Abstract
Objectives
The aim was to define the effectiveness of tofacitinib and to characterize the patient population receiving tofacitinib in a real-world cohort clinical setting for ulcerative colitis (UC) in Finland.
Methods
This is a retrospective non-interventional multicenter patient chart data study conducted in 23 Finnish Inflammatory Bowel Disease (IBD) centers. Baseline demographic and clinical data, clinical remission, steroid-free remission rate and time to tofacitinib discontinuation, colectomy or UC-related hospitalization were studied.
Results
The study included 252 UC patients of which 69% were male. Most patients had extensive disease (71%) and were bio-experienced (81%). Tofacitinib demonstrated positive treatment outcomes with clinical response, clinical remission, and steroid-free clinical remission at one year in 33%, 34% and 31% of patients, respectively. Moreover, 64% of patients in pMayo remission at week 16 from the start of tofacitinib were still in remission at one year. Only no or mild disease activity compared to moderate activity at baseline was associated with a higher probability of achieving remission according to pMayo at six months, p = .008. Hospitalizations and/or colectomies during the study period (before treatment discontinuation/end of follow-up) were low (n = 24), with less than 5 colectomies.
Conclusions
In this real-world cohort, including a majority of bio-experienced UC patients, tofacitinib was effective in achieving steroid-free clinical remission in a third of the population at one year. A majority of patients in remission at week 16 were also in remission at one year. Results are in line with earlier published real-world studies. Registration: ClinicalTrials.gov NCT05082428.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by cycles of remission, and exacerbation of inflammation, with a substantial impact on quality of life (QoL) [Citation1]. Typical symptoms include bloody stools, diarrhea, urgency, and fatigue, and often the disease has considerable consequences for work participation and sick leave [Citation2]. The symptoms vary based on the severity and extent of the disease. The goal of treatment is the resolution of symptoms and deep remission, defined as durable symptomatic and endoscopic remission without corticosteroid therapy, as well as improvement of quality of life, and prevention of complications from the disease, such as surgeries, dysplasia, colorectal carcinoma and hospital admissions [Citation3,Citation4].
Since the late 1990s, anti-tumour necrosis factor (TNF) treatment has significantly improved treatment outcomes. After that, other biological treatment options such as vedolizumab and ustekinumab have become available for the treatment of UC. However, approximately one-third of patients do not respond to biologic treatment and up to 20% per year lose response to anti-TNF agents after an initial improvement (secondary non-responders), leading to a dose escalation or a switch to another drug class [Citation5]. Therefore, alternative treatment options for patients with UC are needed.
Tofacitinib is an oral Janus kinase (JAK) inhibitor for the treatment of UC that mediates signal-transduction activity of multiple cytokines that are integral to lymphocyte activation, function, and proliferation [Citation6,Citation7]. The efficacy and safety of tofacitinib has been demonstrated as induction and maintenance therapy in 3 Phase 3, randomized, placebo-controlled trials (RCT) in patients with moderate to severe UC [Citation8–10]. However, the study design and procedures in RCTs may not always reflect clinical practice and treatment patterns. Thus, it is of interest to clinicians and patients to describe outcomes in the environment where the drug will be used. Therefore, real-world evidence (RWE) has become increasingly important in providing additional evidence of treatment effectiveness in clinical practice.
The aim of our study was to assess the real-world effectiveness of tofacitinib as well as to characterize the patient population receiving tofacitinib for UC in a real-world clinical setting in Finland.
Material and methods
Study design
This is a retrospective non-interventional multicenter patient chart data study conducted in 23 Finnish IBD centers. Per the Act on Secondary Use of Health and Social Data 552/2019 (in Finnish: Laki sosiaali- ja terveystietojen toissijaisesta käytöstä), no consent, institutional review board or independent ethics committee was required. We included all adult (age ≥18 years) patients with a confirmed UC diagnosis (ICD-10: K51) between January 2010 and December 2021 who were prescribed tofacitinib for UC after 01 March 2019 (national reimbursement decision). In Finland tofacitinib is reimbursed for the treatment of moderate to severe ulcerative colitis in adults, when response to either conventional immunosuppression therapy or biological therapy has been insufficient, the response has been lost, or the treatment has not been tolerated. The reimbursement is restricted to a medical statement from a specialized medical care unit or from a gastroenterology specialist. Data collection was completed on 01 November 2022. The main exclusion criteria were less than eight weeks of follow-up and a history of panproctocolectomy, ileal pouch-anal anastomosis (IPAA) or ileostomy.
