Abstract
Background
Fewer adenomas are detected at colonoscopy in women compared to men and failure to detect adenomas and sessile serrated polyps is associated with an increased risk of post-colonoscopy colorectal cancer. The aim of this study was to investigate whether this was in part due to the greater difficulty of conducting colonoscopy in women, with the difference being more apparent in colonoscopies conducted by less skilled endoscopists.
Material and methods
Cross-sectional exploratory analysis of data on 16,551 individuals undergoing a primary colonoscopy (PCOL group) or colonoscopy after positive faecal immunochemical test (FIT group) within the randomized controlled trial SCREESCO. Endoscopist adenoma detection rate (ADR; low or high) was determined based on each endoscopist’s colonoscopies performed in SCREESCO. In each study group, the relationship between the sex difference in colonoscopy outcome and endoscopist ADR was assessed using multiplicative interaction tests.
Results
Endoscopists performed equally many colonoscopies in men and women (median 52% men). There were no signs of effect modification of the risk ratio of any finding (men vs women) by endoscopist ADR in the PCOL group (p = 0.33) or the FIT group (p = 0.30). The proportion of incomplete index colonoscopies was lower in men than in women in both groups and there was no effect modification by endoscopist ADR in either the PCOL group (p = 0.41) or the FIT group (p = 0.96).
Conclusions
This study provides no evidence that endoscopist skill measured by ADR underlies the sex difference in adenoma detection at colonoscopy. This study has trial number NCT02078804 and is registered with ClinicalTrials.gov.
Introduction
A colonoscopy is the final step in the great majority of colorectal screening programs and equal opportunities for men and women to have a significant lesion detected by colonoscopy is an important public health issue in countries that promote colorectal cancer screening. The adenoma detection rate (ADR) tends to be higher in men than women [Citation1–6] and the age-standardized incidence of colorectal cancer (CRC) internationally with 21.1 vs 15.7 cases in men vs women per 100 000 in 2018 [Citation7] and in most European countries, e.g. around 50 vs 40 cases per 100 000 in Sweden during 2000–2016 [Citation8]. Thus, a higher yield of malignant and pre-malignant lesions in men than in women is expected in a screening program. There are, however, other important differences between men and women that may influence different diagnostic yields by colonoscopy. Colonoscopy can be technically more difficult in women than men [Citation1,Citation4], and women have an increased need for analgesia and sedation [Citation9,Citation10], leading to a different probability of complete examination [Citation1]. A considerable variation in colonoscopy quality among different endoscopists has been demonstrated in several countries [Citation5,Citation6,Citation11,Citation12]. Endoscopist performance may affect male and female screenees differently given the more technically challenging procedure in women. Current guidelines stipulate that endoscopist ADR should be at least 25% and current cecum intubation rate target standard is at least 95% [Citation13].
In a study based on SCREESCO (SCREEning of Swedish COlons), a randomized controlled screening study with two intervention groups, two rounds of biennial high-sensitive FIT testing two years apart or once-only colonoscopy at the age of 60, we observed differences in yield in men and women and indications that these were influenced by intervention group [Citation14].
The primary aim of the current study was to assess the impact of endoscopist skill measured by ADR in any of the two intervention groups for the yield of colonoscopy in men versus women participating in SCREESCO. We hypothesized that part of the expected sex difference in colonoscopy findings is due to the greater difficulty in performing colonoscopy in women, and hence would be more apparent amongst those screened by an endoscopist with low ADR.
