Abstract
Background and aims
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a precursor of pancreatic cancer. While earlier research has shown a high prevalence of synchronous/metachronous extrapancreatic tumors in IPMN patients, these studies have often been small with retrospective data collection. The aim of the study was to examine absolute and relative risks of non-pancreatic gastrointestinal (GI) cancer precursors and mortality in histologically confirmed IPMN.
Methods
Through the nationwide ESPRESSO histopathology cohort, we retrieved data on IPMN between 1965 and 2016. Each index case was matched to ≤5 general population controls. Through Cox regression, we estimated hazard ratios (HRs) for future GI cancer precursors and death.
Results
A total of 117 patients with IPMN and 539 age- and sex-matched controls were included. Over a median of 2.1 years of follow up, we confirmed two (1.7%) incident GI cancer precursors in IPMN vs. four (0.7%) in controls, corresponding to an HR of 1.89 (95%CI = 0.34–10.55). By contrast, IPMN patients were at increased risk of death (HR 3.61 (95%CI = 1.79–7.27)). The most common cause of death in IPMN was pancreatic cancer (n = 14; 45.2% of all deaths).
Conclusions
We found no association between IPMN and other GI cancer precursors. This argues against comprehensive routine surveillance for other GI cancer precursors in IPMN patients. Mortality was increased in IPMN with pancreatic cancer being the most common cause of death, indicating the need for lifelong follow up in all resected and non-resected patients with IPMN. However, results should be confirmed in larger cohorts.
Keywords:
Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is characterized by cystic dilatation of the main or branch pancreatic duct and is a precursor of pancreatic cancer [Citation1,Citation2].
The recent use of high-quality cross-sectional imaging and the increase in preventive health check-ups among healthy individuals have increased the detection of IPMN [Citation3]. Jaundice, the presence of a mural nodule (≥5mm) or a solid component on imaging, positive cytology, or a main pancreatic duct measuring ≥10mm have all been identified as risk factors for malignancy [Citation2].
The risk of IPMN progression increases over time, and current European guidelines therefore stipulate routine follow-up of patients until patients are no longer fit for surgery (follow-ups at 6 and 12 months after IPMN, and then annually) [Citation2].
Previous research has shown a high prevalence (10–52%) [Citation4,Citation5] of synchronous/metachronous extrapancreatic tumors among patients with IPMN. One review published in 2010 showed that colorectal cancers were the most common extrapancreatic malignancies associated with IPMN in Western populations (range: 3–12%), while gastric cancers were reported in 6–15% of Asian patients with IPMNs (before or after IPMN diagnosis), but only in <1% of patients in Western studies [Citation5].
A systematic review [Citation4] in 2015 identified 15 studies on the association between IPMN and extrapancreatic malignancies (14 reported an elevated risk of extrapancreatic malignancy, particularly gastric and colorectal cancer, while the largest and only prospective study so far failed to detect any association) [Citation6]. However, most previous studies were retrospective with a weak level of evidence, and data remain inconclusive [Citation4]. Since the natural history of IPMN, as well as the vast majority of gastrointestinal (GI) tumors, share the common so-called adenoma–carcinoma sequence, where the ultimate form of malignant progression is invasive carcinoma [Citation4,Citation7], the presence of synchronous/metachronous precursors for cancer in different digestive organs is possible. The implications of these findings may be relevant because inclusion in various systematic cancer screening programs may be warranted for IPMN patients. However, there is a lack of information on the risk of precursors for GI cancer in patients with IPMN.
A meta-analysis on 937 patients with worrisome features or high-risk stigmata (predictive factors for malignancies of IPMN) who underwent non-operative management for any reason (for instance, patients may not have had surgery because of advanced age or co-morbidities) showed a low IPMN-related mortality (particularly for branch-duct IPMN), but a much higher risk of death from other causes [Citation8]. However, patients in these studies were usually aged 60-70 years with no follow-up beyond six years, and these factors may have impacted the outcome. Another limitation of the mentioned meta-analysis was missing data on grade of dysplasia as well as publication bias owing to a lack of interest in reporting data on a population that was being managed outside the guidelines, particularly if negative outcomes occurred during follow-up [Citation8]. IPMN with high-grade dysplasia (HGD) is a major concern for clinicians, and there is a lack of information regarding the outcome of IPMN of low-grade dysplasia (LGD). Studies from the USA [Citation9] and Japan [Citation10,Citation11] have shown recurrence in the remnant pancreas in 4-28% of patients with LGD who were followed for 48-71 months after resection.
