Abstract
The objective of this study was to investigate whether oxyanionic phosphate (P) and sulfate (S) fertilizer management could influence selenium (Se) uptake by wheat (Triticum aestivum) in medium and high Se areas. Field studies were established at two locations for two growing seasons in central South Dakota, USA. Phosphate fertilizer was applied using three different methods (banded with seed, surface-broadcasted in the fall, or surface-broadcasted in the spring) using six different P rates. Sulfate fertilizers were broadcasted at four rates in the fall. Selenium concentration in wheat grain was significantly influenced by the interaction of P application methods and rates, but it was dependent on location. Grain Se concentration decreased in high Se availability soil when P fertilizer was applied, due to the dilution effect. Grain Se concentration and uptake was significantly decreased as S applications increased due to the competition effect, but the depression was apparent in high Se availability soil. The results from this study showed that P and S fertilizer management can influence Se level in wheat grain grown in naturally high Se areas, even though overall grain Se level was strongly associated with location variation.
Introduction
Selenium (Se) is an essential micronutrient for humans and animals, although Se has not been proven to be essential for plants (Terry et al. Citation2000). Interest in Se has increased over the past few decades because it has many potential benefits for improving human health. Studies have demonstrated that higher Se intake is associated with reduced cancer risk (Finley and Davis Citation2001; Brandt et al. Citation2003; Combs Citation2004; Whanger Citation2004), and enhancement of the anti-viral infection and immune systems in humans (Beck Citation2001; Arthur et al. Citation2003). The recommended daily intake (RDI) of 55 µg Se day−1 is readily met in most North Americans, but intake for a large number of people in Europe and Asia does not approach the RDI. Combs (Citation2001) estimated that approximately one billion people around the world may have inadequate intake of Se. In addition, the vast majority of the world's population consumes food at suboptimal Se levels. Furthermore, the Se content in world food supplies may be decreasing due to the influence of acid rain, soil acidification, and intensive crop production on the availability of soil Se over time (Frost Citation1987; Gissel-Nielson 1998).
As the worldwide demand for agricultural products with high Se content increases, wheat (Triticum aestivum) is considered one of the most efficient Se accumulators among common cereal crops (Lyons and Graham Citation2003). High Se areas can be considered Se resources for agricultural products. However, there is a wide variation in the Se content of wheat due to geographical differences in amounts and bioavailability of soil Se. Improper crop management can reduce Se content in staple food because uptake and accumulation of Se in plant tissues are influenced by numerous factors including plant available Se content in soil, plant species, soil pH, soil compaction, and irrigation (Johnsson Citation1991; Terry et al. Citation2000; Ogaard et al. Citation2006; Zhao et al. Citation2007).
Uptake and accumulation of Se by plants is also influenced by the interaction between Se and other competitive oxyanions in soil such as phosphate (P) and sulfate (S). However, many of these studies show conflicting results. Phosphate can influence Se sorption on soil surface since orthophosphate anions will readily displace sorbed Se from the clay mineral and organic matter surface and compete with Se for inner surface complexation sites on soil surfaces (Neal Citation1995). Carter et al. (Citation1972) showed that Se concentration in alfalfa (Medicago sativa) grown in alkaline and acid conditions increased when P fertilizer was applied. However, Mora et al. (Citation2008) showed that there was no effect of fertilizer P addition on Se uptake in white clover (Trifolium repens L.) in non-limed soil. Some other studies showed that P can reduce the uptake and accumulation of Se in plants (Liu et al. Citation2004; Mora et al. Citation2008). The interaction between S and Se uptake and accumulation in plants has been shown in positive and negative correlation (Cruz-Jimenez et al. Citation2005; Cartes et al. Citation2006; Huang et al. Citation2008; Mackowiak and Amacher Citation2008; McGregor et al. Citation2008; Stroud et al. Citation2010). For example, Adams et al. (Citation2002) found that Se concentration in wheat grain significantly decreased from 0.09 µg Se g−1 in the absence of applied S to roughly 0.04 µg Se g−1 with S applications, while a positive correlation between S and Se uptake was reported by several researchers (Dhillon and Dhillon Citation2000; White et al. Citation2004; Galeas et al. Citation2007; Stroud et al. Citation2010). The conflicting results for the interaction of P, S, and Se for plant uptake are confusing.