Material and methods
Study population
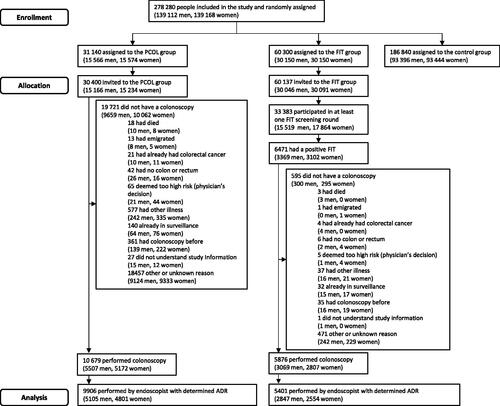
SCREESCO is a randomized controlled trial registered with ClinicalTrials.gov, NCT02078804. It included subjects in 18 of 21 regions of Sweden where 74.5% of the national population live, and where screening for colorectal cancer was not previously offered [Citation14,Citation15]. Sixty-year-old men and women born 1954 − 1958 without a previous diagnosis of colorectal or anal cancer or participation in the ongoing Nordic-European Initiative on Colorectal Cancer trial [Citation11] were randomized to either once-only primary colonoscopy (PCOL group), invitations to two rounds of two-sample high-sensitive FIT using a home test kit two years apart (FIT group), or no intervention (control group) with a ratio of 1:6 for PCOL versus control and 1:2 for FIT vs control. The study started in March 2014, and all colonoscopy procedures were completed in December 2020.
Participants in the PCOL group provided written consent in conjunction with the colonoscopy. Participants who were invited to FIT screening provided informed consent by returning the completed test. People who were assigned to the control group were not individually informed about participation in the study, and as there was no intervention in this group, consent was not required. The Ethics Committee at Karolinska Institutet (Stockholm, Sweden) approved the study (2012/2058-31/3). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
In this study, we included those randomized to either of the intervention groups who had undergone a colonoscopy in the SCREESCO study performed by an endoscopist with determined ADR (defined below). In the FIT group, data was obtained only for the first screening round (out of the two) in which the individual underwent a colonoscopy [Citation14].
Evaluation of colonoscopy findings
Pathological evaluation defined colorectal neoplastic lesions as CRCs, advanced adenomas, adenoma, and sessile serrated polyps (SSPs). Advanced adenomas were defined as adenomas with a diameter ≥10 mm, or containing high-grade dysplasia, or with a villous component. Advanced SSPs constituted a separate group, defined as advanced at a diameter of 10 mm or more. Pathologists with expertise in gastrointestinal pathology participated in the evaluation of the lesions removed at colonoscopy. The endoscopists recorded the lesions regarding size and location (right-sided [proximal to the splenic flexure], left-sided, or rectal). Further pathological details from subsequent therapy were also recorded.
Endoscopist ADR and CIR
Certified endoscopists (gastroenterologists, surgeons or registered nurses) had performed >100 colonoscopies per year and had performed >1000 in total before SCREESCO. Endoscopist ADR was defined by the ADR in all its first-time colonoscopies (a screenee’s first colonoscopy performed within SCREESCO) as previously described [Citation12]. Endoscopist ADR was defined only for endoscopists that had performed at least 40 first-time colonoscopies out of which at least 20 in each group. The ADR was extracted separately by group (PCOL and FIT) for each endoscopist and the endoscopist ADR was defined as ‘High’ if adenoma detection rate was both at least 20% in the PCOL group and at least 35% in the FIT group, and otherwise as ‘Low’.
We additionally extracted the endoscopist CIR based on first-time colonoscopies, overall and separately by study group and by endoscopist ADR.
Statistical analysis
We analyzed the following outcomes: any finding (also categorized according to the worst finding: cancer, advanced adenoma, or non-advanced adenoma); finding at least 3 adenomas; advanced SSP; and having incomplete index colonoscopy. We analyzed each study group separately since women tend to be disadvantaged compared to men in CRC screening involving faecal immunochemical testing (FIT) with lower sensitivity and positivity explained by lower faecal hemoglobin concentrations [Citation16], and different attitudes towards the procedure [Citation17,Citation18,Citation19] may account for a lower attendance rate for colonoscopy in women [Citation14]. Crude prevalence risks for each outcome were calculated separately for men and women in each study group.
