Abstract
Objectives
Comprehensive follow-up data from the largest hospital district in Finland was used to assess hospital-based healthcare resource utilization (HCRU) and expenses, incidence and prevalence, survival, and effect of comorbidities/complications on survival of adult patients with intestinal failure due to short bowel syndrome (SBS-IF).
Methods
This study utilized electronic healthcare data covering all ≥18-year-old patients with SBS-IF at the Hospital District of Helsinki and Uusimaa in Finland between 2010 and 2019. Patients were followed from SBS-IF onset until the end of 2020 or death and compared to birth year and sex-matched control patients without SBS-IF.
Results
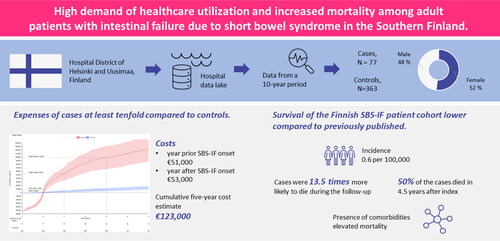
The study included 77 patients with SBS-IF (cases) and 363 controls. Cases had high HCRU; the cumulative expenses were about tenfold compared to the controls, at the end of the study (€123,000 vs. €14,000 per patient). The expenses were highest during the first year after SBS-IF onset (€53,000 per patient). Of the cases with a median age 62.5 years, 51.9% died during study time. The median survival was 4.4 years from SBS-IF onset and cases died 13.5 times more likely during the follow-up compared to controls. Mortality risk was lower in female cases (hazard ratio (HR) 0.46; 95% confidence intervals (CI) 0.24, 0.9) and higher with presence of comorbidities (Charlson comorbidity index HR 1.55; 95% CI 1.2, 2.0) and mesenteric infarction (HR 4.5; 95% CI 1.95, 10.36). The incidence of adult SBS-IF was 0.6 per 100,000 adults.
Conclusion
Our study demonstrates a high demand for healthcare support and elevated mortality in adult SBS-IF-patients. Our results suggest that the presence of comorbidities is a key driver for mortality.
Introduction
Short bowel syndrome (SBS) is the most frequent cause of intestinal failure (IF) [Citation1]. SBS-IF is a rare and highly disabling condition in which individuals cannot absorb nutrients adequately due to functional or physical loss of portions of the small bowel (the remaining <200 cm) [Citation2, Citation3]. Adult patients with SBS-IF constitute a heterogenous group due to variable underlying causes of SBS as well as differences in length, anatomy, and health of the remnant intestine. The most common causes of SBS-IF in adults include inflammatory bowel disease, mesenteric vascular disease, cancer, and traumatic injuries [Citation4–6].
The primary therapy for SBS-IF is parenteral nutrition (PN), of which the patients are dependent on until the intestine has adapted to the resection [Citation3, Citation7]. Management of patients with SBS-IF is complex and multifaceted, involving nutrition support, fluid and electrolyte management, pharmacological therapies, and requires a multidisciplinary approach [Citation8]. Despite advances in treatment, adult patients with SBS-IF have significant morbidity [Citation9], decreased survival [Citation4, Citation10–14], and increased healthcare utilization [Citation4, Citation15, Citation16].
SBS-IF does not have an International Classification of Diseases 10th Revision (ICD-10) code. Thus, various inclusion criteria and proxies, such as home parenteral nutrition (HPN) [Citation17], have been used for retrospective registry studies on SBS-IF. We have previously studied pediatric patients with SBS-IF in Finland [Citation18], however, the adult patient population differs largely from the pediatric. In Finland, management of pediatric SBS-IF patients is centralized in the Hospital District of Helsinki and Uusimaa (HUS), while management of adult patients is more fragmented [Citation19]. The characteristics of the Finnish adult SBS-IF patients have been studied before [Citation1, Citation9]. However, real-world evidence (RWE) on incidence, healthcare resource utilization, and survival of adult patients with SBS-IF in Finland is lacking.
