests, an upper endoscopy with electrical tissue impedance spectroscopy (ETIS) and esophageal biopsies. The primary outcome was the esophageal sensitivity based on perfusion sensitivity score. Secondary outcomes included mucosal barrier function and reflux symptoms and correlations between the different outcomes.
Results
Perfusion sensitivity score was not significantly different during treatment with Ziverel (106 (73–115)) and placebo (102 (67–110)) (p = 0.508) along with total RDQ score (2.6 (1.9–3.3) vs 2.8 (1.6–3.5) p = 0.456). ETIS showed comparable values during treatment with Ziverel (13514 (8846–19734)Ω·m) and placebo (13217 (9127–24942)Ω·m (p = 0.650)). Comparing Ziverel and placebo no difference was seen in transepithelial electrical resistance (TEER) 203 (163–267) Ω.cm2 vs 205 (176–240) Ω.cm2 (p = 0.445) and fluorescein flux 775 (17–6964) nmol/cm2/h vs 187 (4–12209) nmol/cm2/h (p = 0.638).
Conclusion
Ziverel did not show a benefit on acid sensitivity, reflux symptoms or esophageal mucosal integrity compared to placebo in PPI-refractory patients with reflux symptoms.
Trial registration: Netherlands Trial Register number: NL7670
Keywords:
Introduction
Gastroesophageal reflux disease (GERD) stands as a prominent gastrointestinal condition in the Western world [Citation1]. Managing GERD predominantly relies on proton pump inhibitors (PPIs), which effectively resolve mucosal lesions in most patients [Citation2]. However, approximately one third of patients with both erosive and non-erosive GERD continues to experience reflux symptoms, despite daily PPI use [Citation3,Citation4]. The etiology of refractory GERD is complex, and esophageal hypersensitivity is thought to contribute to reflux perception, playing a role in PPI-resistant symptoms [Citation5–7]. Normally, diffusion of luminal contents into the mucosa is prevented by the apical membranes and tight junctions of esophageal epithelial cells [Citation8]. However, prolonged acid and weak acid reflux may result in microscopic damage to the esophageal epithelium, leading to impairment of the mucosal barrier function and allowing acid penetration into the submucosal layer resulting in afferent nerves activation [Citation6,Citation9]. As a result, impaired mucosal barrier function may represent a potential target for intervening in reflux disease.
Ziverel is a formulation of hyaluronic acid and chondroitin sulfate in combination with a highly bioadhesive agent (poloxamer 407) that coats the esophageal wall and thereby acts as a physical barrier to reflux [Citation10]. Furthermore, due to its adhesive properties, poloxamer 407 prolongs the effects of both hyaluronic acid and chondroitin sulfate; two glycosaminoglycans with the biological ability to stimulate tissue repair and regeneration [Citation11,Citation12]. It is hypothesized that Ziverel tackles esophageal hypersensitivity by protecting the mucosal barrier function. Three randomized-controlled trials indeed demonstrated that Ziverel combined with PPIs provided a superior control of reflux symptoms compared to placebo in both patients with erosive [Citation13] and non-erosive [Citation14,Citation15] reflux disease. Also, an ex vivo study in pigs has shown that Ziverel prevents acid perfusion-induced mucosal barrier damage in the esophagus [Citation16]. More information on efficacy and mechanisms of action of Ziverel is warranted. We aim to assess the effect of Ziverel on esophageal sensitivity to acid, mucosal barrier function and reflux symptoms in patients with PPI-refractory reflux disease and to correlate changes in sensitivity, barrier function, and symptom perception.
Methods
Study design
We performed a single-center, double-blind, randomized placebo-controlled, crossover trial in patients with PPI-refractory reflux symptoms between 02 September 2019 and 20 January 2022. The study was conducted according to the principles of the Declaration of Helsinki, complied with Good Clinical Practice (GCP) and the Dutch Act on Medical Research Involving Human Subjects (WMO). The local Medical Ethics Committee approved the study (2018#B201960) on the 25 January 2019. Written informed consent was obtained from all patients before study participation. The study was prospectively registered in the Dutch trial registry (Trial NL7670, trialregister.nl). All authors had access to the complete study data and reviewed and approved the final manuscript.