We computed the adjusted risk ratio (RR = RiskMen/RiskWomen) with 95% CIs estimated in binary regression models with log link (i.e. a log-linear model in the probability of the outcome). For PCOL and FIT groups in turn, effect modification by endoscopist ADR was assessed by testing the presence of multiplicative interaction between screenee sex and endoscopist ADR on the risk of a particular finding. Each analysis was adjusted for calendar period of colonoscopy (May 2014-April 2016, May 2016-April 2018, and May 2018-July 2020) since selection of participants induces an association between sex and calendar period due to heterogeneous probability to participate [Citation14], and changes and improvements in the health care system possibly affecting yield (Supplementary Figure 1).
We performed two sensitivity analyses. The first was based on the ADR cutoffs for 25% in the PCOL group and 40% in the FIT group. Secondly, we alternatively used the raw ADR proportion without cutoffs in the analyses of interaction.
We additionally performed a test of multiplicative interaction between each endoscopist’s overall adenoma detection rate and their detection rate in males and females separately, using the same type of regression model as above, adjusted for calendar period of colonoscopy, in total and by study group. The association between endoscopist adenoma detection rate and proportion of colonoscopies performed in men, in total and by study group, was assessed using a linear regression model.
The analyses were performed using R (4.1.3).
Results
Cohort
In the SCREESCO study, 31 140 individuals were randomly assigned to the PCOL group, out of which 15 566 (50.0%) were men and 15 574 (50.0%) were women, and 60 300 to the FIT group, out of which 30 150 (50.0%) were men and 30 150 (50.0%) were women (). A total of 16 555 individuals underwent a colonoscopy, out of which 8576 (51.8%) were men and 7979 (48.2%) were women. Seventy-eight endoscopists performed at least 20 first-time colonoscopies in each study group in 15 307 (92%) individuals that underwent colonoscopy, out of which 41 (52.5%) had low ADR and 37 (47.5%) had high ADR.
Baseline characteristics
In the PCOL group, there were 2161 men (50.8%) and 2090 (49.2%) women who underwent a colonoscopy performed by an endoscopist with low ADR, and 2944 (52.1%) men and 2711 (47.9%) women who underwent a colonoscopy performed by an endoscopist with high ADR (). In the FIT group there were 1204 men (51.2%) and 1146 (48.8%) women, and 1643 (53.9%) men and 1408 (46.1%) women, correspondingly. Men and women were similar with respect to all baseline characteristics within each stratum defined by study group and endoscopist ADR, including age at colonoscopy and calendar period of colonoscopy.
Table 1. Screenee and practitioner characteristics for SCREESCO study colonoscopies, stratified by study group (PCOL group = primary colonoscopy group, FIT group = faecal immunochemical testing group), endoscopist ADR (high ADR = adenoma detection rate is at least 20% in the primary colonoscopy group, at least 35% in the faecal immunochemical testing group), and screenee sex.
Endoscopist adenoma detection rate
The median number of first-time colonoscopies per endoscopist was 156.5 (Q1-Q3: 110.0-236.0) and most endoscopists performed approximately equally many colonoscopies in men and women (median 52% men; Q1-Q3: 49.2–54.8%) (Supplementary table 1). The median ADR was higher in men (median: 32.6%; Q1-Q3: 26.2–39.8%) compared to women (median: 22.9%; Q1-Q3: 17.9–28.9%). The median endoscopists CIR was 96.9 (Q1-Q3: 94.0–98.5) and this was similar in endoscopists with low and high ADR.
There were no signs of association between endoscopist ADR and proportion colonoscopies performed in male screenees (p-value slope = 1.00 in all; 0.14 in PCOL group; 0.78 in FIT group) (Supplementary Figure 2). There was a comparable increase in endoscopist adenoma detection rate in male and female screenees with increasing overall adenoma detection rate in both groups combined (p-value multiplicative interaction test: 0.18), and in the PCOL and FIT groups separately (p-value multiplicative interaction test: 0.37 and 0.24) (Supplementary Figure 3).
