Abstract
The basic principle for the treatment of idiopathic diarrhoea (functional diarrhoea K59.1) is to delay transit through the gut in order to promote the absorption of electrolytes and water. Under mild conditions, bulking agents may suffice. With increasing severity, antidiarrhoeal pharmaceuticals may be added in a stepwise manner. In diarrhoea of unknown aetiology, peripherally-acting opioid receptor agonists, such as loperamide, are first-line treatment and forms the pharmaceutical basis of antidiarrheal treatment. As second-line treatment opium drops have an approved indication for severe diarrhoea when other treatment options fail. Beyond this, various treatment options are built on experience with more advanced treatments using clonidine, octreotide, as well as GLP-1 and GLP-2 analogs which require specialist knowledge the field.
KEY MESSAGES
Chronic diarrhoea without an established cause is common.
There are a small number of clinical trials, often with a limited number of patients or healthy volunteers.
Treatment is often carried out on a trial-and-error basis, with considerable variation in the choice of treatment.
There is a paucity of guidelines, and there is a gap in knowledge concerning treatment goals, such as the frequency, consistency and form of stool.
The stepwise approach to the treatment of chronic idiopathic diarrhoea described in this article is based on clinical knowledge and experience.
Introduction
Chronic idiopathic diarrhoea is common and affects 5% of the population, diagnosed according to ICD-10 as functional diarrhoea (K59.1). Diagnosis is often challenging and treatment often needs to be initiated prior to identification of the actual cause. With this article, we provide recommendations for a stepwise approach to the use of antidiarrhoeal pharmaceuticals. This proposal is based on knowledge and experience of diarrhoea in clinical practice and thus not on the usual evidence process.
Character of faecal matter and underlying mechanisms
Diarrhoea involves changes in stool frequency, consistency, and quantity. It is defined as the passage of three or more loose or watery stools per day, exceeding a total of 200 grams or 200 millilitres [Citation1]. The classification of diarrhoea further distinguishes between three main types: acute diarrhoea, lasting less than 2 weeks; persistent diarrhoea, ranging from 2 to 4 weeks; and chronic diarrhoea, persisting for more than 4 weeks [Citation2].
Diarrhoea is a symptom which reflects an imbalance between the absorption and secretion of water in the gut. With a normal daily intake and gastrointestinal secretion of about 9 litres, approximately 98% of this is subsequently absorbed, resulting in a manageable consistency of stools. It has recently been shown that the faecal water content varies within a narrow range in patients experiencing diarrhoea [Citation3]. From a water content of 70.5 ± 0.8% in normal faeces (Bristol stool form 3–5), the water content was found to be 74.6 ± 1.6% in loose or watery stool (Bristol stool form 6–7), estimating that the water content is greater than 78% in half of subjects with diarrhoea [Citation3].
The attending clinician often faces a condition that requires immediate treatment. After exclusion of an inflammatory condition, the diarrhoea, be it of secretory, osmotic or motility character, should be treated according to empirical standards. Clinical experience teaches that it is often necessary to address the problem with symptomatic treatment without certain knowledge of the cause of the diarrhoea.
The problematic nature of diarrhoea within the fields of gastroenterology, surgery, and oncology is enigmatic with a profound knowledge gap of how to tackle the problem, spanning from life-threatening dehydration, over inconvenience to low quality of life. The aim of this article was to create a stepwise treatment approach to address the problem on the basis of physiological mechanisms and pharmacological control.
The clinical problem
When investigating chronic diarrhoea, the question is usually whether it is a case of inflammatory or functional bowel disease. The situation becomes increasingly challenging if tests for faecal calprotectin, transglutaminase IgA antibodies, 7α-hydroxy-4-cholesten-3-one, (7-hydroxy-cholestenone), or alternatively, the75Se-homotaurocholic acid (75Se-HCAT) test, faecal PCR analysis of pathogenic intestinal bacteria, Clostridioides difficile toxin, direct microscopic examination of parasites, and colonoscopy with biopsies are inconclusive. Furthermore, intestinal stenoses and fistulae should be excluded as causes of diarrhoea. Following a negative workup, symptomatic treatment is the remaining course of action.
Treatment steps
In the treatment of patients with severe diarrhoea, it is important to escalate therapy in a stepwise manner. The severity of diarrhoea is estimated to be on the basis of the shape of the stool, using the Bristol stool form scale. Keeping a stool diary for 1–2 weeks may help to obtain a clear picture of the problem. According to the Bristol stool form scale [Citation4], stool types 6 and 7 (i.e., loose or watery stools) are primarily associated with diarrhoea, while stool type 5 (“somewhat loose stools”) is sometimes also included in the diarrhoea concept.