Data collection
The data were collected from hospital electronic health records from all patient visits during the study period. The data have been generated as part of standard clinical care, treatment, and follow-up of ulcerative colitis patients in Finland. Gastroenterologists collected the data into an electronic data mining tool (eDMT), or Microsoft Excel form designed for the study. All investigators attended a training session before the start of the study to avoid possible errors in the data collection. Patients were followed until the end of tofacitinib use or data collection date.
Definitions
Clinical response and remission were assessed based on the partial Mayo (pMayo) score, including stool frequency, rectal bleeding, and physician’s global assessment, ranging from 0 to 9 [Citation11]. Endoscopic Mayo-subscore of ≥2 and fecal calprotectin (FC) >250 mg/kg were used as indicators of active disease. Clinical remission was defined as a pMayo score of <2 with a rectal bleeding sub score of 0. When endoscopic data were available, the full Mayo score was utilized for further analyses, with the definition of remission as ≤2 points with no individual sub score exceeding 1, and with a rectal bleeding sub score of 0. Sustained remission was defined as patients being in remission throughout the estimated time intervals.
Treatment response was defined as a pMayo score decrease of ≥2 points and ≥25% from baseline, with an accompanying decrease of ≥1 point on the rectal bleeding sub score or an absolute rectal bleeding score of ≤1. Patients in remission and not having required corticosteroid treatment within four weeks prior to the contact were stated as being in steroid-free remission.
Statistical analyses
Analyses were performed using R, version 4.0.3. Small patient numbers (1–4) were replaced by ‘<5’ and the corresponding percentages were replaced by ‘-,’ according to the guidelines set by permission authority Findata.
Data was analyzed at baseline, at week 8, at week 16, at week 24 and at week 52. For treatment outcomes at a specific timepoint, the measures were handled by carry forward imputation, i.e., the latest available measure was assumed to be unchanged until otherwise proven. The treatment outcomes were reported for all patients on treatment at the timepoint, and the corresponding proportions were computed of all patients on follow-up.
The competing-risk time-to-event model was fitted to estimate time to clinical response, with time-to-event defined as time from index (initiation of tofacitinib) to clinical response (event), treatment discontinuation (competing event), or end of follow-up (censoring event). Patients with unknown pMayo score at baseline were excluded. The cumulative incidence as a function of time for both types of events was assessed, and the Aalen-Johansen state probabilities of the multistate model were illustrated. Time to composite endpoint (the earliest event of treatment discontinuation, colectomy, or UC hospitalization) (event) or end of follow-up (censoring event) from index was assessed with the Kaplan–Meier method. A log-binomial generalized linear model was fitted to estimate which baseline clinical factors are associated with remission at 6 months from the index.
Results
Patient characteristics
The study included a total of 252 patients from 23 Finnish IBD centers in 16 out of 21 Finnish hospital districts with a catchment area covering 91% of the Finnish population. Patient characteristics are summarized in . More than two thirds (69%) of patients were male; the median age was 36 years (IQR 27-49), and the median disease duration was five years (IQR 2-11). A vast majority had extensive disease (71%), whereas disease limited to the rectum (2%) was rare. Active disease at baseline was demonstrated clinically by an elevated pMayo score (median 5, IQR 3–6, data available in 81% of patients), and biochemically by a substantially elevated FC (median 1275 mg/kg, IQR 620–2672 mg/kg, data available in 75% of patients), and a CRP slightly above reference (median 4 mg/l, IQR 4–9.5 mg/l, data available in 79% of patients). However, it is notable that 6% (14) of patients were in clinical remission at baseline. These patients were included in all analyses but were not able to reach a clinical response per our definition. All results included in the research protocol as well as patient numbers and missing values for baseline characteristics and outcomes at different data points are reported in the supplementary materials (Tables S3–S5).