Yield by sex and endoscopist ADR in the PCOL group
Yield was generally higher, up to twice as high for some lesions, for individuals investigated by a high-ADR compared to a low-ADR endoscopist for both men and women and in both the PCOL group and the FIT group. In the PCOL group, the number of men and women with any finding among those investigated by a low-ADR endoscopist was 468 (21.7%) and 306 (14.6%) correspondingly, compared to 1002 (34.0%) men and 676 (24.9%) women among those investigated by a high-ADR endoscopist (). There were no signs of effect modification of the risk ratio of any finding (men vs women) by endoscopist ADR in the PCOL group (p = 0.33) (). There were also no effect modification of the risk ratio of finding advanced or non-advanced adenoma, at least 3 adenomas, and advanced SSPs.
Figure 2. Forest plot of adjusted risk ratios (RR) of men vs women (reference) for each outcome for screenees in the PCOL group stratified by endoscopist ADR (low = blue, high = red). analyses were adjusted for calendar period of colonoscopy. The p-value from the multiplicative interaction test of endoscopist skill and sex is colored black. PCOL group = primary colonoscopy group.
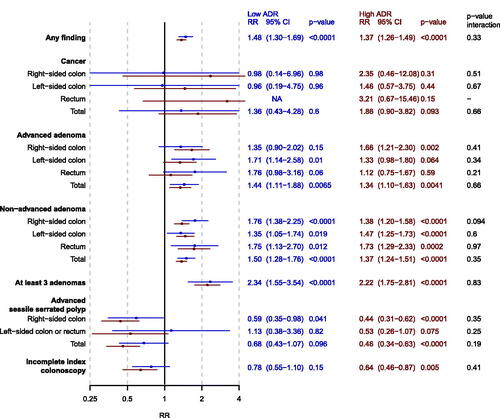
Table 2. Yield by sex, study group and endoscopist ADR. PCOL group = primary colonoscopy group, FIT group = faecal immunochemical testing group.
Yield by sex and endoscopist ADR in the FIT group
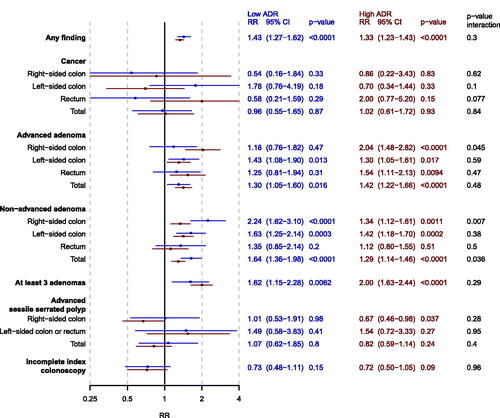
In the FIT group, there were 452 (37.5%) men and 300 (26.2%) women who had any finding among those investigated by a low-ADR endoscopists and 870 (53.0%) men and 562 (39.9%) women among high-ADR endoscopists (). There were no signs of effect modification of the risk ratio of any finding (men vs women) by endoscopist ADR (p = 0.3). The risk for cancer detection in the rectum was higher for men than women among high-ADR endoscopists (n = 14; 0.9% men, n = 6; 0.4% women, RR = 2.00, 95% CI 0.77–5.20) and lower for men compared to women among low-ADR endoscopists (n = 6; 0.5% men, n = 10; 0.9% women, RR = 0.58, 95% CI 0.21–1.59; p-value multiplicative interaction test = 0.077) ( and ). The risk ratio of right-sided non-advanced adenoma was modified by endoscopist ADR (p-value multiplicative interaction 0.007), with less of a sex-difference in detection rate among high-ADR endoscopists (n = 268; 16.3% men, n = 171, 12.1% women, RR = 1.34, 95% CI 1.12–1.61) compared to low-ADR endoscopists (n = 114; 9.5% men, n = 49, 4.3% women, RR = 2.24, 95% CI 1.62–3.10). The risk ratio of right-sided advanced adenoma was also modified by endoscopist ADR (p-value multiplicative interaction 0.045), with a greater sex-difference among high-ADR endoscopists (n = 119; 7.2% men, n = 50, 3.6% women, RR = 2.04, 95% CI 1.48–2.82) compared to low-ADR endoscopists (n = 43; 3.6% men, n = 35, 3.1% women, RR = 1.18, 95% CI 0.76–1.82). There were no effect modification of the risk ratio of finding at least 3 adenomas and advanced SSPs.