The effects of P and S fertilizer application on Se uptake have been widely studied, but most studies were carried out with the application of Se fertilization, while few studies were conducted in soils with naturally medium or high Se levels. Wheat producers in high Se areas such as the Northern Great Plains, USA, are continually seeking improvements in production practices to enhance grain Se content, since there is interest in developing a consistent grain supply of Se-rich wheat for markets in Europe and Asia. Some soils in western South Dakota are naturally high in plant available Se, yet this can be highly variable within field locations. It was important to investigate how production practices like nutrient management issues may influence wheat grain Se concentration grown in soils with different available Se levels. Therefore, the objective of this study was to investigate the effect of oxyanionic fertilizers of P and S on Se uptake by wheat in naturally medium and high Se soils.
Materials and methods
Field study characteristics
Two field sites of differing soil Se availability were selected in South Dakota, USA, for the 2007 and 2008 growing seasons. Carter soil (very-fine, smectitic, Mesic Vertic Paleustolls) was the dominant soil series at the Lyman County site (44°01′N, 99°38′W). Millboro soil (fine, smectitic, Mesic Typic Haplusterts) was the dominant soil series at the Tripp County site (43°30′N, 100°03′W). Fields at both sites were established after a crop rotation of soybean (Glycine max) and winter wheat was cultivated under a no-till system.
Hard white winter wheat (variety Wendy) was selected due to its high yield potential at both sites. Wheat was planted in September at 128 kg ha−1 seeding rate each year with a no-till drill with 17-cm row spacing and harvested in July following in each growing season. Each treatment plot (1.5 m × 7.6 m) was established in a completely randomized block design with four replications.
During the experiment period both sites received above-average annual precipitation compared to the long-term (30 years) annual precipitation of 487 mm for Lyman County and 603 mm for Tripp County. The wheat crop survived the winter weather in the dormant state with little winter-kill observed at both sites in either year. The temperatures at the early development period were greater for 2008 than 2007, and the accumulated growing degree days (GDDs) at both sites were slightly above normal both years compared to the long-term average of 1,192 and 1,217 GDDs for the Lyman and Tripp sites, respectively.
The soil had pH ranges of 7.3–7.9 (0–15 cm depth), high clay content, and low orthophosphate levels for both sites (). Mean total Se in soil was 1.2 and 3.7 µg Se g−1 for the Tripp and Lyman sites, respectively. The total soil Se in the Tripp site was within the worldwide Se concentration range (0.01 and 2.0 µg Se g−1), but the Lyman site Se was substantially higher than the normal range. In both soils, Se was predominantly present in plant non-available form fractions, which accounted for 72 to 78% of Se in the 0–15 cm depth and 62 to 69% in the 15–60 cm depth (). Overall extractable Se was ranked for extraction fractions in this order: total Se > conditionally available Se > plant available Se.
Table 1. Selected physical and chemical properties of soils used in this field study
Table 2. Mean soil selenium (Se) concentrations by sequential fractionation as plant available, conditionally available, and total Se forms sampled at two depths, at various times during the growing season, and at two locations in 2007 and 2008
Phosphate and sulfate fertilizer applications
The recommended P fertilizer application rate (100%) at both sites was 63 kg ha−1 which was based on the Olsen-P soil test for a grain yield goal of 5,400 kg ha−1 (Gerwing and Gelderman Citation2005). Six P application rates were applied from 0 to 300% of the recommended P rates. These rates included 1) Control (no P applied), 2) 100% (63 kg ha−1), 3) 150% (94 kg ha−1), 4) 200% (126 kg ha−1), 5) 250% (157 kg ha−1), and 300% (188 kg ha−1) of the recommended application rate. The P rates were applied as triple-super phosphate by one of these methods: 1) banding with seed, 2) surface-broadcasted in fall, or 3) surface-broadcasted in the spring. Urea was surface-broadcasted in the fall according to the soil test nitrogen (N) recommendation rate for the selected grain yield goal.