The aim of this study was to describe the HCRU and related expenses of the SBS-IF adult patients treated at HUS. In addition, overall survival (OS), occurrence of septic infections, SBS incidence, and prevalence were assessed. Due to the Finnish national healthcare system, all patients have right to equal access to care, and the treatment of patients living at the same region is centralized to one hospital. Thus, our study was able to capture all adult SBS-IF patients living in the HUS area.
Materials and methods
Study design, population, and data source
This retrospective registry study utilized electronic medical records gathered from the HUS data lake containing data from secondary healthcare. HUS is the largest hospital district in Finland covering a population of about 1.6 million. For description of the data source and the Finnish national primarily taxation funded healthcare see our previous study [Citation18]. The study was conducted with permission from HUS (data permit no. HUS/141/2020) by the provision of the Act on the Secondary Use of Health and Social Data (Finland’s Ministry of Justice 552/2019), therefore no informed consent from the patients was required. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. Data with personal identification (ID) code was solely handled by the personnel at the HUS data lake or by the HUS investigators, and pseudonymised at the HUS data lake. Only pseudonymised data required for the subsequent analyses was released into a secure, remote data environment to which only the HUS personnel and individuals listed in the study permission had access. Only aggregated, anonymous results were pulled out of the environment for reporting. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Inclusion criteria
This study covers adult SBS-IF patients treated at HUS and living in the HUS area between 2010 and 2019. The patient cohort was formed based on the procedure codes for central venous catheter (CVC) insertion or removal and text search for short bowel (). The patients were required to be ≥18 years at inclusion. Two HUS specialists (authors SP ja AP) validated the included patients. The validation comprised checking the patients had undergone small bowel resection, the length of small bowel was <200 cm, and the patients had received parenteral support (PS) for IF for at least three consecutive months. Follow-up of each patient started at the first mention of short bowel in the medical records or at the first record of an inclusion procedure code (but no earlier than January 1st, 2010). Follow-up ended at death, or end of study (December 31st, 2019), except for HCRU analyses where follow-up was terminated at the date of the last healthcare contact.
Figure 1. Flowchart of the data formation (excluded data, blue boxes) and analyses (grey boxes). CVC, Central venous catheter; HUS, Hospital District of Helsinki and Uusimaa;, * = any symbol.
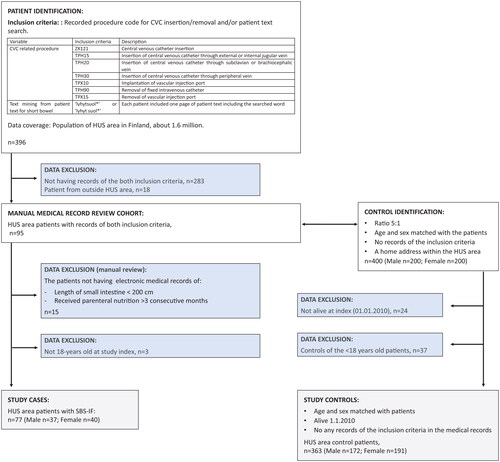
Sex and birth year matched controls were randomly selected in a 5-to-1 ratio from individuals with records in the HUS data lake and home address within the HUS area. Controls were followed from the index date of the matched patient. A few controls were excluded after matching as they had deceased prior to the index date of the corresponding patient. Thus 17 patients had 4 controls, 1 had 3, and 1 had 2. The controls represent adults without SBS-IF who had been treated at HUS secondary healthcare during the same period as the corresponding patient cases.
Outcomes
The primary outcome of the study was the hospital-based HCRU and expenses. The secondary outcomes were SBS-IF incidence, prevalence, survival and effect of co-morbidities or complications on survival.
Patient characteristics, healthcare resource utilization and expenses
Demographic and clinical characteristics were assessed from HUS data lake and reported at index. The Charlson comorbidity index (CCI) was calculated according to method by Quan et al. [Citation20] based on the ICD-10 -codes recorded within two years prior to index. Patients with cancer were individuals with any records of ICD-10-codes starting with ‘C’, excluding C44 (non-melanoma skin cancer), within two years prior to index.