Figure 3. Forest plot of adjusted risk ratios (RR) of men vs women (reference) for each outcome for screenees in the FIT group stratified by endoscopist ADR (low = blue, high = red). analyses were adjusted for calendar period of colonoscopy. The p-value from the multiplicative interaction test of endoscopist ADR and sex is colored black. PCOL group = primary colonoscopy group.
Incomplete colonoscopies
The risk of incomplete index colonoscopy was lowest in men in the PCOL group investigated by an endoscopist with high ADR (n = 63; 2.1%) and highest for women in the FIT group investigated by an endoscopist with low ADR (n = 49; 4.3%) (). The corresponding risk ratio (men vs women) was not modified by endoscopist ADR in neither the PCOL group (p-value multiplicative interaction test = 0.41) nor the FIT group (p-value multiplicative interaction test = 0.96) ( and ).
Sensitivity analyses
The results in the two sensitivity analyses were similar to the above with respect to all outcomes, and in particular regarding the analysis of interaction (data not shown).
Discussion
Summary of findings
Yield was up to twice as high for individuals investigated by a high-ADR compared to low-ADR endoscopist. Overall, there is no convincing evidence of a sex-difference in colonoscopy findings being avoided by endoscopists with high ADR. As expected, women had a consistently lower risk of a positive colonoscopy finding, and a higher risk of incomplete index colonoscopy compared to men, with no evidence that this was due to the procedure requiring a greater level of ADR when conducted in women.
Interpretation of findings
We hypothesized that women would be disadvantaged by low-performing endoscopists when screened by FIT beyond what would be expected from the difference in disease incidence between men and women for the following reasons: women tend to be disadvantaged compared to men in CRC screening involving FIT [Citation16], colonoscopy can be technically more difficult in women than men [Citation1,Citation4], which may entail a disadvantage since colonoscopy quality varies among endoscopists [Citation5,Citation6,Citation11,Citation12]. However, our findings go against this hypothesis and instead indicate that a high endoscopist ADR increases yield proportionately in men and women.
The risk of having an incomplete index colonoscopy was consistently higher for women compared to men, higher in the FIT group and higher among low ADR endoscopists, but there were no signs of effect modification by endoscopist ADR of the risk ratio between men and women.
Previous research
We are not aware of any previous studies of interaction effects on yield between endoscopist ADR and screenee sex. The adenoma detection rate has previously been shown to be higher in men than women several other studies [Citation1–8,Citation20–23], and varies by screening modality which was confirmed in our analysis of the SCREESCO study and in a previous analysis of SCREESCO [Citation14]. A previous study of negative colonoscopies and endoscopist adenoma detection rate in the United States showed that higher adenoma detection rate was associated with lower risk of post-colonoscopy CRC and that this association did not depend on the screenee’s sex [Citation24]. However, the balance between male and female screenees for each endoscopist was not shown and endoscopist adenoma detection rate was not calculated separately by screenee sex. Any attempt to classify endoscopist performance should therefore account for both the (in)balances of colonoscopies with respect to screenee sex and screening modality. There is therefore also a need for sex-specific cutoffs of adenoma detection rate when assessing endoscopist performance [Citation1,Citation23].