A separate set of field plots examined the response of grain Se content to S treatments. Gypsum (CaSO4·2H2O) was applied by surface-broadcasting at the rate of 0 (control), 20, 40, and 80 kg S ha−1 after wheat planting to obtain a wide range of sulfate availability. Phosphorus fertilizer was banded with the seed at both sites at the rate of 63 kg ha−1 to the soil test P recommendation. Fertilizer N was applied as a surface-broadcast application of urea after planting in the fall according to the soil test N recommendation rate. In 2008, the field sites were replanted in the same treatment plots and the same fertilizer treatments were applied as in 2007 to determine if high residual nutrient levels from the previous fertilization may influence Se uptake and accumulation in wheat grain.
Soil and plant analysis
Soil was sampled with a hydraulic soil probe at the pre-plant and post-harvest times. Four cores per plot were sampled and divided into surface (0–15 cm) and sub-surface (15–60 cm) depth intervals. Soil samples were air-dried (35°C), ground to pass a 2-mm sieve, and analyzed for physical and chemical properties. Soil pH was quantified in a 1:1 soil-to-water ratio suspension using an AR 50 pH/EC meter. Soil sulfate, nitrate, and phosphate were extracted using monobasic calcium phosphate, 2M KCl, and 0.5M sodium bicarbonate, respectively, and determined by a QuikChem FIA+ flow injection analyzer (Lachat Inc., Loveland, CO). A portion of the bulk air-dried soil sample was ground to pass a 0.25 mm diameter sieve. Soil Se was sequentially extracted by a modified fractionation method suggested by Chao and Sanzolone (Citation1989) to determine the following soil Se fractions: 1) 0.1M KH2PO4 extractable Se (plant available Se), followed by 2) 4M HCl extractable Se (conditionally available Se). A separate soil sample was extracted by 3) a mixture of HNO3 + HF + HClO4 for determination of total Se in soil.
Wheat grains were collected from the whole plot in each treatment. Wheat grain was ground using an Udy mill and stored in the dark at room temperature (23 ± 2°C) in airtight plastic bags until analyzed. The method developed for the determination of Se concentration in wheat grain was based on the digestion of 0.5 g of samples with concentrated HNO3 and H2O2 (9 and 1 mL, respectively) in a closed-vessel microwave oven (CEM Corporation, Matthews, NC). Selenium in the solution was determined by an atomic absorption spectrometer with a continuous flow hydride generator (GBC Instruments, Australia). Selenium uptake was calculated by multiplying the grain yield dry matter, corrected for 0% moisture, by the Se concentration. Two standard reference materials obtained from the National Institute of Standards and Technology (NIST 1567 wheat flour, 1.1 µg Se g−1) and South Dakota Wheat Commission (wheat flour, 3.4 µg Se g−1) were used to validate the precision of the method by adding check samples periodically in the analysis procedure.
Statistical analysis
The data was statistically analyzed using analysis of variance (ANOVA) and general linear model (GLM) procedures with the SAS statistical program (SAS institute Citation1988). Means were separated using the LSD0.05 option when the F-test was statistically significant for main effects with the ANOVA or GLM applications. Correlation analysis (PROC REG) determined the strength of the relationships between plant components and soil chemical properties.
Results and discussion
Effect of phosphate fertilizer applications
Grain Se concentration was influenced by P application, but it was dependent on location (). Selenium concentration in grain was significantly decreased at the Lyman site when P fertilizer was applied, but grain Se concentration at the Tripp site was not significantly changed by P application. At the Lyman site Se concentration in wheat grain grown without P application (control plants) averaged 14.7 µg Se g−1, whereas grain Se concentration decreased to 10.8 µg Se g−1 with the P fertilizer application. However, there was no further decrease in the grain Se concentration with the P application over the 100% recommended rate. This result conflicted with several other studies which found that the addition of P fertilizer increased Se concentration in plant tissue. For example, Liu et al. (Citation2004) found that P addition increased absorption and accumulation of Se in rice (Oryza sativa) shoots when the P supply increased. Selenium concentration in alfalfa grown in alkaline and acid conditions also increased after P fertilizer applications (Carter et al. Citation1972). Carter et al. (Citation1972) provided two possible explanations: 1) P addition replaced soil adsorbed Se since P had higher adsorption capacity than Se: Phosphate replaced the Se form on the exchange site and subsequently increased soil solution Se for plant use; or 2) the P application may have stimulated plants to absorb more Se because of increased root proliferation. It is well documented that the presence of P in soil could decrease Se sorption on soil surfaces and increase Se concentration in soil solution, thereby increasing Se uptake in plants (Nakamaru and Sekine Citation2008). Lee et al. (Citation2011) also observed that P application significantly decreased Se sorption on South Dakota soils. However, in this study soil pH was not significantly changed with P application () and the soil Se fractions were not significant with P application. The lower grain Se concentration by P application may be attributed to the dilution effect, because grain yield in the control was significantly lower than in the P application plots () and the increased biomass may have diluted the grain Se concentration. This was supported by the grain Se uptake which was not significantly different with P fertilizer application. The lower grain yield was observed in the control plants, which may have resulted in their higher grain Se concentration. A similar result was found by Mora et al. (Citation2008) who observed the dilution effect on white clover. The clover showed reduced Se concentration due to the dilution effect when P was applied to soil.