Patient selection
We included patients with typical reflux symptoms (acid regurgitation and/or heartburn) under PPI treatment for at least 3 months. Exclusion criteria included the use of other medication than PPI with an effect on gastrointestinal sensitivity, motility or secretion that could not be stopped for the duration of the study, the presence of other gastroesophageal diseases including Barrett’s esophagus or a history of gastric or esophageal surgery. Women who were pregnant, breastfeeding, or fertile (not using contraception) were also excluded.
Study protocol and randomization
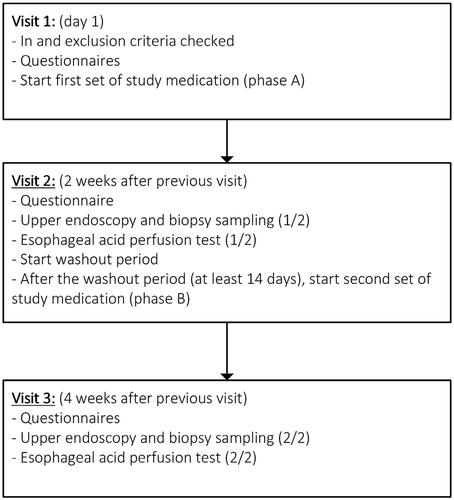
Patients were allocated randomly in a double-blind fashion in a 1:1 ratio to one of two treatment groups. The trial pharmacist at the hospital conducted the randomization process. The first group was treated with Ziverel (4 sachets of 10 mL daily) for 14 days, followed by a second 14-day period in which patients received placebo. The second set of study medication was started after a washout period of at least 14 days. In the second group, the treatment order was reversed. At the end of each 14-day treatment period questionnaires were filled in and endoscopy and the modified Bernstein test to evaluate esophageal sensitivity for acid were performed. Patients continued 2 times daily standard dose of PPI for the entire duration of the study. A schematic study outline can be found in .
Investigational product
The investigation product is Ziverel. Ziverel is a formulation of hyaluronic acid and chondroitin sulfate in combination with a highly bioadhesive agent (poloxamer 407). The placebo was indistinguishable from Ziverel due to similar organoleptic characteristics. Labeled study medication was provided by Norgine, Amsterdam, The Netherlands. Ziverel and placebo were delivered at the AMC Clinical Pharmacy, where they were stored and dispensed. The dosage (40 mL/day) and duration (14 days) were based on the results of the two previous randomized controlled trials evaluating Ziverel and its effect on symptoms in patients with gastroesophageal reflux [Citation14,Citation15].
Esophageal acid perfusion (esophageal sensitivity)
The modified Bernstein procedure was used to evaluate the esophageal sensitivity. A water-perfused manometry catheter was passed transnasally into the esophagus. The catheter was positioned in a manner whereby one of the side holes of the catheter was 5 cm above the lower esophageal sphincter (LES). After an adaptation period of 5 min, saline was infused into the esophagus through the channel of the catheter corresponding to the side hole 5 cm above the LES for 5 min, followed by 0.1 M of hydrochloric acid (pH 1.1) for a period of 15 min. When retrosternal discomfort or pain was reported the test was stopped earlier. Both saline and hydrochloric acid were infused at a rate of 8 mL/min. Patients were unaware of the nature of the solution infused. Retrosternal sensations during the acid perfusion test were rated on a visual analogue pain rating scale (VAS) and the time to first perception, discomfort and/or pain were noted. In addition, the perfusion sensitivity score (PSS) was calculated; ((total acid perfusion time − lag time to discomfort) × maximum VAS) [Citation17]
Symptom scores
Patients completed the standardized validated reflux-disease questionnaire (RDQ) [Citation18]. The RDQ consists of 12 questions assessing the severity and frequency of 3 GERD-related symptom domains (heartburn, regurgitation and epigastric pain). The mean of three dimensions gives a score ranging from 0 to 5. Furthermore, after both phase A and B, patients were asked two additional questions about the average duration of effect of a single dose and whether recurrence of symptoms interrupted sleep.
Measuring esophageal mucosal barrier function
Electrical tissue impedance spectroscopy (ETIS)
Electrical tissue impedance spectroscopy (ETIS) is an instrument used for the in vivo assessment of tissue impedance during endoscopy, it produces a measure of mucosal integrity. A four-electrode-tipped probe (Medical Engineering Section, Royal Hallamshire Hospital, Sheffield, UK) was advanced through the working channel of an endoscope to perform ETIS of esophageal mucosa. In our center, ETIS was previously used to study mucosal barrier function in GERD and eosinophilic esophagitis [Citation19–21].
