Abstract
To evaluate the hypothesis that plant-mediated oxygen supplies decrease methane (CH4) production and total global warming potential (GWP) in a tropical peatland, the authors compared the fluxes and dissolved concentrations of greenhouse gases [GHGs; CH4, carbon dioxide (CO2) and nitrous oxide (N2O)] and dissolved oxygen (DO) at multiple peatland ecosystems in Central Kalimantan, Indonesia. Study ecosystems included tropical peat swamp forest and degraded peatland areas that were burned and/or drained during the rainy season. CH4 fluxes were significantly influenced by land use and drainage, which were highest in the flooded burnt sites (5.75 ± 6.66 mg C m−2 h−1) followed by the flooded forest sites (1.37 ± 2.03 mg C m−2 h−1), the drained burnt site (0.220 ± 0.143 mg C m−2 h−1), and the drained forest site (0.0084 ± 0.0321 mg C m−2 h−1). Dissolved CH4 concentrations were also significantly affected by land use and drainage, which were highest in the flooded burnt sites (124 ± 84 μmol L−1) followed by the drained burnt site (45.2 ± 29.8 μmol L−1), the flooded forest sites (1.15 ± 1.38 μmol L−1) and the drained forest site (0.860 ± 0.819 μmol L−1). DO concentrations were influenced by land use only, which were significantly higher in the forest sites (6.9 ± 5.6 μmol L−1) compared to the burnt sites (4.0 ± 2.9 μmol L−1). These results suggest that CH4 produced in the peat might be oxidized by plant-mediated oxygen supply in the forest sites. CO2 fluxes were significantly higher in the drained forest site (340 ± 250 mg C m−2 h−1 with a water table level of −20 to −60 cm) than in the drained burnt site (108 ± 115 mg C m−2 h−1 with a water table level of −15 to +10 cm). Dissolved CO2 concentrations were 0.6–3.5 mmol L−1, also highest in the drained forest site. These results suggested enhanced CO2 emission by aerobic peat decomposition and plant respiration in the drained forest site. N2O fluxes ranged from −2.4 to −8.7 μg N m−2 h−1 in the flooded sites and from 3.4 to 8.1 μg N m−2 h−1 in the drained sites. The negative N2O fluxes might be caused by N2O consumption by denitrification under flooded conditions. Dissolved N2O concentrations were 0.005–0.22 μmol L−1 but occurred at < 0.01 μmol L−1 in most cases. GWP was mainly determined by CO2 flux, with the highest levels in the drained forest site. Despite having almost the same CO2 flux, GWP in the flooded burnt sites was 20% higher than that in the flooded forest sites due to the large CH4 emission (not significant). N2O fluxes made little contribution to GWP.
1. INTRODUCTION
Tropical peatland ecosystems in Southeast Asia cover 24.8 million ha, comprising 56% of the tropical and 6% of the global peatland area. This ecosystem’s high carbon density allows for a large regional peat carbon store of 68.5 Pg, equivalent to 77% of the tropical and 11–14% of the global peat carbon store (Page et al. Citation2011), which is comparable to the total fossil fuel emissions released throughout the world over nine years (van der Werf et al. 2008). Given the size of this carbon reservoir, tropical peatlands play a critical role as a global sink for gaseous carbon. Inversely, if environmental conditions (e.g., land use type, groundwater level) change, these peatlands may become a major source of gaseous carbon and greenhouse gases (GHGs), such as carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O).
In recent decades, considerable portions of peatland in Southeast Asia have been reclaimed to support agroforestry and rice paddy development. The deforestation and development of peatlands is usually accompanied by the draining of water from these areas, releasing much carbon through aerobic peat decomposition. In addition, the water table level can decrease drastically during exceptionally long dry periods, which frequently occur in El Niño and Southern Oscillation (ENSO) years (Hirano et al. Citation2007, Citation2012; van der Werf et al. 2008).