The treatment proposal is based on the knowledge and experience of diarrhoea in clinical practice rather than an evidence-processing method ().
Figure 1. Stepwise treatment protocol for diarrhoea. The treatment options above the line are the primary options with a therapeutic indication; those below the line are secondary option without an approved indication. The arrow illustrates increased severity of diarrhoea.
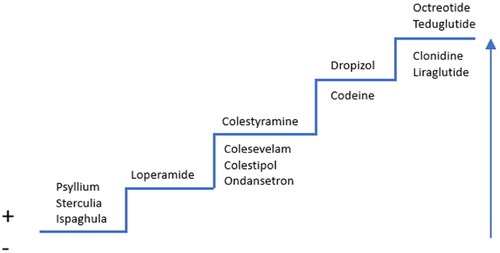
The first step is treatment with the soluble fibres in psyllium and ispaghula, which are fermented to form a viscoelastic water-binding mass which increases the transit time and, resultingly, water absorption [Citation1,Citation5]. Sterculia are insoluble fibres with a high ability to bind water and normalise the consistency of stool [Citation6]. There is compelling scientific support for treatment with these products in constipation, but weak support in chronic diarrhoea. Nutritional management of functional diarrhoea with low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) from a registered dietitian may be employed but there is no "one-size-fits-all" approach to this management. However, low FODMAP may shape personalised nutrition interventions to alleviate symptoms.
In the next step, loperamide is used. This pharmaceutical has an established place in treatment. Loperamide stimulates µ-type opioid receptors in the gut, reducing secretion and motility and increasing water absorption [Citation7,Citation8]. It has a high protein binding rate (95%) and a half-life of 9 to 14 h. Passage across the blood-brain barrier is limited and a “kick” effect is absent at therapeutic doses. In acute diarrhoea, the loperamide dose is 2 tablets of 2 mg after each loose stool until acceptable consistency is reached (the maximum daily dose is 16 mg). In case of prolonged diarrhoea, it is usually recommended not to wait for a diarrhoea episode before the administration of a new dose. Instead, a suitable regular dose that can counteract diarrhoea without inducing constipation should be identified. Loperamide is less effective in severe forms of diarrhoea [Citation9]. High doses (40-800 mg) have been shown to be able to cause changes such as QT prolongation and torsades de pointes in ECG [Citation10]. Compared with tablets, uptake of loperamide is faster and the treatment effect is more marked when given in capsule form [Citation11].
Bile salt malabsorption develops in diseases of the distal ileum, after intestinal resection, and after irradiation of the ileum. Bile salt malabsorption also occurs after cholecystectomy in the presence of pathological intestinal flora and intestinal dysmotility, functional diarrhoea, and in IBS-D as a probable result of idiopathic overproduction of bile. Bile salt-binding resins may have a positive effect in these conditions [Citation12]. Cholestyramine is indicated in bile salt-induced diarrhoea. Its use is sometimes impaired by undesirable effects such as abdominal pain, nausea, and an unpleasant taste. Treatment is initiated with ½-1 sachet of 4 g once or twice daily, and the dose is subsequently titrated to the desired effect. Colestipol has also been used to treat bile salt malabsorption. Colesevelam, which is a more potent bile salt sequestrant and has a more pleasant taste, offers a new option. In many cases, high doses of colesevelam are required initially, and the dose is later adjusted to lower level, 3 tablets of 625 mg twice daily. A particular problem with bile salt sequestrants is that they reduce the bioavailability of other pharmaceuticals and fat-soluble vitamins. To minimise the risk of interactions, any other medication should be taken 1 h before or 4 h after the bile salt sequestrant. This effect appears to be less pronounced with colesevelam [Citation13].
5-HT3 receptor antagonists may improve stool consistency, especially in IBS-D where serotonin is considered significant for the symptom progression [Citation14]. Ondansetron has been shown to be effective in IBS-D in a small number of studies [Citation14–16]. Low initial doses of 4 mg once or twice daily are frequently sufficient. To achieve a full effect, the dose is later titrated to 4-8 mg up to 3 times daily.