Ussing chamber experiments
For the purposes of this study, six biopsies were obtained during upper endoscopy using a Jumbo biopsy forceps. Four biopsies were used for Ussing chamber experiments. The biopsies were taken at 5 cm from the Z-line in a four-quadrant fashion from macroscopically normal mucosa. Immediately after collection, the biopsies were stored in oxygenated Meyler buffer on ice. Within 15 min the biopsies were transferred to the Ussing chambers. In the Ussing chambers the biopsies were submerged in oxygenated modified Meyler buffer, kept at a temperature of 37 °C. The modified Meyler buffer consists of; 105 mM NaCl, 4.7 mM KCl, 1.3 mM CaCL2·2H2O, 1.0 mM MgCl2·6H2O, 20.0 mM NaHCO3, 0.4 mM NaH2PO4·H2O, 0.3 mM Na2HPO4·2H2O, and 10.0 mM HEPES. The pH of the solution was adjusted to 7.4, and it was continuously bubbled with carbogen gas (95% O2-5% CO2).
The tissue was short-circuited using two sets of electrodes connected to a dual voltage clamp (World Precision Instruments, Berlin, Germany) This allowed us to directly measure the voltage deflection caused by a constant bipolar current of 20 µA. Thus, using Ohm’s law, the transepithelial electrical resistance can be computed. Throughout the experiment the TEER was measured every 15 min, these values were averaged for each patient. To determine the paracellular permeability for small molecules, fluorescein (376 Da, 0.5 mg/mL) was added to the mucosal side of the biopsy and was measured at the serosal side. Samples from the serosal side were collected every 15 min for the duration of the experiment; 90 min. The transepithelial flux of the fluorescent marker was measured with a CLARIOstar analyzer (BMG‐labtech, Offenburg, Germany) and is used to determine paracellular permeability.
Statistical analysis
Sample size
For calculation of the sample size a prospective observational study in which the esophageal acid sensitivity was assessed in 13 patients with GERD who were dependent on PPIs was used [Citation22]. Esophageal acid sensitivity was measured before and after a radiofrequency energy delivery procedure. Since we were interested in esophageal acid sensitivity in GERD (both erosive and non-erosive) patients on PPIs, our calculation was based on information in the patient group before this procedure. As there is no previous data regarding PSS in our specific patient population, we based our calculations on the mean lag time to heartburn perception, a variable incorporated in the PSS. Authors found, during acid perfusion, a mean lag time to heartburn perception of 9.5 min with a standard deviation of 2.3 min. An improvement in mean lag time of 20% was considered as the minimal clinically important difference. Using this percentage, a paired two-tailed T-test with a power of 90% and a significance level of 5%, a sample size of 18 subjects was calculated. Taking into account a lost to follow-up percentage of 20%, the total number of subjects required for our study is 22 subjects.
Endpoint definition
The primary endpoint is esophageal sensitivity based on perfusion sensitivity score. Secondary endpoints included time to acid perception, discomfort or pain, maximum VAS-score during the esophageal acid perfusion test, gastroesophageal reflux symptoms, esophageal barrier function based on electrical tissue impedance during endoscopy, transepithelial electrical resistance and fluorescein flux measured during Ussing chamber experiments.
Statistical analysis
Descriptive statistics were presented as percentage for categorical data and as mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables. Analysis was performed using the paired Student t-test for parametric data and the Wilcoxon signed rank test for non-parametric data. The log-rank test was used to compare lag times to perception, discomfort and pain. The correlation between fluorescein influx (permeability), PSS score (sensitivity) and RDQ-GERD (symptoms) data was tested using the Spearman’s correlation test. A p-value of <0.05 was considered significant. SPSS statistics (version 27; SPSS, Chicago, Illinois, USA) was used for statistical analysis.
Results
Patient characteristics
A total of 15 patients (3 men, median age 48 (33-63)) of the planned 22 were included in the study. During the COVID pandemic the study was temporarily put on hold and the study medication expired. This led to termination of the study prior to completion. Seven patients were randomized to receive Ziverel followed by placebo, 8 patients received placebo followed by Ziverel. One patient withdrew consent after the first treatment period and was only treated with Ziverel. All patients reported symptoms of heartburn and/or acid regurgitation under stable PPI treatment for at least 3 months. All patients used a standard b.i.d. dose of a PPI prior to and during the study period. At baseline the duration of reflux symptoms was 60 (14-98) months and a RDQ-score of 2.67 (2.25–3.75) was reported. The main baseline parameters can be found in .