Table 1. Baseline demographics of patients with UC treated with tofacitinib in Finland.
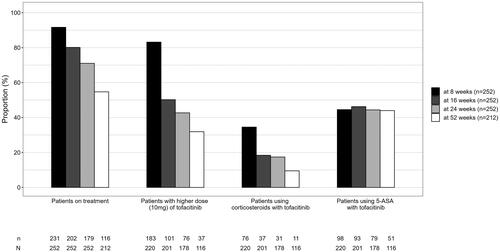
A total of 91% (229/252) patients had previously been treated with corticosteroids and 88% (222/252) had received thiopurines. Moreover, a large majority of patients, 81% (204/252), had previously been exposed to biological treatment, 63% (159/252) to infliximab, 24% (61/252) to adalimumab, 8% (20/252) to golimumab, 34% (85/252) to vedolizumab, and 8% (19/252) to ustekinumab. On average, 1.4 ± 1.0 biologicals had been used prior to tofacitinib. At baseline, 60% (n = 135) of patients were using corticosteroids.
Tofacitinib dose and dose changes
One-year follow-up data were available for 212 patients. Tofacitinib dosing patterns and concomitant corticosteroid and 5-ASA use at different data collection points are shown in . Since this study describes treatments patterns in a real-life clinical setting, steroid tapering was not mandatory. In general, after initiation of tofacitinib, the aim is to taper the daily dose of corticosteroids with 5 mg every one to two weeks. At w8 35% (n = 76), at w16 18% (n = 37), at w24 17% (n = 31) and at w52 10% (n = 11) of patients were still on corticosteroids. Moreover, at w52, 24% (n = 51) of patients were still taking 5-ASA as a concomitant treatment. For all patients on treatment, the proportion of patients who have had a per label recommended induction dose of 10 mg twice daily for the whole eight or 16 weeks without any attempts to lower the dose were 70% (168/241) and 36% (91/251) respectively (Table S4). Notably, 14 patients received a tofacitinib induction dose of 10 mg twice daily for a maximum of 7 weeks. Patients with a higher dose (10 mg) of tofacitinib of the whole cohort and of those on treatment at different time points during the study are presented in the supplemental material (Table S4).
Clinical outcomes
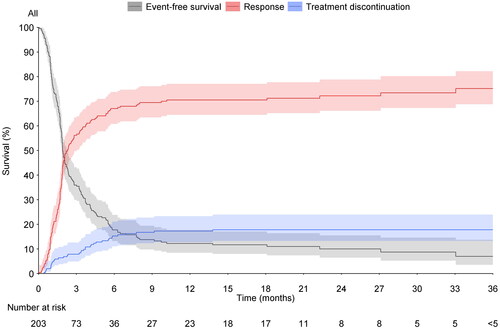
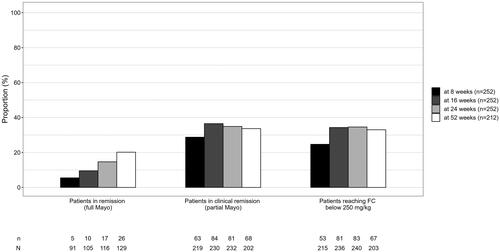
Out of those patients with available data by respective time points, 29% (63/219) of patients were in clinical remission (using pMayo) at w8, 37% (84/230) at w16, 35% (81/232) at w24, and 34% (68/202) at w52. Competing risk time- to -event model of time to response or discontinuation is shown in . In patients using corticosteroids at baseline, less than five patients reached steroid-free clinical remission at w8, 29% (36/124) of patients reached steroid-free clinical remission at w16, 26% (32/124) at w24, and 30% (32/108) at w52. With respect to clinical response, requiring both baseline data and respective timepoint data, 37% (76/203) had a response at w8, 45% (91/203) at w16, 43% (87/203) at w24, and 33% (58/174) at w52. Steroid-free clinical remission was achieved in 11% (23/219) of patients at w8, 32% (73/230) of patients at w16, and 30% (70/232) of patients at w24, and 31% (63/202) of patients at w52.