HCRU was assessed based on the recorded inpatient days and hospital outpatient clinic contacts at the HUS specialized healthcare. The number of hospitalization days and outpatient clinic contacts were assessed cumulatively one year prior to index and up to five years post index using mean cumulative functions [Citation21]. The inpatient and outpatient contacts were priced according to Mäklin et al. [Citation22]. The prices included the average expenses of all procedures, treatments, medications, and other contact related expenses in each specialty. All expenses reported in this paper are in 2020 euros (€) and estimated using same methods as in our previous study [Citation18].
Yealy incidence and prevalence
Yearly and overall incidence and prevalence of SBS-IF were calculated. The incidence was calculated utilizing the number of all adults at the HUS area at the end of each year, obtained from Statistics Finland [Citation23].
Septicaemia screening
The occurrence of septicaemias was assessed based on the positive microbiological blood culture results. The exact origin of the blood sample (i.e. CVC or peripheral vein) was not assessed. Negative and unclear results were discarded. For conservative estimates, the results positive for the epidermidis species were discarded as these may have represented skin contaminations. At least 28 days were required between two positive blood cultures to be considered a new septicaemia. Septicaemia was not assessed for the controls.
Mortality analyses
Overall survival (OS) was defined as time from index until the end of follow-up using Kaplan–Meier method. Results were reported up to 5 years. OS overall and OS stratified by several variables in the cases and controls were explored. The variables were: CCI, age group, prevalent cancer (a cancer diagnosis recorded up to 2 years pre-index), mesenteric infarction (MI; acute or chronic mesenteric infarction; ICD-10-code K55*), acute kidney failure (AKF; ICD-10-code N17), chronic kidney failure (CKF; ICD-10-code N18), and occurrence of the first septicaemia. For MI, AKF, CKF, and first septicaemia the OS was calculated from the first occurrence of the complication instead of the index, if the condition occurred after index, to avoid immortality survival bias.
The association of case-control-status, patient characteristics and complications with OS was further studied using a Cox proportional hazards models. The fixed effect hazard ratio (HR) for the case-control-status was obtained from a mixed effects Cox model with a random effect for the case-control-pair identifier, which corresponded with the case-control matching. No other covariates were included. For age, sex, prevalent cancer, and CCI both univariable models (results not shown) and multivariable model including all variables simultaneously were calculated. For the complications, that is, AKD, and CKD separate models with time-varying covariates adjusted by age, sex, and cancer status were fit. Similarly, the cumulative number of septicaemias was modeled as a time-varying covariate and the fit was adjusted with patient age, sex, and cancer status. Proportional hazard assumptions were confirmed visually (not shown).
Statistical analyses
Statistical analyses were performed using R version 4.0.2 [Citation24]. Statistical significance level 0.05 was assumed through-out and the p-values were not adjusted for multiple testing. Results representing low patient numbers were replaced by <5 in the results tables and the corresponding percentages are not shown. The 95% confidence intervals (CI) were included. Only existing data were used, with no imputation of missing values. The proportion of missing values were reported, when applicable.
Results
Patient characteristics
Seventy-seven (77) adult patients with SBS-IF and 363 control patients were included in the analyses (). Approximately half of the patients were female, and the median age was 62.6 (). The median follow-up time was 1.7 and 3.5 years for cases and controls, respectively. Over half of the cases (n = 44) had a CCI of over one. Half of the cases (47.5%) and minority of controls (6.1%) had a cancer diagnosis. Of the cases 74.0% (n = 57) did not have any, 16.9% (n = 13) had one, and 9.1% (n = 7)) had at least two septicaemia during follow-up. AKF was present in 19.5% (n = 15) and CKF in 9.1% (n = 7) of the cases.
Table 1. Demographic and clinical characteristics. Small patient groups (<5 patients) have not been reported in detail. Occurrence of septicaemia was not studied in controls.