Table 1. Baseline characteristics of included patients (n = 15).
Esophageal acid sensitivity
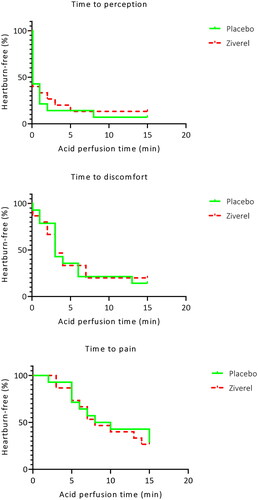
The esophageal acid sensitivity test resulted in the perception of acid in 13 out of 15 patients (86.7%). The median time to acid perception was comparable during Ziverel and placebo treatment, 0 (0–1.5) minutes and 0 (0–1) minutes (p = 0.752). Twelve out of 15 patients (80%) experienced discomfort with a comparable median time to discomfort of 3 (1.25–4) minutes during Ziverel and 3 (1.5–5.5) minutes during placebo treatment (p = 0.672), as shown in . Pain was reported by 11 of 15 patients during treatment with Ziverel and by 10 of 15 patients during treatment with placebo. The median time to pain did not significantly differ during these treatment periods; 7 (5–10) minutes and 6.5 (5–11.25) minutes (p = 0.438). No significant difference in maximum VAS was reported during Ziverel treatment 8.1 (6.7–8.6) and placebo treatment 8.1 (6.3–8.9) (p = 0.925). In addition, the perfusion sensitivity score (PSS) was not significantly different during treatment with Ziverel 106 (73–115) and placebo 102 (67–110) (p = 0.508).
Questionnaire
Reflux symptoms based on total RDQ score did not significantly differ during Ziverel treatment vs placebo treatment; 2.6 (1.9–3.3) vs 2.8 (1.6–3.5). In addition, all RDQ subscores (heartburn, regurgitation, GERD and dyspepsia) showed no significant difference, as shown in .
Table 2. RDQ score and subscores after 4 weeks of treatment.
Patients reported a median effect duration of a single dose of Ziverel of 1 (0–18) minute and a duration of 8 (0–60) minutes of placebo (p = 0.563).
Esophageal mucosal integrity
Analysis of extracellular in vivo tissue impedance (ETIS) showed comparable values during Ziverel treatment (13514 Ω·m (8846–19734) and placebo treatment (13217 Ω·m (9127–24942) p = 0.650. In vitro transepithelial electrical resistance (TEER) did not significantly differ during Ziverel and placebo treatment (median (IQR) 203 (163–267) Ω.cm2 and 205 (176–240) Ω.cm2 respectively (p = 0.445). Furthermore, no difference in paracellular permeability measured by fluorescein flux was seen during Ziverel (775 (17–6964) nmol/cm2/h) and placebo (187 (4–12209) nmol/cm2/h) (p = 0.638) treatment.
Post hoc analysis
A post hoc analysis did not show a statistically significant linear relationship between fluorescein influx (permeability) and PSS score (sensitivity) during Ziverel use (r=.130, p = 0.687). No significant linear relationship was seen either between fluorescein influx and RDQ-GERD score (r=.335, p = 0.222). Moreover, no significant linear relationship between PSS score and RDQ-GERD score was present (r=.046, p = 0.887).
Adverse events
During the course of the trial, no serious adverse events occurred. Two adverse events took place. Worsening of reflux symptoms during treatment with Ziverel was reported by one patient. Another patient experienced pain after endoscopy at the end of the Ziverel treatment period. Both complaints resolved without intervention. Informed consent was withdrawn by one patient during the study due to personal reasons.