For these reasons, we examined absolute and relative risks of non-pancreatic GI cancer precursors and mortality in a nationwide cohort of patients with histologically confirmed IPMN. To better understand the temporality of this association we examined IPMN and (I) earlier/concomitant GI cancer precursors, as well as (II) future GI cancer precursors.
Methods
Study population
Patients with IPMN were identified through the Epidemiology Strengthened by histoPathology Reports in Sweden (ESPRESSO [Citation12]) study, representing GI biopsies from all 28 Swedish pathology departments during the period 1965–2017. For each index individual, local histopathology IT personnel retrieved data on personal identity number [Citation13], date of biopsy, topography (where the biopsy was obtained), morphology (biopsy appearance), and – where available – free text. Since IPMN was recognized as a novel entity during the 1990s [Citation14], we restricted our study period to 2000–2016. We excluded patients with HGD to avoid the risk of misclassification (we wanted to include only patients with GI precursors and therefore excluded those with advanced dysplasia and possible transformation to invasive cancer).
Exposure and outcome measures
In Sweden, GI biopsies and surgical specimens are classified according to the Systematized Nomenclature of Medicine – Clinical Terms (SnoMed CT) classification system, which was jointly developed by the National Health Service (NHS) in England and the College of American Pathologists [Citation14]. We identified IPMN and GI cancer precursors using relevant SnoMed codes and topography codes from pathology reports (Supplementary Table 1).
Controls/reference individuals
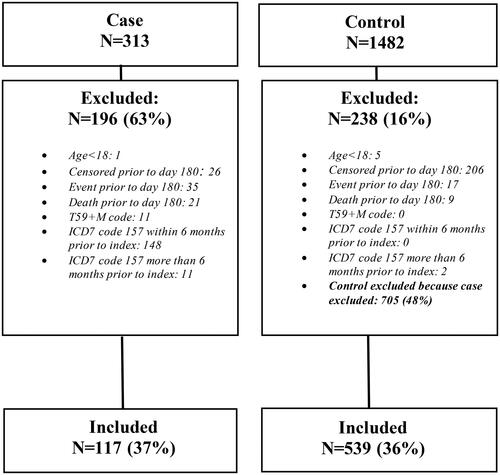
Each (index) individual with a histopathology report was matched with up to five controls for age, sex, calendar year (of biopsy), and county of residence from the Swedish Total Population Register. Flow chart of patients (subject disposition) is presented in .
Statistical analysis
We used logistic regression to calculate odds ratios (ORs) for prior or concomitant other GI cancer precursors lesions. The prior/concomitant analysis was limited to before and up to 180 days after pancreatic biopsy, consistent with the international definition of synchronous cancer ≤6 months of the index diagnosis [Citation15].
Future GI cancer precursors were assessed from day 181 onwards. Follow-up continued to the first diagnosis of (other) incident GI precursor, death, emigration, or end of follow-up (31 December 2016).
Using Cox proportional hazards regression models, we then estimated hazard ratios (HRs) for other future GI precursors in IPMN, as well as for mortality compared with the general population. We conditioned on matching set. Due to the low number of events for the primary outcome, no further adjustment was possible in the GI precursor analysis. For mortality, we also performed an analysis adjusted for education and the Charlson Comorbidity Index [Citation16]. Data on education were retrieved from the LISA database [Citation17]. Since chronic obstructive pulmonary disease (COPD) is attributable to cigarette smoking we used it as a proxy for heavy smoking in our study [Citation18]. We used a standardized list of International Classification of Diseases (ICD) codes to identify alcohol-related disorders and diseases as already published in the previous publication of our group [Citation19].
For all outcomes, we also calculated incidence rates per 1000 patient years with 95% confidence intervals.
Ethics
This project was approved by the Research Ethics Committee in Stockholm, Sweden. Due to the registry-based nature of the study, informed consent was waived [Citation20].