Incidence and prevalence
The annual number of new adult patients with SBS-IF during 2010-2019 varied between <5 to 13 (Supplementary Table S1). The overall incidence was 0.6 cases per 100,000 adults, and the annual incidence varied between 0.3 and 1.0. On average there were eight new cases annually. Adult SBS-IF prevalence was 42 in HUS in Finland in 2019.
Expenses and number of hospital-based healthcare resource utilization
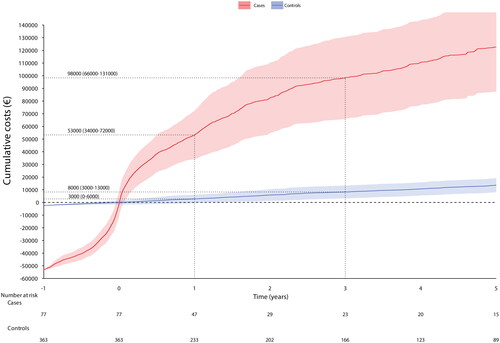
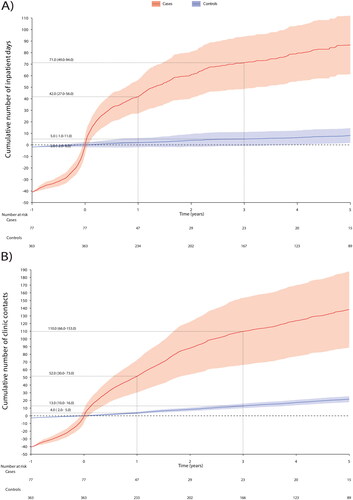
The cases incurred each year significantly more HCRU than the controls (). The HCRU expenses started to cumulate before the SBS-IF onset (a year before index €50,990; 95% CI €42,470, €59,509 per patient (PP)) (Supplementary Table S2) and peaked at the index. An average PP HCRU expenses during the first year after index was €53,000 (95% CI €34,000, €72,000) for cases and €8,000 (95% CI €3,000, €13,00) for controls. The expenses accumulation of the cases was slower after the second year from index. The HCRU and expenses of the controls remained approximately at the same level during follow-up (about €2,700 each year), however, a small positive trend occurred during the last year of follow-up. HCRU expenses comprised of inpatient days and clinic contacts, of which the cumulative number is shown in and Supplementary Table S3. The estimate of 5-year cumulative number of inpatient days was 86.7 (95% CI 88.7, 187.6) and outpatient contacts 138.1 (95% CI 61.3, 112.1). Difference in HCRU between patients with and without cancer diagnoses was non-significant (Supplemental Figure S1).
Overall survival
Forty (51.9%) cases and 32 (8.8%) controls died during the follow-up. The median survival of the cases was 4.4 years from index (95% CI 1.8, 8.3), while the median survival of the controls was not reached (). For cases, the 1-, 2-, and 5-year survival probabilities were 74.7% (95% CI 63.3, 83.1), 58.4% (95% CI 45.6, 69.2), and 47.9% (95% CI 34.7, 60.0), respectively, and for controls 99.1% (95% CI 97.2, 99.7), 97.8% (95% CI 95.3, 98.4), and 96.9% (95% CI 94.2, 98.4), respectively.
Figure 4. The Kaplan–Meier overall survival from (A) all cases and controls (no stratification), by Charlson comorbidity index (B) cases and (C) controls, by age group (D) cases and (E) controls, by cancer status (F) cases and (G) controls. A person was defined having prevalent cancer if they were recorded with any cancer diagnosis code starting with C* excluding C44 (non-melanoma skin cancer).