Speculatively, there could be a relationship between high and low ADR endoscopists with regard to how the screenee experienced the colonoscopy, which in turn could be related to how difficult the colonoscopy was. This could be assessed in future studies and used as a method further elude whether the difficulty of doing colonoscopy in women is behind a part of the sex difference in yield.
Strengths and limitations
In SCREESCO, randomization of screening modality was balanced between men and women. The sample size was large, and SCREESCO was performed in a screening-naïve population spread across almost all regions of Sweden. This makes the study representative of a national, diverse population, and suitable for sub-analyses such as sex comparisons. Lesion detection rates in SCREESCO were largely acceptable with some room for improvement [Citation3]. The endoscopists in SCREESCO had to certify that they had an experience of >100 colonoscopies per year and >1000 in total before SCREESCO, which is a strength.
These requirements of endoscopist experience also constitute a limitation of this study, since they ensure a limited ADR range so that endoscopists classified as low ADR were still experienced. This limited our ability to assess effect modification by extremes of endoscopist ADR. However, since any endoscopist with this experience was allowed to participate, this provided a significant range of ability among the endoscopists in terms of performance.
We estimated the ADR of the endoscopists using their ADR based on their first-time colonoscopies performed in SCREESCO since information on endoscopist ADR was not available prior to the start of the study in 2014, which is a limitation. The reliability of endoscopist ADR depends on the number of colonoscopies it is based on [Citation25], and in our study the number of colonoscopies per endoscopist was generally lower than the recommended 500 for precise estimation of ADR [Citation25] but comparable with other studies [Citation26–28]. The use of the estimated ADR could be a source of bias in our study if female screenees were more often assigned to high-ADR endoscopists. However, screenees or endoscopists were not generally able to choose one another, endoscopists performed approximately equal numbers of colonoscopies in men and women, and endoscopist adenoma detection rate was not associated with the proportion of colonoscopies performed in men.
The cutoffs used to classify endoscopist ADR (low- or high-ADR) were low compared to recent recommendations [Citation12,Citation29]. We chose the lower cutoffs to get approximately equally many endoscopists in each class based on that endoscopists ADR in SCREESCO were low, although comparable to the Swedish and Norwegian arms of the NordICC study [Citation11]. The sensitivity analyses indicated that the results were robust with respect to the choice of cutoff.
The results of this study have implications for other settings where screening by primary colonoscopy and/or FIT is being used in a screening naïve population. However, the age at colonoscopy of screenees in this study was between 59 and 65 years, so our findings may not generalize to younger and/or older subgroups or to individuals with a screening history.
Conclusions
We did not find evidence that the sex differences in colonoscopy yield are modified by endoscopist ADR. Our hypothesis that part of the sex difference in colonoscopy yield is due to the procedure being more difficult in women is not supported. We cannot, however, exclude a disadvantage for women screened by FIT. Endoscopist adenoma detection rate varied according to screening participant’s sex and screening modality, so we recommend that assessment of colonoscopy quality should account for these factors in future studies. High quality screening procedures are important for all participants alike, so upskilling of all screening endoscopist to achieve a more uniform standard would be desirable.
Author contributions
Marcus Westerberg: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing
Lars Holmberg: Conceptualization, Supervision, Writing – review & editing
Anders Ekbom: Conceptualization, Supervision, Writing – review & editing
Chris Metcalfe: Writing – review & editing
Robert Steele: Writing – review & editing
Anna Forsberg: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
Clinical trial registry
This study has trial number NCT02078804 and is registered with ClinicalTrials.gov at https://www.clinicaltrials.gov/ct2/show/nct02078804.
Ethical approval
The Ethics Committee at Karolinska Institutet (Stockholm, Sweden) approved the study (2012/2058-31/3).