Results
Background results
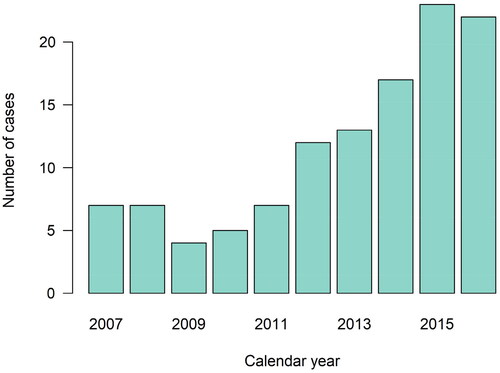
A total of 117 patients with IPMN and 539 age- and sex-matched controls were included in the main analysis (see supplemental Table S1 and for inclusion and exclusion criteria). The number of patients with IPMN increased from 2009, peaking in 2015 (2016 was not complete due to reporting from parts of pathology departments during that year) – . Among patients with IPMN, the mean age at diagnosis was 68.3 years and 48% were female.
Patients with IPMN were also more likely to have prior respiratory disease, diabetes, COPD, obesity/dyslipidemia, and alcohol-related disease. Of note, almost all IPMN cases (97%) had ≥3 healthcare visits within the last year before diagnosis. A Charlson score of ≥3 was seen in 28% of IPMN cases, compared to in 11% of controls. Additional baseline characteristics are presented in .
Table 1. Baseline characteristics of study cohort.
Main results
IPMN cases were at increased risk of earlier GI cancer precursors compared to controls from the general population (OR = 6.67; 95%CI = 3.45–14.29).
Over a median of 2.1 years of follow up, we identified two (1.7%) incident GI cancer precursors among patients with IPMN compared to four (0.7%) in the control group, corresponding to an HR (stratified for matching factors) of 1.89 (95%CI = 0.34–10.55) ().
Table 2. Description of events (GI cancer precursors) among the study population.
Patients with IPMN were however at increased risk of death (HR = 3.61; 95%CI = 1.79–7.27) ( and and ). In a sensitivity analysis, we also adjusted for education and the Charlson Comorbidity Index. This adjustment did not impact on the risk estimates (adjusted HR = 3.33; 95%CI = 1.36–8.13).
Figure 3. Cumulative incidence curves for GI precursor lesions and all-cause mortality in patients with IPMN compared to controls. IPMN: intraductal papillary mucinous neoplasm; GI: gastrointestinal.
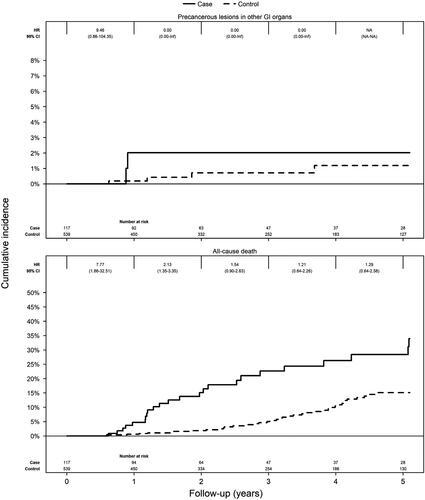
Table 3. Risk of all-cause and cause-specific mortality in patients with IPMN and matched general population comparators (Cox model).
Table 4. Description of deaths among the study population.
Pancreatic cancer was the most common cause of death in IPMN (n = 14; 45.2% of all deaths). Pancreatic cancer was the cause of death in only two (2.7%) controls.
Extrapancreatic cancers were found in eight (6.8%) IPMN patients and 21 (3.9%) controls.
Discussion
In this population-based cohort study composed of all adults in Sweden with histologically verified IPMN and matched controls from the general Swedish population, IPMN was not associated with cancer precursors in other GI organs. GI cancer precursors were few, and further analyses of subgroups were not possible. Our results contrast with previous studies that have shown significantly higher risks of GI cancer precursors (adenomatous polyps of the colon and Barrett’s metaplasia) in IPMN than in controls either with pancreatic cancer or originating from the general population [Citation4,Citation21,Citation22].
We found extrapancreatic malignancies in 6.8% of IPMN patients, compared to 3.9% in controls.