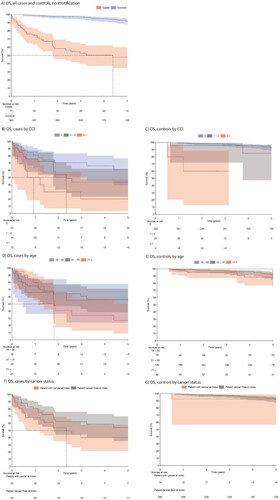
The presence of comorbidities lowered survival (log rank p < 0.05) (). Survival did not differ between age groups (log rank p > 0.05) (). The effect of cancer diagnosis on survival in the cases, was not statistically significant (log rank p > 0.05) (). Median survival of cases with AKD was 2.1 years (95% CI 0.9, NA), for those with CKD 1.9 years (95% CI 0.7, 4.3), and those with MI 0.9 years (95% CI 0.3, 5.7). Median survival for cases with first septicaemia was 0.8 years (95% CI 0.4, 5.7) (Supplemental Figure S2 A-D).
Multivariable analysis of factors associated with survival
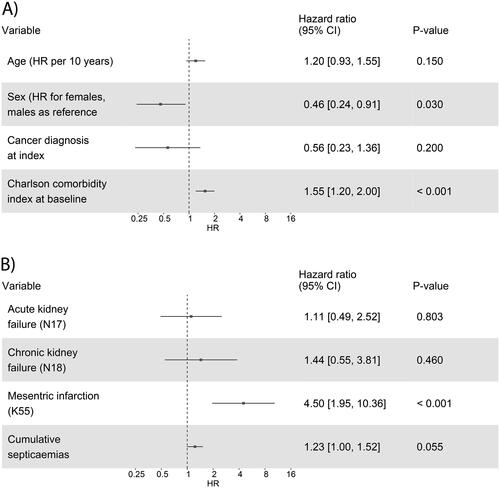
Based on a Cox model for the association of case-control-status with the overall survival, the cases were 13.5 times more likely to die during the follow-up (HR 13.5; 95% CI 11.8, 15.1; p-value <0.001). The multivariable model including variables age, sex, prevalent cancer, and CCI, showed that male sex (HR for females 0.46; 95% CI 0.24, 0.91; p < 0.05) and higher CCI (HR 1.55; 95% CI 1.2, 2.0; p < 0.001) were associated with mortality (). Age and cancer diagnosis at index were not associated with mortality (p > 0.05). The association of co-diagnoses on mortality was further studied in a separate model, showing that the presence of MI associated with a 4.5 times higher risk of death (HR 4.5; 95% CI 1.95, 10.36, p < 0.001), while AKD, CKD and cumulative number of septicaemias did not show an association ().
Figure 5. Cox multivariable fit models for association of (A) age, sex, cancer diagnosis, and Charlson comorbidity index, and (B) acute and chronic kidney failure, mesenteric infarction, and cumulative septicaemia with overall survival, adjusted by age, sex, and cancer status. The hazard ratios (HR), 95% confidence intervals (CI), and p-values are reported.
Discussion
To our knowledge, this is the first study reporting comprehensive real-world follow-up data on expenditure and survival of the adult patients with SBS-IF from Finland. Data for this study was retrieved from the largest hospital district in Finland, HUS, treating an average of 8 new adult patients with SBS-IF each year. During 2010–2019, a total of 77 adult SBS-IF-patients originating from the HUS primary catchment area were treated and followed-up at HUS. Our estimate on the incidence of adult SBS-IF (6 new SBS-IF cases per 1 million) obtained from a 10-year period is in line with previous studies from Europe (between 5 and 80 cases per 1 million) [Citation2, Citation4].
The expenses of the management of the adult patients with SBS-IF are at least tenfold compared to controls. Our estimate for 5-year cumulative expenses was €123,000, of which the first year covered €53,000. The accumulation of expenses was slower after the first year so that the expenses of second and third year together equaled the expenses of the first year. The previously estimated expenses covering only hospitalizations was US $34,130 PPY [Citation4]. Noteworthy, our estimate consisted of both inpatient days and hospital outpatient contacts. The estimates of expenses presented in our study are underestimations of the actual expenses because only mean prices of contacts were utilized for calculations. Importantly, the reported estimations of expenses are based on hospital-based HCRU alone, and do not cover HPN and home care that significantly increase HCRU [Citation25]. According to our own (unpublished) estimates, only the expenses of HPN medicines, solutions and devices vary between €23,000-67,000 PPY, even if the expenses of HPN nursing is not included. The specialty care hospital-based HCRU and home visits are included in expenses reported in the present study, even if HPN-related expenses are not.