Figure 2. Competing risk time-to-event model of time to response (red line) or discontinuation (blue line). proportion of patients who have not reached a response, nor discontinued is shown in grey. Shaded areas = 95% confidence interval.
When evaluating the proportion of patients in sustained remission from w8, 84% (53/63) were still in remission at w16, 63% (40/63) at w24, and 51% (28/55) at w52. Moreover, the proportion of patients in sustained remission from w16 to w24 was 76% (64/84), and 64% (45/70) to w52. Steroid-free sustained remission from w16 to w24 was achieved in 73% (53/73) patients and to w52 in 61% (37/61) patients.
Of the 171 patients with a baseline FC more than 250 mg/kg indicating active disease, 18% (31/171), 32% (55/171), 34% (58/171), and 31% (44/141) achieved an FC less than 250 mg/kg at w8, w16, w24, and w52, respectively. The proportion of patients in remission according to pMayo or full Mayo or reaching FC level less than 250 mg/kg at different timepoints are presented in . Mean FC changes from baseline and responders defined by FC reduction of ≥50%/75%/90% compared to baseline at different time points are presented in the supplementary materials (Table S4).
Figure 3. Proportion (n divided by N) of patients in remission at different timepoints according to pMayo, full Mayo or achieving FC level <250mg/kg.
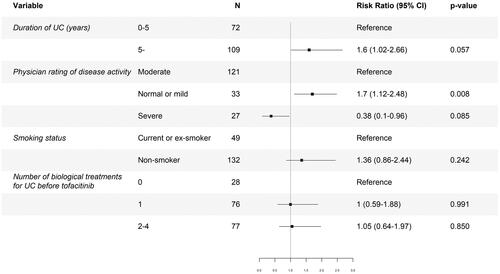
We tested the effect of duration of disease, disease activity, smoking status, and use of biological treatments before tofacitinib on clinical outcomes in multivariate regression analysis, . The number of biological treatments used for UC prior to tofacitinib did not emerge as a risk factor for failing to achieve remission at 6 months. Only no or mild disease activity compared to moderate activity at baseline was associated with a higher probability of achieving remission according to pMayo score at 6 months (risk ratio 1.7 [1.12–2.48]), p = .008.
Treatment discontinuation, hospitalization, or colectomy
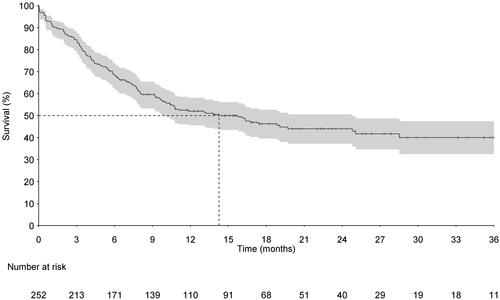
Time to tofacitinib discontinuation, colectomy, or UC-related hospitalization was studied as a time to composite end-point mode. This endpoint consists mostly of drug discontinuations. A total of 125 patients discontinued tofacitinib treatment during the analyzed observation period. The reasons for tofacitinib discontinuation were not collected. The numbers of hospitalizations and/or colectomies during the study period (before treatment discontinuation/end of follow-up) were low (n = 24), with less than 5 colectomies. The median time to treatment discontinuation, hospitalization, or colectomy was 14.3 months (IQR 10.0–24.8 months), .
Discussion
In this real-world, retrospective, observational study we demonstrated the treatment persistence and effectiveness of tofacitinib in a population of highly refractory UC patients. To our knowledge, our study is one of the largest of recently published real-world studies, providing valuable insight on the effectiveness of tofacitinib.