When Sugiyama et al. examined 42 patients with IPMN (16 had benign IPMN and 26 had malignified IPMN), they detected colorectal adenomas in 21% of IPMN patients [Citation21]. However, this study did not include general population controls, and mixing patients with benign and malignant IPMN significantly limits their conclusions. Reid-Lombardo et al. included 471 patients diagnosed with IPMN at the Mayo Clinic in 1994–2006, and compared them with 471 pancreatic cancer patients and 1413 individuals from the general referral population [Citation22]. They found that the proportion of IPMN patients with extrapancreatic neoplasm diagnosed before or at the time of IPMN was 52%, compared with 36% of patients with pancreatic cancer and 43% of the general population [Citation22]. The Mayo Clinic study showed that the most common benign neoplasms in the IPMN group were adenomatous colon polyps (24% versus 16% in the pancreatic cancer group and 15% in the general population) and Barrett’s metaplasia of the esophagus (IPMN: 4%, pancreatic cancer: 0.6%, general population: 1%), so the authors concluded that an IPMN diagnosis necessitated both a colonoscopy and a detailed history of upper GI symptoms/risk factors and potentially an upper endoscopy [Citation22]. However, the Mayo Clinic study was limited by its retrospective design (information regarding prior cancer history might have been missed and not documented) and its limited data on the frequency of follow-up of patients outside the Mayo Clinic. Besides, patients treated at highly-specialized referral centers may have increased comorbidity and closer surveillance after diagnosis due to a more severe disease than the average IPMN patient [Citation22]. The vast majority of previous studies have focused on extrapancreatic cancers and not on GI cancer precursors in IPMN patients, and have been performed retrospectively with a weak level of evidence [Citation4]. Many IPMN studies also use individuals with pancreatic tumors as controls. This is not optimal due to substantial differences in epidemiology (i.e. sex distribution), pathomorphology, genetics, and prognosis [Citation4].
We noticed a significantly higher mortality among IPMN patients. Despite excluding IPMN with HGD (to avoid bias from possible misdiagnosis), pancreatic cancer was the most common cause of death in IPMN. Considering current guidelines [Citation2] for the management of pancreatic cystic lesions (annual check-ups even after pancreatic resection), we suspect that the recommended surveillance in IPMN may have failed and that pancreatic cancers were not recognized in a timely manner, or that the initial resection of the pancreatic tumor was not radical (R0).
Three features are most commonly reported as being associated with progression in the pancreatic remnant: the presence of HGD in resected specimens, margin positive resection, and a family history of pancreatic cancer [Citation23]. The role of LGD and outcome after resection has not been fully elucidated. Fuji et al. found recurrence in the remnant pancreas in 10% of patients with LGD during the median follow up of 71 months after resection [Citation10]. In another study from Japan, Hirono et al. found different types of recurrence (intermediate-grade dysplasia, HGD, invasive IPMN, pancreatic cancer), of remnant pancreas in 5.5% of patients during the median follow up of 53 months, however neither of them has LGD at initial operative treatment [Citation11]. In recently published data from Johns Hopkins’ Hospital, 449 consecutive IPMN patients undergoing resection were analyzed (including 319 patients with low/intermediate grade of dysplasia), with a median follow-up of 48.9 months; noninvasive progression was found in 81 (25%) and invasive progression in seven (2%) patients with low/intermediate grade of dysplasia [Citation9]. The same study showed that the overall rate of progression (in all IPMN patients) was 27.6% (3.6% progressed to invasive carcinoma and 24.1% to noninvasive IPMN), and that progression to invasive carcinoma occurred at a median of seven years after surgery [Citation9].
We had no information regarding the pathological subtypes of IPMN. In their meta-analysis, Koh et al. included 14 studies with a total of 1199 patients and showed that pancreaticobiliary subtype was associated with the greatest likelihood of invasion among all IPMN subtypes, whereas the gastric subtype was associated with the least likelihood of invasion [Citation24].
Our study lacked information on margin status after surgery. Kim et al. analyzed 353 patients with LGD and found that R0 resection was achieved in 79% of patients, and there were three recurrence cases (1.1%) in the resection margin or remnant pancreas [Citation25]. A multicenter study performed at eight academic medical centers from the Central Pancreas Consortium in USA included 502 patients who underwent surgery for IPMN, showing that the majority of positive margins were associated with LGD, but margin positivity was not associated with recurrence of either IPMN or invasive cancer [Citation26].
It is important to emphasize that pancreatic cancer arising in the remnant pancreas is not always a relapse of the preceding primary cancer. Luchini et al. used an integrated histopathological and molecular approach in patients with metachronous pancreatic ductal adenocarcinoma lesions, describing two distinct potential processes (some are true recurrences of the initial primary in the remnant pancreas, while others are true second primaries) [Citation27]. Gotoh et al. collected clinicopathological data from patients after pancreatectomy for a second high-risk lesion in the remnant pancreas, and performed mutational and immunohistochemical analysis of major genes associated with pancreatic cancer as well as targeted next-generation sequencing, showing that genetic assessment might help differentiate metachronous multifocal high-risk lesions from local recurrence [Citation28].