Table 3. Phosphate (P) fertilization influence on wheat (Triticum aestivum) grain yield, grain selenium (Se) concentration, and grain Se uptake for 2007 and 2008
In this study, P fertilizer was applied with three different methods, but banding with seed application of P fertilizer resulted in the same grain Se concentration as did application by broadcasting. This result contrasted with our hypothesis that banding P application with seeds can displace sorbed soil Se fractions in early development stages of wheat and will subsequently increase grain Se concentration. However, banding application maximized the contact of P with the wheat root at the root development stages, and thus the plants applied with banding showed a trend of lower grain Se concentration compared with other methods, even though this effect was not significant (p = 0.13). Banding P application usually gives higher crop yields as compared to broadcasting of P fertilizer, especially on low and medium P-testing soils. Total P uptake by wheat was significantly higher in broadcasting in spring compared to banding P application (). In this study, banding P application method had low efficiency for P uptake by wheat due to soil properties such as high clay contents, low P levels, and high soil pH.
Grain Se concentration was strongly influenced by location variation due to the difference in plant available Se content in soils (). This data is consistent with the review study by Combs (Citation2001), which showed that the Se contents in wheat vary according to the amounts of Se available in soils. Mean (1.52 µg Se g−1) grain Se concentration at the Tripp site was significantly higher compared to wheat grain produced in low Se areas [for example, as low as 0.11 µg Se g−1 in New Zealand (Combs Citation2001)], but the mean (11.47 µg Se g−1) grain Se concentration grown in the Lyman site was approximately seven-fold higher than in the Tripp site (). The greater extractable soil Se fractions in the Lyman site were reflected in this concentration difference regardless of the application of P fertilizer, which indicates that a location which has different amounts of soil Se is the most important single factor for Se concentration in plants.
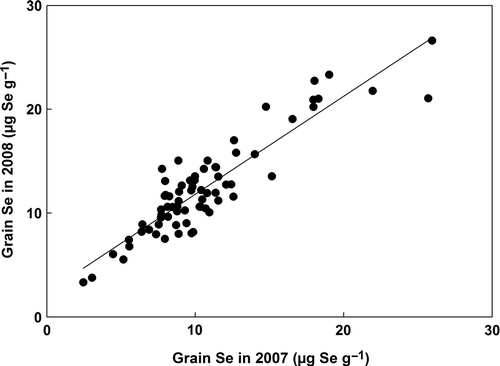
Even though grain Se concentration was diluted with increased biomass within any growing year, grain Se concentration was not correlated with grain yield between growing years. The mean grain Se concentration ranged from 2.5 to 26.6 µg Se g−1 at the Lyman site. Grain Se concentration was influenced by growing year; it was significantly higher (10.6 vs. 11.5 µg Se g−1) in 2008 than 2007, even though grain Se concentration was highly correlated (R 2 = 0.93) between 2007 and 2008 (). Overall, at both sites grain yield was significantly higher in 2008 compared to 2007 (data not shown), but grain Se concentration and Se uptake were significantly higher in 2008. This result showed that grain Se uptake can be influenced by crop environment between growing years, although there is no immediate evidence of the difference.
Figure 1. The relationship of wheat (Triticum aestivum) grain selenium (Se) concentration between the 2007 and 2008 harvest years, across all phosphate treatments.