During the past decade, treatment options in IBD have expanded beyond anti-TNFs to include anti-integrins, IL12/23 inhibitors, and JAK inhibitors. Despite promising RCT data on their efficacy, in daily clinical practice new drugs are much more often first introduced in patients who have failed earlier and better-established treatments. Since the features and the course of the disease in these patients can be thought to be more aggressive, the results from RCTs cannot be applied directly. Therefore, studies that evaluate the effectiveness of new treatment options in the real-world setting are relevant to confirm the clinical benefit of a given therapy and to guide therapeutic decisions.
In our study, the proportion of patients in clinical remission at w8 (29%) and at w16 (37%) was in line with most studies, demonstrating clinical remission rates from 21% to 57% at w8 and from 32% to 51% during weeks 12–16 [Citation12–16]. However, results in these studies vary relatively widely, with remission rates up to 61% in a Korean study [Citation17]. The variations can be explained at least by different patient populations but also by different definitions for remission. It should therefore be noted that the definition of clinical remission in our study was stricter than in earlier published studies, which may explain the difference.
Despite the potentially rapid onset of response to tofacitinib demonstrated in the RCTs, it is important to acknowledge that response to tofacitinib can also occur with a significant delay, as shown in a small proportion of our patients finally responding far beyond 6 months from the start of tofacitinib. Moreover, 12% of our patients had not received a response as defined in this study, but were still on treatment at 52 weeks, indicating that they were already in remission before the initiation of tofacitinib or they had received other benefits from the treatment.
Furthermore, we demonstrated a steroid-free remission rate of 32% at w16, which fits well with the steroid-free remission rates of 25.0 and 44.3% in two real-world meta-analyses [Citation18,Citation19]. Moreover, the w16 steroid-free remission rate in our study exceeded the w8 remission results of the randomized, double-blind, placebo-controlled OCTAVE (tofacitinib) induction 1 and 2 trials of 18.5% and 16.6%, respectively [Citation10]. An extended induction dose beyond 8 weeks in approximately one third of the patients in our cohort may explain these differences. However, the steroid-free clinical remission rate of 31% at w52 in our study was lower than the steroid-free remission rates from 32% to 61% demonstrated by earlier studies [Citation12–17,Citation20]. This may be explained by the more severe disease phenotype in our patients, based on the multiple biological treatments used prior to the start of tofacitinib. In our study, nearly one-third achieved 52w steroid-free remission. Only a few real-world studies have been published on outcomes of tofacitinib treatment beyond one year. A Dutch multicenter study [Citation21] showed a steroid-free clinical remission rate of 31.8% after 24 months of treatment, with approximately half of all patients (55.5%) having discontinued tofacitinib after a median duration of 13 weeks.
Increasing evidence suggests additional clinical benefits in achieving histologic healing in IBD, particularly UC [Citation22]. Patients who achieve histologic healing have less long-term disease complications and are less likely to develop colorectal dysplasia. Moreover, in UC patients who achieve endoscopic mucosal healing, histologic healing is further protective against disease flares, medical escalation, hospitalization, surgery, and corticosteroid use [Citation23,Citation24]. So far only one study with tofacitinib exists to demonstrate an endo-histological healing rate of 11% at w8, 25% at w24, and 38% at w52 [Citation12]. Due to a low number of performed endoscopies and a probable simultaneous sampling bias, as active and more severe disease most probably easier triggers an endoscopic assessment, histological remission was not selected as an assessed outcome in our study.
Based on our findings, only absent or mild disease activity compared to moderate disease activity at baseline was associated with higher clinical remission rates according to pMayo at 6 months from the index, whereas duration of disease, smoking status, and history of biological treatment did not show any associations with clinical remission. A retrospective British observational cohort study [Citation13] showed higher baseline CRP level and younger age to be associated with increased primary non-response rates, whereas in the Spanish Eneida Registry [Citation15] baseline pMayo score was identified as the only short-term predictor of steroid-free remission. Also, the male sex has previously been associated with a reduced steroid-free remission rate in a meta-analysis [Citation11]. However, in the OCTAVE induction 1 and 2 trials, tofacitinib efficacy was consistent regardless of CRP levels at baseline, baseline steroid use, or previous exposure to anti-TNF [Citation10].