Additional explanations for the high number of pancreatic cancers in IPMN include risk factors such as diabetes, COPD (proxy for heavy smoking), obesity, and alcohol-related disease, for which we adjusted our calculations. Unfortunately, we lacked data on actual alcohol consumption. Since IPMN recurrence may occur even five years after IPMN resection [Citation2,Citation29], lifelong surveillance is recommended following resection of an IPMN, as long as the patient is fit and willing to undergo surgery if indicated [Citation2].
We acknowledge some limitations of our study. We lacked data on number and size of IPMN, dilatation of the main pancreatic duct, and presence of enhancing mural nodules in the cyst, as well as information on CA19-9 levels (all of which are important parameters in the context of worrisome features for malignant alteration of IPMN) [Citation2]. Another limitation is that we could not distinguish between IPMN patients diagnosed through biopsy and those undergoing surgery. We had no data on the type of IPMN (branch duct vs main duct or mixed-type IPMN). This distinction has also been lacking in other studies [Citation4], and represents an important knowledge gap considering the significant difference between these IPMN entities in terms of natural history and prognosis.
Follow-up time in our study was limited (median 2.1 years, mainly because of high mortality rates, but also because most IPMN cases were diagnosed in 2010 or later and our follow-up ended in late 2016), and this may have contributed to the low number of GI cancer precursors. The low statistical power also limited our opportunities to examine precursor subtypes. Finally it should be noted that our study was observational and cannot determine causality.
Among the strengths of this paper is the histological verification of IPMN. Other strengths are the population-based setting, the large sample size, and the many sensitivity analyses. Our study paves the way for future studies that may bridge knowledge gaps and help physicians apply a personalized approach to surveillance of IPMN patients. A better understanding of risk factors for IPMN progression is needed, as well as strict compliance with existing guidelines for patients undergoing partial pancreatic resection for IPMN [Citation2].
Conclusions
We found no association between IPMN and other GI cancer precursors. This argues against comprehensive routine surveillance for other GI cancer precursors in IPMN patients, but it is notable that there were few events, and our results should be confirmed in larger cohorts.
Mortality was increased in IPMN, and the high mortality from pancreatic cancer is a concern and should spur clinicians to carefully follow up both resected and non-resected patients with IPMN.
Transparency
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors contribution
All authors conceived and designed the study. MV and JFL wrote the first draft of the paper. JFL supervised the project. MV and JFL funded the study. MT carried out the statistics. All authors interpreted the data and contributed to the writing of the paper. All authors revised and approved the final version. JFL takes responsibility for the integrity of the data and the accuracy of the data analyses. JFL is the guarantor of the data.
Ethical approval
This study was approved by the Stockholm Ethics Review Board (2014/1287-31/4 and 2018/972-32).
Grants and financial support
The Swedish Cancer Foundation (JFL).
| Abbreviations | ||
| COPD | = | chronic obstructive pulmonary disease |
| ESPRESSO | = | Epidemiology Strengthened by histoPathology Reports in Sweden |
| GI | = | gastrointestinal |
| HGD | = | high-grade dysplasia |
| HR | = | hazard ratio |
| ICD | = | International Classification of Diseases |
| IPMN | = | intraductal papillary mucinous neoplasm |
| LGD | = | low-grade dysplasia |
| OR | = | odds ratio |
| SnoMed CT-System | = | Systematized Nomenclature of Medicine – Clinical Terms. |
Supplemental Material
Download MS Word (15.3 KB)Disclosure statement
Dr. Ludvigsson has coordinated an unrelated study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from the Janssen corporation. Dr Ludvigsson has also received financial support from MSD to develop a paper reviewing national healthcare registers in China. Dr Vujasinovic and Dr Löhr have received lecture fees from Abbott and Viatris. Dr Vujasinovic and Dr Löhr have also received financial support for writing a scientific book from Viatris. All other authors declare that they have no conflicts of interest and nothing to declare.
Data sharing statement
Other researchers can apply for our data through the different Swedish pathology departments, and through the Swedish National Board of Health and Welfare.
References
- Tanaka M, Kobayashi K, Mizumoto K, et al. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2005;40(7):669–675. doi:10.1007/s00535-005-1646-4.
- European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi:10.1136/gutjnl-2018-316027.