Wheat grain yields and grain Se concentrations were used for a correlation matrix with the soil chemical properties at post-harvesting time (). Soil pH in the 0–15 cm depth was positively correlated (r = 0.67) with grain Se concentration, while grain Se concentration was not significantly correlated with soil pH in the 15–60 cm depth. This is probably related to Se sorption properties on soils because Se sorption decreased as soil pH increased, so more Se was available in soil solution and ultimately for the plants. Soil electrical conductivity (EC) values in the soil surface layer were not correlated with grain Se concentration, but correlations of soil EC at the 15–60 cm depth with grain Se concentration (r = 0.80) were significant.
Table 4. Correlation coefficient matrix between chemical properties in post-harvest soils and grain yield and grain selenium (Se) concentration for combined years of this study
Grain Se concentration was directly influenced by the plant available and conditionally available Se in soils within a site, even though plant available and conditionally available Se were not consistently changed by P application (). Grain Se concentration was moderately correlated with plant available and conditionally available Se in soils at post-harvest time. After the first wheat harvest in 2007, the plant available Se concentration ranged from 0.05 to 0.54 µg Se g−1 and 0.03 to 1.56 µg Se g−1 at the 0–15 cm and 15–60 cm depths, respectively. The conditionally available Se ranged from 0.03 to 1.56 µg Se g−1 at the 0–15 cm depth and 0.25 to 3.04 µg Se g−1 at the 15–60 cm depth. The range of plant available and conditionally available Se concentrations at the 15–60 cm depth was greater and wider than in the 0–15 cm depth layer. Grain Se concentration was strongly correlated with plant available Se (r = 0.87) and conditionally available Se (r = 0.91) in the 15–60 cm depth, while total soil Se was not significantly correlated with grain Se at either depth. Measuring the distribution of Se fraction in a soil rather than the total Se content is vital for understanding the bioavailability of Se for plants. Many studies revealed that soluble or plant available Se rather than total Se in soils was highly correlated to Se uptake by plants (Zhao et al. Citation2005; McGregor et al. Citation2008). For example, Zhao et al. (Citation2005) found that there was a significant correlation between Se uptake and plant available Se, indicating that plant available Se could predict Se concentration in plants. In this study, Se concentration in grain was also directly influenced by the plant available and conditionally available Se concentration in soil, even though plant available and conditionally available Se was not significantly changed by P applications. The relationship between grain Se concentration and soil fractions was stronger at the 15–60 cm depth than at the 0–15 cm depth. This may be due to the movement of soluble Se into the 15–60 cm depth and greater root density in the surface soil, which would deplete greater levels of plant available Se. A spatial distribution study in South Dakota has shown that unavailable Se remained in the upper layer while available and conditionally available Se were transported to the lower layers (Doolittle et al. Citation1995). The post-harvest extractable Se concentration at any depth in 2008 was not significantly different from the pre-plant soil Se levels. In addition, the total Se in soils was not significantly different between pre-planting and post-harvest. A similar result was observed when extractable soil Se was measured after two years of cropping cultivation and found not to be significantly different from the pre-plant levels, even though Se uptake by plants occurred (McGregor et al. Citation2008). The plant available and conditionally available Se can be absorbed by plant roots; thus the concentration of these Se fractions probably decreased. We assumed that the plant-available and conditionally available Se differences (ΔSe) between pre-planting and post-harvesting were absorbed and accumulated in plant tissue; thus these should be highly correlated. However, ΔSe was not significantly correlated with grain Se concentration (data not shown). The correlation coefficient matrix showed that the plant available and conditionally available Se concentrations at the 15–60 cm depth at post-harvesting time would be good estimators for the grain Se concentration.
Effect of sulfate fertilizer applications
Contrary to the effect of P application, grain yield was not significantly influenced by S application at either site (); thus grain Se concentration was not significantly influenced by increasing grain yield with S fertilizer application. Selenium concentration in wheat grain was decreased with the application of S fertilizer, but it was also dependent on location. Grain Se concentration was significantly decreased with increasing S fertilizer application at thenLyman site, but grain Se concentration at the Tripp site was not influenced by S application (). At the Lyman site, grain Se concentration was depressed by about 20% with each 20 kg S ha−1 fertilizer application rate, and overall, 40% of grain Se concentration was depressed with S application compared to the control plants. On the other hand, overall grain Se concentration with S application was not statistically significant at the Tripp site, although it was 25% lower in grain Se concentration compared to the control plants. This result indicates that S fertilizer application may influence grain Se concentration, but depression of grain Se concentration was apparent in soil with greater Se availability. The results from this study corresponded to those of other researchers, who also found that application of S significantly reduced accumulation of Se by plant tissues (Adams et al. Citation2002; White et al. Citation2004; Huang et al. Citation2008). For example, Huang et al. (Citation2008) showed that increasing S concentration in solution significantly decreased Se concentrations in shoots and roots of corn (Zea mays).