Discussion
In this study, we found that Ziverel showed no significant additional effect over placebo on esophageal acid sensitivity, occurrence of reflux symptoms or mucosal integrity. We hypothesized that Ziverel would provide symptom relief in patients with refractory symptoms under PPI treatment and had the ability to reduce the permeability of injured mucosa. This hypothesis is based on previous studies showing symptom relief in patients with non-erosive reflux disease (NERD) when Ziverel was added to PPI therapy and an animal study [Citation14–16] In our trial we were not able to reproduce these results. One of the factors may be related to the characteristics of the patients included in this study. Inclusion was based on the presence of reflux symptoms only, no objective measurements were done prior to inclusion. During the study, none of the patients had erosive esophagitis so all patients suffered from non-erosive reflux disease. Our research group has previously described that paracellular permeability is significantly higher in erosive esophagitis patients, but not in NERD patients, compared with healthy controls [Citation23]. It could be hypothesized that the effect of Ziverel on tissue repair is less evident and more difficult to measure in NERD patients compared to GERD patients. When comparing our ETIS results to previously reported ETIS values from our center a difference is seen. The earlier measured median/mean ETIS values in patients with reflux disease were 5621 (3299) Ω.m and 8834 (2542) Ω.m in healthy volunteers. We found higher ETIS values in both treatment groups (Ziverel (13514 Ω·m (8846–19734) and placebo (13217 Ω·m (9127–24942)). This is most likely caused by the characteristics of the gel in both the verum and the placebo [Citation24]. A baseline measurement without Ziverel or placebo treatment would have provided us with more insight into this theory.
Furthermore, the two randomized controlled trials [Citation14,Citation15] with Ziverel that were conducted prior to this trial focused exclusively on reflux symptoms to measure effect. In these trials Ziverel was combined with PPI to treat reflux disease patients. In the ex vivo study [Citation16] Ziverel monotherapy was used to evaluate the effect on mucosal barrier damage. In our trial the effect of Ziverel on mucosal barrier function was tested in patients who are on concomitant PPI therapy. As previously mentioned PPIs are able to resolve mucosal lesions in the majority of GERD patients [Citation2]. It is possible that, in the patients who participated in our trial, the microscopic mucosal lesions that impair mucosal integrity had already been healed by PPI therapy. Therefore the effect of Ziverel seen in the previously performed trials could have a different mechanism of action than hypothesized.
Some limitations to this study must be acknowledged. First, this trial was terminated prior to full completion. Due to a temporary halt during the COVID pandemic resulting in expired study medication, it was not possible to treat all planned patients, therefore this trial may be underpowered. Another limitation is related to patient selection; patient selection was based on symptoms rather than objective measurements such as 24-h pH-impedance monitoring or esophagitis during endoscopy.
One of the strengths of this trial is the fact that an array of in and ex vivo modalities were combined to fully evaluate the effect of Ziverel on the mucosal barrier function, including the Ussing chamber technique. The Ussing chamber is still considered the gold standard to determine intestinal barrier function including epithelium permeability and has the potential to diligently measure changes [Citation25]. Given that no effect was seen with any of these investigational techniques, we can be confident that a strong effect is not present.
Conclusion
Ziverel did not show a benefit on acid sensitivity, reflux symptoms or esophageal mucosal integrity compared to placebo in PPI-refractory patients with reflux symptoms.
Specific author contributions
RON, RW, AS and AB played a role in planning of the study. RON, JO and TK had a role in conducting the study. RON and TK were involved in the acquisition of data. RON, TK and AB had a role in collecting and/or interpreting data. RON and TK played a role in drafting the manuscript. RW, AS and AB played a role in reviewing and revising the manuscript for important intellectual content. All authors approved the final draft submitted.
| Abbreviations | ||
| ETIS | = | extracellular in vivo tissue impedance |
| GERD | = | gastroesophageal reflux disease |
| LES | = | lower esophageal sphincter |
| NERD | = | non-erosive reflux disease |
| PPI | = | proton pump inhibitor |
| PSS | = | perfusion sensitivity score |
| RDQ | = | reflux disease questionnaire |
| TEER | = | transepithelial electrical resistance |
| VAS | = | visual analogue scale |
Disclosure statement
RON, RW, AS and TK have no financial or personal competing interests. AB received research funding from Nutricia, Norgine, SST, Thelial, Sanofi, Dr Falk Pharma and Bayer and received speaker and/or consulting fees from Laborie, Medtronic, Dr. Falk Pharma, Calypso Biotech, Alimentiv, Regeneron/Sanofi, AstraZeneca. JO received speaker and consulting fees from Laborie.
Additional information
Funding
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880. doi: 10.1136/gutjnl-2012-304269.
- Chiba N, De Gara CJ, Wilkinson JM, et al. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112(6):1798–1810. doi: 10.1053/gast.1997.v112.pm9178669.
- Sigterman KE, van Pinxteren B, Bonis PA, et al. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;2013(5):CD002095.