The number of inpatient days observed in this study was higher than in a study conducted in the US (first year 42 vs. 14.7 days PPY) [Citation4]. However, we counted all cause hospitalizations while Siddiqui and colleagues counted only hospitalizations related to SBS-IF. A study conducted in Australia reported a median rate of two readmissions with average length of stay 11 days each [Citation16], which were at same magnitude as the numbers observed in our study (three hospital admissions PPY). In addition to hospitalizations, our cases had an average of 37 outpatient clinic contacts PPY. Due to rarity of the syndrome, adult patients with SBS-IF are often managed by healthcare providers with limited experience on the disease [Citation16, Citation19, Citation26]. High HCRU expenses related to this patient group suggest the need to provide services that are efficient, high quality, economically viable, and improve patient outcomes.
While SBS-associated mortality has decreased during the last decades [Citation4, Citation26], the cases were over ten times more likely to die during the follow-up. Half of the cases died during follow-up. Moreover, we observed a relatively low OS rate. In our study, 2- and 5-year survival of the adult SBS-IF patients was 60% and 49%, which is lower than in previous studies, ranging from 80–90% and 60–87%, respectively [Citation10–14, Citation17, Citation27]. Obviously, study methodology [28] and patient population characteristics, such as age, co-diagnoses, PN administration or weaning-off, and bowel anatomy, have an impact on survival in each study. Our patient cohort represents adult SBS-IF cases (SBS Type II and III) from a whole hospital district and general surgical/medical units, as opposed to previously reported cohorts (SBS Type III) from specialized IF units and HPN registries. Our data also include cases who did not survive to be discharged with HPN.
We associated presence of comorbidities, higher CCI and MI, with higher risk of death. Previously, patients with sepsis, liver disease, and metastatic cancer had significantly higher in-hospital mortality compared to patients without these conditions [Citation4]. The cumulative number of septicaemia was not associated with survival in our study, which was probably affected by small patient number (74% did not have septicaemia). The likelihood of having CVC-related bloodstream infections, decreased kidney function, or abnormal liver test results increase with the number of catheter days [Citation9], however, the number of catheter days was not known in our study. Surprisingly, cancer diagnosis at index did not influence survival or HCRU in our study. Contrary to a previous study [Citation13], OS was not affected by age. This may be because of our relatively small sample size and the underlying disease having greater impact than age at index.
Study strengths and limitations
A strength of this study was the RWD extracted from the largest hospital district in Finland. Most of the Finnish healthcare units outside HUS manage only one to two adult patients with SBS-IF [Citation19]. Thus, a nationwide study could not provide additional information compared to the current study setting. The health record data available via data lake technology enable extraction and analysis of large data sets. Due to the Finnish healthcare system, this study covers all adult SBS-IF patients living in the HUS area 2010–2019. The results represent a good overview of adult SBS-IF management from a time period proceedings a new treatment option glucagon-like peptide-2 analogue teduglutide, providing a valuable baseline and an interesting comparison for future research. Previously, the characteristics of the Finnish IF patients were concluded to be similar to other Western countries [Citation1], indicating generalizability of the current findings.
The retrospective data used were initially recorded for purposes other than research, which may cause some limitations. We may have missed a few patients with SBS-IF with the inclusion criteria used in this study. Thus, the number of patients with SBS-IF at HUS during study time is higher than 77. We have listed the number of patients with diagnoses that typically cause SBS-IF. All the SBS-IF patients had records of at least one of the SBS-IF causing diagnoses in their medical records at index, however, the cause of each patients’ SBS-IF could not be validated by a specialist. Thus, the definitive etiology of SBS-IF remains unknown. Further, causes of death, SBS type and the length of small bowel were unavailable. We did consider using variables additional to sex and birth year when matching the controls, however, the previously mentioned SBS-IF cause or bowel length could not be used. Another limitation of this study is that all-cause HCRU was reported, not SBS-IF specific. However, SBS-IF is not a single disease and SBS-IF patients typically have several concomitant disorders. Hence, reporting all-cause and not disease-specific HCRU is justified. Information on PN was incomprehensively reported at HUS data lake. Thus, we reported hospital-based HCRU and were not able to describe PN related expenses. Further, PN dependence could not be studied here, which both would be important topics for further studies.