Table 5. Sulfate (S) fertilization impacts on wheat (Triticum aestivum) grain yield, grain selenium (Se) concentration, and grain Se uptake for 2007 and 2008
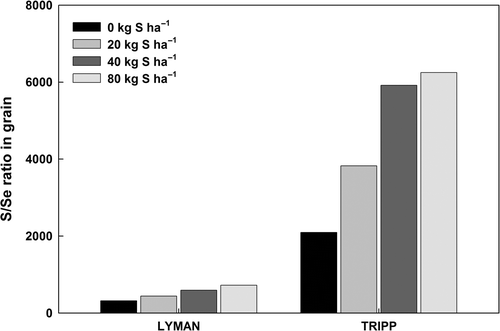
In this study, the application of S fertilizer was significant only in the Lyman site, which had high Se availability in soil. Selenium concentration in plants tissues may be suppressed following application of S fertilizer due to direct antagonism between S and Se uptake processes. Selenium, especially selenate, has similar chemical properties to S, and both are transported and assimilated mainly into S-containing amino acids and proteins as a result of the S assimilation pathway (Hawkesford and Zhao Citation2007). Sulfate applications did not significantly change the soil pH and soil Se fractions (data not shown), indicating that plant available Se in soil was not influenced by S application. However, the application of S fertilizer significantly increased available S content in soil and thus increased S/Se ratio in soils, which was probably attributable to suppressed Se uptake in S application. Sulfate uptake by plants is not affected by the level of Se in the soil. Plant roots absorb both S and Se from soil solution through the use of high and low affinity S-transporters that are localized in cortical cells and root epidermis, where the ratio of Se/S in plants was found to be selective. Bell et al. (Citation1992) found that non-Se accumulator plant species discriminate against Se uptake relative to S, whereas primary Se accumulators preferentially absorb Se over S. In this study, S concentrations in grain were steadily increased with S application in soils at both sites, and S concentration in grain was increased up to 6,000 times more than grain Se concentration. The ratio between S and Se concentration in grain was significantly increased with S application at both sites, but this effect was more apparent at the Tripp site, which had lower available Se in soil compared to the Lyman site (), indicating that the depression of grain Se concentration by S fertilizer application was greater in high Se availability soil.
Figure 2. The effect of sulfate (S) fertilizer application on the ratio of S and selenium (Se) in wheat (Triticum aestivum) grain for two growing seasons.
Sulfate applications significantly increased extractable S in post-harvest soil compared to pre-planted soil samples in 2007. However, S fertilizer application had little effect on soil pH and soil Se fractions over the two-year experimental period. There was little effect on the extractability of soil Se and Se uptake by wheat grain was decreased with S fertilizer application due to the competition effect between soil Se and S. The residual soil S from prior S applications in 2007 did not affect Se uptake in 2008, even though extractable S in soil was significantly increased by S application for any one year. Sulfate fertilizer application significantly decreased grain Se concentration as well as grain Se uptake at the Lyman site, but grain Se concentration was not significantly different between 2007 and 2008 (data not shown). At both sites, grain Se concentration was significantly greater (e.g., 5.01 vs. 4.31 µg Se g−1 at the Lyman site) in 2008 than in 2007; even wheat grain yield was higher (e.g., 5.32 vs. 4.19 Mg ha−1 at the Lyman site) in 2008. Although more grain yield was obtained in 2008, grain Se concentration was significantly higher in 2008, which indicates that grain Se concentration and Se uptake are also strongly influenced by growing conditions. In this study, S fertilizer application did not significantly change the soil pH and soil Se fractions, indicating that extractability of Se was not significantly changed by S fertilizer application, so the resultant decrease in grain Se concentration and Se uptake was probably caused by competitive interactions between soil S and soil Se fractions.