The significance of previous biological exposure for the outcome of tofacitinib therapy remains unclear. Although we could not demonstrate any differences in outcomes between bio-naïve and bio-experienced patients, a recently published Italian retrospective study [Citation24] showed a drop in clinical remission rates from 66.7% in patients naïve to any biological therapy to 21.4% in patients using tofacitinib as fourth-line therapy, and further a significant difference in remission rate in patients taking tofacitinib as a first- or second-line option versus those taking tofacitinib as a third- or fourth-line option.
Our study has some limitations. As for all real-world studies, the major criticism is associated with the retrospective data collection and analysis. Moreover, due to the study setting, the data for collected variables were not completely available for all patients at all time points and the analyses of response and remission did not take the discontinued patients into account. Endoscopic data were available only in a minority, which reflects to clinical practice, where the response to treatment is evaluated based on clinical symptoms combined with changes in FC concentration rather than endoscopies. In addition, due to the multicenter setting and large number of investigators, the analysis may have been impacted by data collection bias. Some safety concerns have been raised as a higher incidence of thrombotic, major adverse cardiovascular events and cancers with tofacitinib than with TNF inhibitors in patients with rheumatoid arthritis in a high cardiovascular risk study population has been presented [Citation25–27]. Even as this risk was not reproduced in the UC studies, the question of safety has raised the expectation of real-world clinical studies. However, as the objective of our study was not to assess the safety of tofacitinib, no safety data were collected. Despite these limitations, we believe that this study, with a large number of patients included, reflects well the real-word situation and provides important data on the effectiveness and use of tofacitinib.
Conclusion
In our refractory population where a majority of patients had a history of at least one biological and tofacitinib was introduced as a late option, tofacitinib demonstrated positive treatment outcomes. The clinical response, clinical remission, and steroid-free clinical remission at one year were 33%, 34% and 31%, respectively. Of those patients having achieved remission at week 16 from the start of tofacitinib, 64% were still in remission at one year. In addition, after the start of tofacitinib, surrogate biomarkers reflecting disease activity decreased significantly.
Ethics statement
This study was approved by the Finnish Health and Social Data Permit Authority, Findata (permit number THL/3255/14.02.00/2021). No ethics approval or informed consent was required by Finnish legislation, as the persons were not contacted, and the study did not affect the treatment of the patients. The study was conducted in accordance with the latest version of the Helsinki Declaration, Good Pharmacoepidemiology Practices, and Data Protection Directive.
Supplemental Material
Download MS Word (55.5 KB)Acknowledgments
The authors would like to thank the following gastroenterologists for their valuable effort in data collection: Tuire Ilus PhD, Anna-Maija Puolanne MD, Jari Timonen MD, Ville Liukkonen MD, Ritva Koskela PhD, Lauri Salminen MD, Inka Koskinen MD, Ulla-Maija Suhonen MD, Hans Henricson MD, Mikko Kellokumpu MD, Mikko Mattila MD, Kristi Kontola MD, Heikki Nuutinen MD, Hannu Olavi Rantala MD, and Taija Havulinna MD.
Disclosure statement
PM has received speaker and consultancy fees from Abbvie, Orion Pharma, Pfizer, Sandoz, Takeda, Tillotts Pharma and advisory board member fees from BMS, Gilead and Janssen-Cilag. HE has received speaker fees from Abbvie and Tillots Pharma, congress fees from Abbvie and Pfizer. JT has received advisory board member fees from Abbvie and Pfizer. AK owns Orion stocks. MH has received consultant honoraria from Tillots Pharma and Janssen and congress fees from Ferring. JP has received consultant honoraria from IQVIA and congress fees from Abbvie, Pfizer and Tillots Pharma. CGB has received speaker and consultant honoraria from Abbvie, BMS, Galapagos, Janssen, Pfizer, and Takeda. MK is employed by Pfizer Oy and owns Pfizer stocks. DH is a former employee of Pfizer AB and owned Pfizer stocks at the time of this work. KK is a former employee of Pfizer Oy and owned Pfizer stocks at the time of this work.