Conclusions
In this real-world longitudinal study, we found that patients fulfilling criteria for SBS-IF (Type II and III) had a high healthcare demand and elevated mortality. Our results indicate a potential unmet need to improve care of these patients.
Author contributions
All authors contributed to conception and design of the study. LUV wrote the first draft of the manuscript. ST performed the statistical analyses. All authors contributed to interpretation of data. All authors critically commented throughout the preparation of the manuscript and approved the final draft of the manuscript.
Ethical approval and patient consent statement
The study was conducted with permission from the Hospital District of Helsinki and Uusimaa (HUS) (data permit no. HUS/141/2020) by the provision of the Act on the Secondary Use of Health and Social Data (Finland’s Ministry of Justice 552/2019), therefore no informed consent from the patients was required.
Supplemental Material
Download MS Word (1.1 MB)Acknowledgements
The authors wish to thank Minna Puttonen, Heidi Virtanen, and Heikki Mäkisalo for their input in the design and execution of this study. We also thank Tanja Nieminen and Tatu Sainio for assisting in evaluating HPN expenses.
Disclosure statement
LUV, MIL, and ST are employed by Medaffcon Oy. BK is employee of Takeda and does not own Takeda stock. LMS has received consulting and lecture fees from Takeda. MPP has received payments from Takeda for lectures, consulting and for acting as principal investigator of this study. SP & AP have received consulting and lecture fees from Takeda and for data validation for this study. VS has received consulting fee from Takeda for this study.
Data availability statement
The original data were obtained from the Hospital District of Helsinki and Uusimaa (HUS). Data can be acquired with data permission by following the guidance and application process of the registry. All authors had access to the pseudonymized aggregate data, whereas pseudonymized single-level registry data were available only to authors who analyzed the data. Only the personnel of the registry had full access to patient data.
Additional information
Funding
References
- Pohju AK, Pakarinen MP, Sipponen TM. Intestinal failure in Finland: prevalence and characteristics of an adult patient population. Eur J Gastroenterol Hepatol. 2021;33(12):1505–1510. doi: 10.1097/MEG.0000000000002082.
- Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247–307. doi: 10.1016/j.clnu.2016.01.020.
- Pironi L, Corcos O, Forbes A, et al. Intestinal failure in adults: recommendations from the ESPEN expert groups. Clin Nutr. 2018;37(6 Pt A):1798–1809. doi: 10.1016/j.clnu.2018.07.036.
- Siddiqui MT, Al-Yaman W, Singh A, et al. Short-bowel syndrome: epidemiology, hospitalization trends, In-hospital mortality, and healthcare utilization. JPEN J Parenter Enteral Nutr. 2021;45(7):1441–1455. doi: 10.1002/jpen.2051.
- Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(1 Suppl):8S–13S. doi: 10.1177/0148607114520994.
- Pironi L, Steiger E, Joly F, et al. Characteristics of adult patients with chronic intestinal failure due to short bowel syndrome: an international multicenter survey. Clin Nutr ESPEN. 2021;45:433–441. doi: 10.1016/j.clnesp.2021.07.004.
- Bielawska B, Allard J. Parenteral nutrition and intestinal failure. Nutrients. 2017;9(5):466. doi: 10.3390/nu9050466.
- Parrish CR, DiBaise JK. Managing the adult patient With short bowel syndrome. Gastroenterol Hepatol (N Y). 2017;13(10):600–608.
- Pohju AK, Hakkarainen AI, Pakarinen MP, et al. Longitudinal evolution of catheter-related bloodstream infections, kidney function and liver status in a nationwide adult intestinal failure cohort. Scand J Gastroenterol. 2022;57(7):763–767. doi: 10.1080/00365521.2022.2039281.