Conclusions
The results of this study showed that oxyanionic P and S fertilizer application had a significant impact on wheat grain Se concentration. Phosphate applications significantly decreased grain Se concentration in high Se availability soil, due to the increase of yield when compared to no P fertilizer application, while the Se uptake in the grain was not significantly different. Contrary to P application, S applications decreased grain Se concentration as well as grain Se uptake in high Se availability soil, due to the increased competition for Se uptake by wheat. The results from this study showed that P and S fertilizer applications in agricultural soil significantly influenced Se uptake and accumulation in plants, even though overall grain Se uptake was strongly associated with location variation.
Acknowledgments
This research was supported by the South Dakota Wheat commission and the South Dakota Agricultural Experimental Station.
References
- Adams , ML , Lombi , E , Zhao , FJ and McGrath , SP . 2002 . Evidence of low selenium concentrations in UK bread-making wheat grain . J. Sci. Food Ag. , 82 : 1160 – 1165 .
- Arthur , J , McKenzie , RC and Beckett , GJ . 2003 . Selenium in the immune system . Am. Soc. Nutr. Sci. , 133 : 1457S – 1459S .
- Beck , MA . 2001 . Antioxidants and viral infections: host immune response and viral pathogenicity . J. Am. Coll. Nutr. , 20 : 384S – 388S .
- Bell , PF , Parker , DR and Page , AL . 1992 . Contrasting selenate sulfate-interaction in selenium-accumulating and nonaccumulating plant species . Soil Sci. Soc. Am. J. , 56 : 1818 – 1824 .
- Brandt , PA , Zeegers , MPA , Bode , P and Goldbohm , RA . 2003 . Toenail selenium levels and the subsequent risk of prostate cancer: A prospective cohort study . Cancer Epidemiol., Biomark. Prev. , 12 : 866 – 871 .
- Carter , DL , Robbins , CW and Brown , MJ . 1972 . Effects of phosphorus fertilization on the selenium concentration in alfalfa . Soil. Sci. Soc. Am. Proc. , 36 : 624 – 628 .
- Cartes , P , Shene , C and Mora , ML . 2006 . Selenium distribution in ryegrass and its antioxidant role as affected by sulfur fertilization . Plant Soil , 285 : 187 – 195 .
- Chao , TT and Sanzolone , RF . 1989 . Fractionation of soil selenium by sequential partial dissolution . Soil Sci. Soc. Am. J. , 53 : 385 – 392 .
- Combs Jr , GF . 2001 . Selenium in global food systems . Br. J. Nutr. , 85 : 517 – 547 .
- Combs Jr , GF . 2004 . Status of selenium in prostate cancer prevention . Br. J. Cancer , 91 : 195 – 199 .
- Cruz-Jimenez , G , Peralta-Videa , JR , de la Rosa , G , Meitzner , G , Parsons , JR and Gardea-Torresdey , JL . 2005 . Effects of sulfate on selenium uptake and chemical speciation in Convolvulus arvensis L . Environ. Chem. , 2 : 100 – 107 .
- Dhillon , KS and Dhillon , SK . 2000 . Selenium accumulation by sequentially grown wheat and rice as influenced by gypsum application in a seleniferous soil . Plant Soil , 227 : 243 – 248 .
- Doolittle , JJ , Huang , X and Bly , AG . 1995 . The spatial variability of selenium in selected soils of Charles mix county, South Dakota . Proc. S.D. Acad. Sci. , 74 : 113 – 122 .
- Finley , JW and Davis , CD . 2001 . Selenium from high-selenium broccoli is utilized differently than selenite, selenate and selenomethionine, but is more effective in inhibiting colon carcinogenesis . BioFactors , 14 : 191 – 196 .
- Frost , DV . 1987 . “ Why the level of selenium in the food chain appears to be decreasing ” . In Selenium in Biology and Medicine , Edited by: Combs , GF , Spallholtz , JE , Levander , OA and Oldfield , JE . 537 – 547 . New York : AVI Van Nostrand Reinhold .
- Galeas , ML , Zhang , LH , Freeman , JL , Wegner , M and Pilon-Smits , EAH . 2007 . Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators . New Phytol. , 173 : 517 – 525 .