Supplemental Material
Download MS Word (17.2 KB)Supplemental Material
Download MS Word (11.9 KB)Supplemental Material
Download PDF (17.4 KB)Supplemental Material
Download PDF (31.2 KB)Supplemental Material
Download PDF (191 KB)Acknowledgments
We are grateful for grants from the 18 Swedish regions, Stockholm County Council, Regional Cancer Center Mellansverige, Swedish Cancer Society, Aleris Research and Development Fund, and Eiken Chemical. We are also grateful to the 33 Swedish hospitals where the colonoscopies in this study were performed. Late Prof. Rolf Hultcrantz is acknowledged for initiating SCREESCO, securing the funding and leading the study through the entire recruitment period and first report.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
For data sharing questions, please contact the study principal investigator at [email protected]. The study protocol and statistical analysis plan are available on request. Deidentified individual participant data that underlie the results reported in this Article (including in the appendix), can be made available to researchers after request to the SCREESCO Steering Committee. Researchers must provide a methodologically sound proposal for a project that conforms with the Swedish Ethical Review Authority permit for the project and will need to sign a data access agreement. Data will be made available at a secure remote server to achieve the aims in the approved proposal. Data will be available from 3 months after publication and until 3 years after publication of the Article. Proposals regarding the data underlying this Article may be submitted up to 2 years after publication. The SCREESCO study will not carry the costs of external projects.
Additional information
Funding
References
- Andersson KL, Ha JB, Abraczinskas DR, et al. Gender differences in colonoscopy: implications for clinical practice and female gastroenterologists. Dig Dis Sci. 2022;67(3):810–816. doi:10.1007/s10620-021-07079-y.
- Bevan R, Blanks RG, Nickerson C, et al. Factors affecting adenoma detection rate in a national flexible sigmoidoscopy screening programme: a retrospective analysis. Lancet Gastroenterol Hepatol. 2019;4(3):239–247. doi:10.1016/S2468-1253(18)30387-X.
- Sekiguchi M, Westerberg M, Ekbom A, et al. Detection rates of colorectal neoplasia during colonoscopies and their associated factors in the SCREESCO study. J Gastroenterol Hepatol. 2022;37(11):2120–2130. doi:10.1111/jgh.15990.
- Singh S, Dhawan M, Chowdhry M, et al. Differences between morning and afternoon colonoscopies for adenoma detection in female and male patients. Ann Gastroenterol. 2016;29(4):497–501. doi:10.20524/aog.2016.0079.
- Gohel TD, Burke CA, Lankaala P, et al. Polypectomy rate: a surrogate for adenoma detection rate varies by Colon segment, gender, and endoscopist. Clin Gastroenterol Hepatol. 2014;12(7):1137–1142. doi:10.1016/j.cgh.2013.11.023.
- James P, Hegagi M, Hegagi M, et al. Variable endoscopist performance in proximal and distal adenoma detection during colonoscopy: a retrospective cohort study. BMC Gastroenterol. 2018;18(1):73. doi:10.1186/s12876-018-0800-4.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in european countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. doi:10.1016/S1470-2045(21)00199-6.
- Cassell BE, Ross K, Chang TY, et al. Predictors of failed conscious sedation in patients undergoing an outpatient colonoscopy and implications for the adenoma detection rate. Sci Rep. 2020;10(1):2167. doi:10.1038/s41598-020-59189-8.
- Childers RE, Williams JL, Sonnenberg A. Practice patterns of sedation for colonoscopy. Gastrointest Endosc. 2015;82(3):503–511. doi:10.1016/j.gie.2015.01.041.
- Bretthauer M, Kaminski MF, Løberg M, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med. 2016;176(7):894–902. doi:10.1001/jamainternmed.2016.0960.
- Sekiguchi M, Westerberg M, Ekbom A, et al. Endoscopist characteristics and polyp detection in colonoscopy: cross-sectional analyses of screening of Swedish colons. Gastroenterology. 2023;164(2):293–295 e4. doi:10.1053/j.gastro.2022.10.003.
- Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49(4):378–397. doi:10.1055/s-0043-103411.