Additional treatment steps may be taken in idiopathic diarrhoea that has been refractory to these pharmaceutical agents. Opium preparations have established effects on diarrhoea which have been known since the nineteenth century. Previously, “opium tincture” could be prescribed as extemporaneous preparations forming the basis of the development of medical grade oral opium drops. The pharmaceutical product contains several opium alkaloids, all of which exert inhibitory effects on motility and secretion [Citation17]. These opium alkaloids stimulate μ-opioid receptors, resulting in a delay in transit time through both the small intestine and the colon. This contributes to an increase in fluid absorption and a subsequent reduction in diarrhoea [Citation18]. Since the pharmaceutical is distributed across the blood-brain barrier, there is a risk for drug dependence. Development of tolerance for the constipation effect in the colon has not been shown [Citation18,Citation19]. The half-life of morphine, one of the constituents in the product, is 2 h, while active metabolites have longer half-lives of approximately 7 h. Opium tincture is indicated for symptomatic treatment of severe diarrhoea in adults when the effect of other anti-diarrhoeal treatments have been insufficient. The regular dose of the medical grade opium tincture is 2.5–5 mg (equal to 5–10 drops), 2–3 times daily with a maximum daily dose of 60 mg.
Codeine is an option in the treatment of prolonged, severe diarrhoea, even though it does not hold this indication. The substance is absorbed rapidly [Citation20] and passes through the blood-brain barrier, affecting opioid receptors in both the brain and the intestine with a widespread inhibitory effect on motility [Citation21]. Around 10% of codeine is bioactivated into morphine and there is a risk of central nervous “kick effect”, abuse, and dependency. The effect of codeine is short in duration with a half-life of 2-3 h. Codeine is administered at a dose of 25 or 30 mg (dose equivalent 6 mg loperamide) 4 times daily [Citation21,Citation22].
On the top of the stepwise treatment approach are products whose use requires specialist expertise in the field. Treatment with octreotide is based on reduced secretion of fluid and electrolytes in the jejunum and ileum [Citation23]. It is indicated for the treatment of hormone producing tumours with secretory diarrhoea. Octreotide has also demonstrated good treatment efficacy in HIV-associated diarrhoea [Citation24] and superior effect to that of loperamide in chemotherapy-induced diarrhoea [Citation25].
Other more tentative treatments include liraglutide (a GLP-1 receptor agonist) which has shown efficacy in short bowel syndrome and bile salt-induced diarrhoea [Citation26,Citation27]. The effect is due to slowed transit through the gastrointestinal tract, which increases the absorption of fluid and electrolytes.
Teduglutide (a recombinant GLP-2 analogue) stimulates the growth of the small intestine, which increases absorption and reduces the loss of fluid and electrolytes in short bowel syndrome [Citation28].
Another possibility to influence motility and absorption is through the stimulation of adrenergic autoreceptors with clonidine as an α2 receptor agonist. The treatment has an antihypertensive effect, but it also suppresses diarrhoea in IBS-D and diabetic enteropathy [Citation29,Citation30].
Combination treatment
There are several treatments available with different pharmacological mechanisms of action, and different treatments can also be combined. For example, standard treatment with fibre supplements can be combined with loperamide or ondansetron. Similarly, colestyramine or colesevelam can be combined with other antidiarrhoeal pharmaceuticals, which should be taken at different time points. In severe diarrhoea, opioid receptor agonists can also be combined with an α2 receptor agonist as an additional therapeutic step
A group of concern consists of patients who, in the final stages of life, receive treatment with high doses of opioid-type analgesics with persisting diarrhoea despite the assumption that all opioid receptors are saturated. In these cases, treatment with an alternative mechanism of action should be explored. Ondansetron and clonidine are options in such situations. Finally, short bowel syndrome is beyond the scope of this article but re-establishment of bowel continuity should always be considered in case of diverted distal bowel.
Conclusion
In patients with diarrhoea without a known cause, treatment is based on the principle that delayed transit through the gastrointestinal tract increases the absorption of fluid and electrolytes. In mild cases, bulking agents may often suffice. Treatment with additional antidiarrhoeal pharmaceuticals can subsequently be started on a trial basis in a stepwise manner. In bile salt-induced diarrhoea, there is a clear indication for bile salt binding resins. In diarrhoea without a known cause, a peripherally acting opioid receptor agonist, such as loperamide, is adequate. In addition, the opium tincture can be used for symptomatic treatment of severe diarrhoea when other antidiarrhoeal pharmaceuticals have proven insufficient. The use of other treatments requires a specialist with specific competence and clinical experience.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15(2):182–193 e3. doi: 10.1016/j.cgh.2016.07.028.
- Camilleri M, Sellin JH, Barrett KE. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology. 2017;152(3):515–532 e2. doi: 10.1053/j.gastro.2016.10.014.
- Nordin E, Hellström PM, Brunius C, et al. Modest conformity between self-reporting of bristol stool form and fecal consistency measured by stool water content in irritable bowel syndrome and a FODMAP and gluten trial. Am J Gastroenterol. 2022;117(10):1668–1674. doi: 10.14309/ajg.0000000000001942.