Several trials have been conducted to restore lowland tropical peatlands (Page et al. Citation2009). For example, restoration efforts have included raising and stabilizing the water table level by blocking drainage canals, which reduces aerobic peat decomposition in the flooded peatlands. However, flooding can also increase anaerobic CH4 production. Soil denitrification, which is a major contributor to N2O production, is also generally promoted under reduced conditions. The radiative forcing effects of CH4 and N2O are 25 and 298 times higher, respectively, than that of CO2 (over a 100-yr time horizon; IPCC Citation2007). Consequently, the increases in the production of CH4 and N2O under flooded conditions could possibly offset decreases in aerobic peat decomposition and associated CO2 emissions. A large portion of previously drained peatland has lost its original vegetation and surface layer through deforestation and/or fire. Under such conditions, the recovery of the water table level will not be able to restore ecosystem function and carbon balance to those associated with original vegetation levels. The change in GHG dynamics in tropical peatland soils after drainage, deforestation, peat fire and re-wetting has not been fully studied to date.
After reviewing the existing data, Couwenberg et al. (Citation2010) have demonstrated that the range of CH4 fluxes in tropical peatlands was one-fifth that observed in temperate and boreal peatlands. They attributed low CH4 emissions to the poor quality of woody tropical peat, which contains higher levels of recalcitrant materials (e.g., lignin). Williams and Yavitt (Citation2010) reported that the biochemical compositions of lignin affect soil methanogenesis. Another explanation involved the oxygen supply from plant roots. Vascular plants in wetlands usually possess special morphological adaptations, such as hypertrophied lenticels, adventitious roots and enlarged aerenchyma (Joabsson et al. Citation1999), which promote gas exchange between the atmosphere and the rhizosphere and allow for the entry of oxygen to the root zone (Megonigal and Day Citation1992; Kozlowski Citation1997). According to a recent review by Laanbroek (Citation2010), the percentage of CH4 oxidized before entering the atmosphere ranges from 0 to up to > 90% of the potential CH4 efflux. Several studies conducted in temperate and boreal wetlands reported that an increase in measurement depth was associated with a decrease in the amount of root tissue and dissolved oxygen (DO) concentrations and an increase in dissolved CH4 concentrations (van der Nat and Middelburg Citation1998; Fritz et al. 2011; Liebner et al. Citation2012). Thus, if plant-mediated oxygen transport and CH4 oxidation also occur in tropical peatlands, significant differences in soil CH4 fluxes, dissolved CH4 concentrations and DO concentrations in peat pore water would be expected between vegetated and non-vegetated lands.
Given these relationships, we hypothesized that (1) DO supplied from plant roots should oxidize CH4 produced in saturated tropical peat soil and (2) CH4 oxidation should reduce total global warming potential (GWP) in a tropical peat swamp forest. To evaluate these hypotheses, we compared DO and GHG fluxes and dissolved concentrations in an ecosystem containing submerged forest vegetation with those in degraded peatland areas that have been burned and/or drained during the rainy season.
2. MATERIALS AND METHODS
2.1. Study site
This study was conducted in a tropical peat swamp area located near Palangka Raya City (02°12′26ʺ S, 55′00ʺ E), Central Kalimantan Province, Indonesia. Most of the peat swamp forest in this region was previously deforested and drained during a national project (i.e., the Mega Rice Project) in the late 1990s. Although the project was terminated in 1999, it left vast tracts of devastated peatland (Notohadiprawiro Citation1998; Muhanmad and Rieley Citation2002). A large portion of the devastated peatland was subsequently abandoned because it was unsuitable for the intended agricultural production. In these areas, several large fires have occurred during the dry season, severely damaging both the vegetation and soil. These fires were mainly ignited as a result of agricultural activities, including land clearance and ash production by local farmers. Some of these fires spread uncontrollably to become wildfires (Usup et al. Citation2004).
Six locations were established as study sites. Three sites were placed in a peat swamp forest that was not affected by deforestation or fire (FW1, FW2, and FD; forest sites). The other three sites were located in a degraded peatland affected by fire (BW1, BW2, and BD; burnt sites). The burnt sites were deforested in 1995 and burned in 1997, 2002 and 2009 during El Niño years. The flooded forest sites (FW1 and FW2) and the flooded burnt sites (BW1 and BW2) were close to the flood plains of the Sebangau and Kahayan Rivers, respectively. Sites FD and BD were located in the middle of a peat dome formed between these two rivers. These two sites are influenced by a large drainage canal, which runs through the peat dome southward to the Java Sea (> 100 km long).