- van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, et al. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16(11):676–689. doi:10.1038/s41575-019-0195-x.
- Pugliese L, Keskin M, Maisonneuve P, et al. Increased incidence of extrapancreatic neoplasms in patients with IPMN: fact or fiction? A critical systematic review. Pancreatology. 2015;15(3):209–216. doi:10.1016/j.pan.2015.03.007.
- Benarroch-Gampel J, Riall TS. Extrapancreatic malignancies and intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2(10):363–367. doi:10.4240/wjgs.v2.i10.363.
- Kawakubo K, Tada M, Isayama H, et al. Incidence of extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60(9):1249–1253. doi:10.1136/gut.2010.227306.
- Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425(4):357–367. doi:10.1007/bf00189573.
- Vanella G, Crippa S, Archibugi L, et al. Meta-analysis of mortality in patients with high-risk intraductal papillary mucinous neoplasms under observation. Br J Surg. 2018;105(4):328–338. doi:10.1002/bjs.10768.
- Amini N, Habib JR, Blair A, et al. Invasive and noninvasive progression after resection of noninvasive intraductal papillary mucinous neoplasms. Ann Surg. 2022;276(2):370–377. doi:10.1097/sla.0000000000004488.
- Fuji T, Umeda Y, Takagi K, et al. Optimal surveillance of intraductal papillary mucinous neoplasms of the pancreas focusing on remnant pancreas recurrence after surgical resection. BMC Cancer. 2022;22(1):588. doi:10.1186/s12885-022-09650-w.
- Hirono S, Kawai M, Okada K-I, et al. Long-term surveillance is necessary after operative resection for intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2016;160(2):306–317. doi:10.1016/j.surg.2016.04.007.
- Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (epidemiology strengthened by histoPathology reports in Sweden). Clin Epidemiol. 2019;11:101–114. doi:10.2147/clep.S191914.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi:10.1007/s10654-009-9350-y.
- Klöppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology. 1998;45(24):1981–1985.
- Lv M, Zhang X, Shen Y, et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96(17):e6799. doi:10.1097/md.0000000000006799.
- Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson Comorbidity Index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. doi:10.2147/clep.S282475.
- Ludvigsson JF, Svedberg P, Olén O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. doi:10.1007/s10654-019-00511-8.
- Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11(1):36. doi:10.1186/1471-2466-11-36.
- Bergman D, Hagström H, Capusan AJ, et al. Incidence of ICD-Based diagnoses of alcohol-related disorders and diseases from Swedish nationwide registers and suggestions for coding. Clin Epidemiol. 2020;12:1433–1442. doi:10.2147/clep.S285936.
- Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. doi:10.2147/clep.S90589.
- Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94(2):470–473. doi:10.1111/j.1572-0241.1999.879_h.x.
- Reid-Lombardo KM, Mathis KL, Wood CM, et al. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251(1):64–69. doi:10.1097/SLA.0b013e3181b5ad1e.
- Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17(5):738–753. doi:10.1016/j.pan.2017.07.007.
- Koh YX, Zheng HL, Chok A-Y, et al. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157(3):496–509. doi:10.1016/j.surg.2014.08.098.
- Kim HS, Han Y, Kang JS, et al. Fate of patients with intraductal papillary mucinous neoplasms of pancreas after resection according to the pathology and margin status: continuously increasing risk of recurrence even after curative resection suggesting necessity of lifetime surveillance. Ann Surg. 2022;276(4):e231–e238. doi:10.1097/sla.0000000000004478.
- Dhar VK, Merchant NB, Patel SH, et al. Does surgical margin impact recurrence in noninvasive intraductal papillary mucinous neoplasms?: a multi-institutional study. Ann Surg. 2018;268(3):469–478. doi:10.1097/sla.0000000000002923.
- Luchini C, Pea A, Yu J, et al. Pancreatic cancer arising in the remnant pancreas is not always a relapse of the preceding primary. Mod Pathol. 2019;32(5):659–665. doi:10.1038/s41379-018-0183-7.
- Gotoh Y, Ohtsuka T, Nakamura S, et al. Genetic assessment of recurrent pancreatic high-risk lesions in the remnant pancreas: metachronous multifocal lesion or local recurrence? Surgery. 2019;165(4):767–774. doi:10.1016/j.surg.2018.10.025.
- Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-Type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg. 2015;220(5):839–844. doi:10.1016/j.jamcollsurg.2015.01.051.