- O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300(6722):439–440. doi: 10.1136/bmj.300.6722.439.
- Nagarajan N, Morden A, Bischof D, et al. The role of fiber supplementation in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(9):1002–1010. doi: 10.1097/MEG.0000000000000425.
- McRorie JW.Jr. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 2: what to look for and how to recommend an effective fiber therapy. Nutr Today. 2015;50(2):90–97. doi: 10.1097/NT.0000000000000089.
- Petruccelli BP, Murphy GS, Sanchez JL, et al. Treatment of traveler’s diarrhea with ciprofloxacin and loperamide. J Infect Dis. 1992;165(3):557–560. doi: 10.1093/infdis/165.3.557.
- Murphy GS, Bodhidatta L, Echeverria P, et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann Intern Med. 1993;118(8):582–586. doi: 10.7326/0003-4819-118-8-199304150-00002.
- Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15(10):e447-60–e460. doi: 10.1016/S1470-2045(14)70006-3.
- Wu PE, Juurlink DN. Loperamide cardiac toxicity: pathophysiology, presentation, and management. Can J Cardiol. 2022;38(9):1378–1383. doi: 10.1016/j.cjca.2022.04.005.
- Doser K, Meyer B, Nitsche V, et al. Bioequivalence evaluation of two different oral formulations of loperamide (diarex lactab vs imodium capsules). Int J Clin Pharmacol Ther. 1995;33(8):431–436.
- Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9(3):332–339. doi: 10.5009/gnl14397.
- Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010;2(1):51–63. doi: 10.1177/1758834009355164.
- Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6(5):545–555. doi: 10.1016/j.cgh.2007.12.015.
- Spiller RC. Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol. 2011;11(1):68–74. doi: 10.1016/j.coph.2011.02.005.
- Steadman CJ, Talley NJ, Phillips SF, et al. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc. 1992;67(8):732–738. doi: 10.1016/s0025-6196(12)60797-6.
- Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737–141736. doi: 10.1155/2014/141737.
- Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2(1):17–21. doi: 10.1038/ajgsup.2014.5.
- Ling GS, Paul D, Simantov R, et al. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627–1636. doi: 10.1016/0024-3205(89)90272-5.
- Findlay JW, Butz RF, Welch RM. Codeine kinetics as determined by radioimmunoassay. Clin Pharmacol Ther. 1977;22(4):439–446. doi: 10.1002/cpt1977224439.
- Schiller LR, Davis GR, Santa Ana CA, et al. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982;70(5):999–1008. doi: 10.1172/jci110711.
- O’Brien JD, Thompson DG, McIntyre A, et al. Effect of codeine and loperamide on upper intestinal transit and absorption in normal subjects and patients with postvagotomy diarrhoea. Gut. 1988;29(3):312–318. doi: 10.1136/gut.29.3.312.
- Högenauer C, Aichbichler B, Santa Ana C, et al. Effect of octreotide on fluid absorption and secretion by the normal human jejunum and ileum in vivo. Aliment Pharmacol Ther. 2002;16(4):769–777. doi: 10.1046/j.1365-2036.2002.01228.x.
- Cello JP, Grendell JH, Basuk P, et al. Effect of octreotide on refractory AIDS-associated diarrhea. A prospective, multicenter clinical trial. Ann Intern Med. 1991;115(9):705–710. doi: 10.7326/0003-4819-115-9-705.
- Zidan J, Haim N, Beny A, et al. Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann Oncol. 2001;12(2):227–229. doi: 10.1023/a:1008372228462.
- Hvistendahl M, Brandt CF, Tribler S, et al. Effect of liraglutide treatment on jejunostomy output in patients with short bowel syndrome: an open-label pilot study. JPEN J Parenter Enteral Nutr. 2018;42(1):112–121. doi: 10.1177/0148607116672265.
- Kårhus ML, Brønden A, Røder ME, et al. Remission of bile acid malabsorption symptoms following treatment with the glucagon-like peptide 1 receptor agonist liraglutide. Gastroenterology. 2019;157(2):569–571. doi: 10.1053/j.gastro.2019.04.002.
- O’Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11(7):815–823 e1–3. doi: 10.1016/j.cgh.2012.12.029.
- Camilleri M, Kim DY, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1(2):111–121. doi: 10.1053/cgh.2003.50019.
- Valdovinos MA, Camilleri M, Zimmerman BR. Chronic diarrhea in diabetes mellitus: mechanisms and an approach to diagnosis and treatment. Mayo Clin Proc. 1993;68(7):691–702. doi: 10.1016/s0025-6196(12)60606-5.