- Scolapio JS, Fleming CR, Kelly DG, et al. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo clinic. Mayo Clin Proc. 1999;74(3):217–222. doi: 10.4065/74.3.217.
- Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117(5):1043–1050. doi: 10.1016/s0016-5085(99)70388-4.
- Salazar E, Clermont-Dejean NM, Schwenger KJP, et al. Patients With severe gastrointestinal dysmotility disorders receiving home parenteral nutrition have similar survival As those With short-bowel syndrome: a prospective cohort study. JPEN J Parenter Enteral Nutr. 2021;45(3):530–537. doi: 10.1002/jpen.1866.
- Joly F, Baxter J, Staun M, et al. Five-year survival and causes of death in patients on home parenteral nutrition for severe chronic and benign intestinal failure. Clin Nutr. 2018;37(4):1415–1422. doi: 10.1016/j.clnu.2017.06.016.
- Dibb M, Soop M, Teubner A, et al. Survival and nutritional dependence on home parenteral nutrition: three decades of experience from a single referral Centre. Clin Nutr. 2017;36(2):570–576. doi: 10.1016/j.clnu.2016.01.028.
- Bering J, DiBaise JK. Short bowel syndrome in adults. Am J Gastroenterol. 2022;117(6):876–883. doi: 10.14309/ajg.0000000000001763.
- Siu AHY, Carey S, Jones L, et al. Detailed analysis of in-hospital costs for adult patients with type III intestinal failure: a single-center study with global implications. JPEN J Parenter Enteral Nutr. 2022;46(3):685–692. doi: 10.1002/jpen.2136.
- Fernandes G, Kaila B, Jeejeebhoy KN, et al. Canadian home parenteral nutrition (HPN) registry: validation and patient outcomes. JPEN J Parenter Enteral Nutr. 2012;36(4):407–414. doi: 10.1177/0148607111434599.
- Puttonen M, Tuominen S, Ukkola-Vuoti L, et al. Pediatric short bowel syndrome: real-world evidence on incidence and hospital resource use from a Finnish data lake. J Pediatr Gastroenterol Nutr. Epub ahead of print 25 July 2023. doi: 10.1097/MPG.0000000000003894.
- Pohju A, Pakarinen MP, Sipponen T. Fragmented management of long-term parenteral support for adult intestinal failure in Finland. Scand J Gastroenterol. 2019;54(4):414–418. doi: 10.1080/00365521.2019.1588370.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data From 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433.
- Nelson W. Recurrent events data analysis for product repairs, disease recurrences, and other applications. Philadelphia: society for Industrial and Applied Mathematics; 2003.
- Mäklin S. Health and social care unit costs in Finland in 2017, 55; 2017.
- Official Statistics Finland: Finnish population by age and region. 1990–2021. https://PxdataStatFi/PxWeb/Pxweb/Fi/StatFin/StatFin__vaerak/Statfin_vaerak_pxt_11rePx/n.d.
- R Core Team. R. A language and environment for statistical computing. 2018.
- Chandra R, Kesavan A. Current treatment paradigms in pediatric short bowel syndrome. Clin J Gastroenterol. 2018;11(2):103–112. doi: 10.1007/s12328-017-0811-7.
- Modi BP, Langer M, Ching YA, et al. Improved survival in a multidisciplinary short bowel syndrome program. J Pediatr Surg. 2008;43(1):20–24. doi: 10.1016/j.jpedsurg.2007.09.014.
- Messing B, Lémann M, Landais P, et al. Prognosis of patients with nonmalignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology. 1995;108(4):1005–1010. doi: 10.1016/0016-5085(95)90196-5.
- Fuglsang KA, Brandt CF, Scheike T, et al. Differences in methodology impact estimates of survival and dependence on home parenteral support of patients with nonmalignant short bowel syndrome. Am J Clin Nutr. 2020;111(1):161–169. doi: 10.1093/ajcn/nqz242.