- Gerwing , J and Gelderman , R . 2005: Fertilizer Recommendation Guide. Cooperative Extension Service at South Dakota State University and US Department of Agriculture EC750. Brookings, SD
- Gissel-Nielsen , G . 1998 . “ Effects of selenium supplementation of field crops ” . In Environmental Chemistry of Selenium , Edited by: Frankenberger , WT and Engberg , RA . 99 – 112 . New York : Dekker .
- Hawkesford , MJ and Zhao , F . 2007 . Strategies for increasing the selenium content of wheat . J. Cereal Sci. , 46 : 282 – 292 .
- Huang , Y , Hu , Y and Liu , Y . 2008 . Interactions between sulfur and selenium uptake by corn in solution culture . J. Plant Nutr. , 31 : 43 – 54 .
- Johnsson , L . 1991 . Selenium uptake by plants as a function of soil type, organic matter content and pH . Plant Soil , 133 : 57 – 64 .
- Lee , S , Doolittle , JJ and Woodard , HJ . 2011 . Selenite adsorption and desorption in selected South Dakota soils as a function of pH and other oxyanions . Soil Sci. , 176 : 73 – 79 .
- Liu , Q , Wang , DJ , Jiang , XJ and Cao , ZH . 2004 . Effects of the interactions between selenium and phosphorus on the growth and selenium accumulation in rice . Environ. Geochem. Health , 26 : 325 – 330 .
- Lyons , GJS and Graham , R . 2003 . Nutriprevention of disease with high-selenium wheat . J. Aust. Coll. Nutr. Environ. Med. , 22 : 3 – 9 .
- Mackowiak , CL and Amacher , MC . 2008 . Soil sulfur amendments suppress selenium uptake by alfalfa and western wheatgrass . J. Environ. Qual. , 37 : 772 – 779 .
- McGregor , AL , Johnson-Maynard , JL , Strawn , DG , Shafii , B and Moller , B . 2008 . Plant uptake and leaching of selenium in manure- and gypsum-amended soils of the western phosphate resource area . Soil Sci. , 173 : 613 – 623 .
- Mora , ML , Pinilla , L , Rosas , A and Cartes , P . 2008 . Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization . Plant Soil , 303 : 139 – 149 .
- Nakamaru , YM and Sekine , K . 2008 . Sorption behavior of selenium and antimony in soils as a function of phosphate ion concentration . J. Soc. Soil. Sci. Plant. Nutr. , 54 : 332 – 341 .
- Neal , RH . 1995 . “ Selenium ” . In Heavy metals in soils , Edited by: Alloway , BJ . 260 – 283 . Glasgow : Blackie Academic & Professional .
- Ogaard , AF , Sogn , TA and Eich-Greatorex , S . 2006 . Effect of cattle manure on selenate and selenite retention in soil . Nutr. Cycl. Agroecosyst. , 76 : 39 – 48 .
- Institute , SAS . 1988: SAS/STAT User's Guide. Release 6.03 ed. SAS inst, Cary, NC
- Stroud , JL , Li , HF Lopez-Bellido , FJ . 2010 . Impact of sulphur fertilization on crop response to selenium fertilization . Plant Soil , 332 : 31 – 40 .
- Terry , N , Zayed , AM , de Souza , MP and Tarun , AS . 2000 . Selenium in higher plants . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 51 : 401 – 432 .
- Whanger , PD . 2004 . Selenium and its relationship to cancer: An update . Br. J. Nutr. , 91 : 11 – 28 .
- White , PJ , Bowen , HC Parmaguru , P . 2004 . Interactions between selenium and sulphur nutrition in Areabidopsis thaliana . J. Exp. Bot. , 55 : 1927 – 1937 .
- Zhao , FJ , Lopez-Bellido , FJ , Gray , CW , Whalley , WR , Clark , LJ and McGrath , SP . 2007 . Effects of soil compaction and irrigation on the concentrations of selenium and arsenic in wheat grains . Sci. Total Environ. , 372 : 433 – 439 .
- Zhao , C , Ren , J , Xue , C and Lin , E . 2005 . Study on the relationship between soil selenium and plant selenium uptake . Plant Soil , 277 : 197 – 206 .