- Forsberg A, Westerberg M, Metcalfe C, et al. Once-only colonoscopy or two rounds of faecal immunochemical testing 2 years apart for colorectal cancer screening (SCREESCO): preliminary report of a randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(6):513–521. doi:10.1016/S2468-1253(21)00473-8.
- Strömberg U, Bonander C, Westerberg M, et al. Colorectal cancer screening with fecal immunochemical testing or primary colonoscopy: an analysis of health equity based on a randomised trial. EClinicalMedicine. 2022;47:101398. doi:10.1016/j.eclinm.2022.101398.
- Clark GR, Steele RJ, Fraser CG. Strategies to minimise the current disadvantages experienced by women in faecal immunochemical test-based colorectal cancer screening. Clin Chem Lab Med. 2022;60(10):1496–1505. doi:10.1515/cclm-2022-0583.
- Ghanouni A, Plumb A, Hewitson P, et al. Patients’ experience of colonoscopy in the english bowel cancer screening programme. Endoscopy. 2016;48(3):232–240. doi:10.1055/s-0042-100613.
- McLachlan SA, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context–a systematic review of the literature. Patient Educ Couns. 2012;86(2):137–146. doi:10.1016/j.pec.2011.04.010.
- Fritzell K, Forsberg A, Wangmar J, et al. Gender, having a positive FIT and type of hospital are important factors for colonoscopy experience in colorectal cancer screening - findings from the SCREESCO study. Scand J Gastroenterol. 2020;55(11):1354–1362. doi:10.1080/00365521.2020.1820568.
- Mohan BP, Khan SR, Daugherty E, et al. Pooled rates of adenoma detection by colonoscopy in asymptomatic average-risk individuals with positive fecal immunochemical test: a systematic review and meta-analysis. Gastrointest Endosc. 2022;96(2):208–222 e14. doi:10.1016/j.gie.2022.04.004.
- Shaukat A, Holub J, Pike IM, et al. Benchmarking adenoma detection rates for colonoscopy: results from a US-based registry. Am J Gastroenterol. 2021;116(9):1946–1949. doi:10.14309/ajg.0000000000001358.
- Cavicchi M, Tharsis G, Burtin P, et al. Difference in physician-and patient-dependent factors contributing to adenoma detection rate and serrated polyp detection rate. Dig Dis Sci. 2019;64(12):3579–3588. doi:10.1007/s10620-019-05808-y.
- Hassan C, Piovani D, Spadaccini M, et al. Variability in adenoma detection rate in control groups of randomized colonoscopy trials: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97(2):212–225 e7. doi:10.1016/j.gie.2022.10.009.
- Schottinger JE, Jensen CD, Ghai NR, et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022;327(21):2114–2122. doi:10.1001/jama.2022.6644.
- Do A, Weinberg J, Kakkar A, et al. Reliability of adenoma detection rate is based on procedural volume. Gastrointest Endosc. 2013;77(3):376–380. doi:10.1016/j.gie.2012.10.023.
- Sarvepalli S, Garber A, Rothberg MB, et al. Association of adenoma and proximal sessile serrated polyp detection rates with endoscopist characteristics. JAMA Surg. 2019;154(7):627–635. doi:10.1001/jamasurg.2019.0564.
- Jover R, Zapater P, Bujanda L, et al. Endoscopist characteristics that influence the quality of colonoscopy. Endoscopy. 2016;48(3):241–247. doi:10.1055/s-0042-100185.
- Mehrotra A, Morris M, Gourevitch RA, et al. Physician characteristics associated with higher adenoma detection rate. Gastrointest Endosc. 2018;87(3):778–786. e5. doi:10.1016/j.gie.2017.08.023.
- Samnani S, Khan R, Heitman SJ, et al. Optimizing adenoma detection in screening-related colonoscopy. Expert Rev Gastroenterol Hepatol. 2023;17(6):589–602. doi:10.1080/17474124.2023.2212159.