- Weijenborg PW, Cremonini F, Smout AJ, et al. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24(8):747–757, e350. doi: 10.1111/j.1365-2982.2012.01888.x.
- Farre R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60(7):885–892. doi: 10.1136/gut.2010.233049.
- Woodland P, Lee C, Duraisamy Y, et al. Assessment and protection of esophageal mucosal integrity in patients with heartburn without esophagitis. Am J Gastroenterol. 2013;108(4):535–543. doi: 10.1038/ajg.2012.469.
- Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61(9):1340–1354. doi: 10.1136/gutjnl-2011-301897.
- Tobey NA, Carson JL, Alkiek RA, et al. Dilated intercellular spaces: a morphological feature of acid reflux–damaged human esophageal epithelium. Gastroenterology. 1996;111(5):1200–1205. doi: 10.1053/gast.1996.v111.pm8898633.
- Farre R, Fornari F, Blondeau K, et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut. 2010;59(2):164–169. doi: 10.1136/gut.2009.194191.
- Tang M, Dettmar P, Batchelor H. Bioadhesive oesophageal bandages: protection against acid and pepsin injury. Int J Pharm. 2005;292(1-2):169–177. doi: 10.1016/j.ijpharm.2004.11.039.
- Volpi N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule. Inflammopharmacology. 2011;19(6):299–306. doi: 10.1007/s10787-011-0098-0.
- Volpi N, Schiller J, Stern R, et al. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16(14):1718–1745. doi: 10.2174/092986709788186138.
- Palmieri B, Corbascio D, Capone S, et al. Preliminary clinical experience with a new natural compound in the treatment of oesophagitis and gastritis: symptomatic effect. Trends Med. 2009;9(4):219–225.
- Palmieri B, Merighi A, Corbascio D, et al. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci. 2013;17(24):3272–3278.
- Savarino V, Pace F, Scarpignato C,. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017;45(5):631–642. doi: 10.1111/apt.13914.
- Di Simone MP, Baldi F, Vasina V, et al. Barrier effect of esoxx((R)) on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012;5:103–107. doi: 10.2147/CEG.S31404.
- Fass R, Pulliam G, Johnson C, et al. Symptom severity and oesophageal chemosensitivity to acid in older and young patients with gastro-oesophageal reflux. Age Ageing. 2000;29(2):125–130. doi: 10.1093/ageing/29.2.125.
- Aanen MC, Numans ME, Weusten BL, et al. Diagnostic value of the reflux disease questionnaire in general practice. Digestion. 2006;74(3–4):162–168. doi: 10.1159/000100511.
- van Hoeij FB, Weijenborg PW, van den Bergh Weerman MA, et al. Mucosal integrity and sensitivity to acid in the proximal esophagus in patients with gastroesophageal reflux disease. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G117–22. doi: 10.1152/ajpgi.00134.2016.
- Weijenborg PW, Rohof WO, Akkermans LM, et al. Electrical tissue impedance spectroscopy: a novel device to measure esophageal mucosal integrity changes during endoscopy. Neurogastroenterol Motil. 2013;25(7):574–578. e457-8. doi: 10.1111/nmo.12106.
- Warners MJ, van Rhijn BD, Verheij J, et al. Disease activity in eosinophilic esophagitis is associated with impaired esophageal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2017;313(3):G230–G238. doi: 10.1152/ajpgi.00058.2017.
- Arts J, Sifrim D, Rutgeerts P, et al. Influence of radiofrequency energy delivery at the gastroesophageal junction (the stretta procedure) on symptoms, acid exposure, and esophageal sensitivity to acid perfusion in gastroesophagal reflux disease. Dig Dis Sci. 2007;52(9):2170–2177. doi: 10.1007/s10620-006-9695-y.
- Weijenborg PW, Smout AJ, Verseijden C, et al. Hypersensitivity to acid is associated with impaired esophageal mucosal integrity in patients with gastroesophageal reflux disease with and without esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(3):G323–9. doi: 10.1152/ajpgi.00345.2013.
- Wong U, Person EB, Castell DO, et al. Improving high-resolution impedance manometry using novel viscous and super-viscous substrates in the supine and upright positions: a pilot study. J Neurogastroenterol Motil. 2018;24(4):570–576. doi: 10.5056/jnm18010.
- Clarke LL. A guide to ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1151–66. doi: 10.1152/ajpgi.90649.2008.