Data availability statement
The data that support the findings of this study were collected by following the guidance and application process of Findata, the central permission authority in Finland. Restrictions apply to the data, where only persons named in the study permission have access to the pseudonymized micro data for the current study, and so are not publicly available.
Additional information
Funding
References
- Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021;53(7):803–808. doi:10.1016/j.dld.2021.03.002.
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi:10.1016/S0140-6736(16)32126-2.
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. doi:10.14309/ajg.0000000000000152.
- Harbord M, Eliakim R, Bettenworth D, et al. Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784. doi:10.1093/ecco-jcc/jjx009.
- Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020;14(5):694–709. doi:10.1093/ecco-jcc/jjz195.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi:10.1111/j.1600-065X.2008.00754.x.
- Fernández-Clotet A, Castro-Poceiro J, Panés J. Jak inhibition: the most promising agents in the IBD pipeline? Curr Pharm Des. 2019;25(1):32–40. doi:10.2174/1381612825666190405141410.
- Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. doi:10.1056/NEJMoa1112168.
- Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17(8):1541–1550. doi:10.1016/j.cgh.2018.11.035.
- Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi:10.1056/NEJMoa1606910.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi:10.1056/NEJM198712243172603.
- Cohen NA, Steinberg JM, Silfen A, et al. Endo-histologic normalization is achievable with tofacitinib and is associated with improved clinical outcomes. Dig Dis Sci. 2023;68(4):1464–1472. doi:10.1007/s10620-022-07716-0.
- Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14(10):1385–1393. doi:10.1093/ecco-jcc/jjaa075.
- Ma C, Panaccione R, Xiao Y, et al. REMIT-UC: real-world effectiveness and safety of tofacitinib for moderate-to-Severely active ulcerative colitis: a Canadian IBD research consortium multicenter national cohort study. Am J Gastroenterol. 2023;118(5):861–871. doi:10.14309/ajg.0000000000002129.
- Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35–42. doi:10.1093/ecco-jcc/jjaa145.
- Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective dutch initiative on crohn and colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–888. doi:10.1111/apt.15689.
- Shin SH, Oh K, Hong SN, et al. Real-life effectiveness and safety of tofacitinib treatment in patients with ulcerative colitis: a KASID multicenter cohort study. Therap Adv Gastroenterol. 2023;16:17562848231154103. doi:10.1177/17562848231154103.
- Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32–40. doi:10.1093/ibd/izab011.
- Lucaciu LA, Constantine-Cooke N, Plevris N, et al. Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211064004. doi:10.1177/17562848211064004.
- Straatmijer T, van Gennep S, Duijvestein M, et al. Real-world clinical and endoscopic outcomes after one year tofacitinib treatment in ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(10):1288–1297. doi:10.1097/MEG.0000000000002028.
- Straatmijer T, van Schaik FDM, Bodelier AGL, et al. Effectiveness and safety of tofacitinib for ulcerative colitis: two-year results of the ICC registry. Aliment Pharmacol Ther. 2023;57(1):117–126. doi:10.1111/apt.17248.
- Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15(10):1557–1564.e1. doi:10.1016/j.cgh.2017.02.016.
- Gupta A, Yu A, Peyrin-Biroulet L, et al. Treat to target: the role of histologic healing in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(9):1800–1813.e4. doi:10.1016/j.cgh.2020.09.046.
- Tursi A, Mocci G, Cingolani L, et al. Use of tofacitinib as first or second-line therapy is associated with better outcomes in patients with ulcerative colitis: data from a real-world study. Expert Opin Pharmacother. 2023;24(14):1649–1656. doi:10.1080/14656566.2023.2230126.
- Kochar BD, Cheng D, Cai T, et al. Comparative risk of thrombotic and cardiovascular events with tofacitinib and anti-TNF agents in patients with inflammatory bowel diseases. Dig Dis Sci. 2022;67(11):5206–5212. doi:10.1007/s10620-022-07404-z.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi:10.1056/NEJMoa2109927.
- Kristensen LE, Danese S, Yndestad A, et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL surveillance. Ann Rheum Dis. 2023;82(7):901–910. doi:10.1136/ard-2022-223715.