In the forest sites, the ground surface was covered by a dense canopy of peat swamp forest, although the composition of tree species was previously affected by selective logging until the late 1990s (Hirano et al. Citation2012). Dominant species present included Tristania whittiana, Combretocarpus rotundatus, Palaquium leiocarpum and Stemonurus scorpioides. In the burnt sites, the ground surface was covered by ferns and grasses, such as Stenochlaena palustris and Nephrolepis sp., with patchy depressions. Trees, such as Combretocarpus rotundatus, were found sparsely. Detailed information about the vegetation around the study sites is provided elsewhere (Page et al. Citation1999; Tuah et al. Citation2001, Citation2003; Jauhiainen et al. Citation2005; Takakai et al. Citation2006; Sundari et al. Citation2012). Based on United States Department of Agriculture (USDA) Soil Taxonomy, the study sites were all classified as Typic Tropofibrists (Takakai et al. Citation2006). The peat depths were 2–3 m at FW1 and FW2 and about 4 m at the other sites. The volumetric carbon density of the peat was 71.5 ± 17.3 kg m−3 (Shimada et al. Citation2001). Annual precipitation and annual mean air temperature over the nine study years (2002–2010) were 2540 ± 596 mm and 26.2 ± 0.3°C, respectively (Sundari et al. Citation2012). The dry season normally begins in June and ends in October. Field observations were conducted during the wet season from December 2011 to May 2012.
2.2. GHG fluxes
GHG fluxes at the ground surface were measured monthly using a closed-chamber method (Morishita et al. Citation2003; Takakai et al. Citation2006; Toma and Hatano Citation2007). A set of cylindrical stainless steel chambers (18.5–21 cm in diameter and 25 cm in height), each with an acrylic lid fixed to a sample collector and a pressure-regulating bag, was used for the flux measurement. Stainless steel collars were permanently planted at three locations at each site. First, 400 mL of the air inside the chamber was taken into a Tedlar® bag as the sample at 0 min before closing the chamber lid. The chamber was then quickly capped with the lid. After 6 min, 250 mL of the air inside the chamber was transferred into another Tedlar® bag. CO2 concentrations in these bags were determined on the day of sampling with a non-dispersive infrared (NDIR) analyzer (ZFP9; Fuji Electric, Japan). The lid was then removed temporarily, and the air inside the chamber was ventilated. Next, 20 mL of the air inside the chamber was periodically collected into pre-evacuated vial bottles with butyl rubber stoppers at 0, 20 and 40 min after closing the lid for CH4 and N2O analyses. CH4 concentrations were determined using a gas chromatograph (GC-8A, Shimadzu, Kyoto, Japan) equipped with (1) a flame ionization detector (FID) maintained at 130°C and (2) a 2-m-long activated carbon column (80/100 mesh; Stainless column, Serial No. D-0015, Shimadzu, Kyoto, Japan) maintained at 70°C with pure nitrogen (N2) gas as a carrier. N2O concentrations were determined with a gas chromatograph (GC-14B, Shimadzu, Kyoto, Japan) equipped with (1) an electron capture detector (ECD) maintained at 340°C and (2) a 1-m-long Porapak N column maintained at 60°C with proportional gas (5% CH4 in argon, Ar). We did not measure fluxes when water table levels exceeded the height of the chamber. As a result, GHG fluxes at several sites were not measured in December 2011 (FW1, BW1 and BW2), February 2012 (BW1), and March 2012 (FW1, FW2 and BW1). Due to a separate technical issue, we could not measure GHG fluxes in January 2012 for any site, with the exception of FW2.
Gas flux was calculated as the change in gas concentration inside the chamber (ΔCc) against closure time (Δt) according to the following equation:
where F is the gas flux (g C or g N m−2 h−1), ρg is the gas density (0.536 × 103 g m−3 for CO2-C and CH4-C; 1.259 × 103 g m−3 for N2O-N), V/A is equivalent to the height of the chamber from the water or ground surface (m) and T is the air temperature during the sampling period (°C). Air temperature was measured with a digital thermometer (TESTO 625, Lenzkirch, Germany) before closing and after opening the chamber, and the mean of both temperatures was used in Eq. 1. A positive flux is indicative of gas emissions from the soil or water surface into the atmosphere, while a negative flux indicates gas uptake from the atmosphere. The total flux in GWP was calculated as the CO2-equivalent carbon flux (CO2-eq g C m−2 h−1) according to the following equation:
where 25 and 298 are the factors used for converting CH4 and N2O fluxes, respectively, to their GWPs over a 100-yr time horizon (IPCC Citation2007); and (16/12), (44/28) and (12/44) are the conversion factors for C to CH4, N to N2O and CO2 to C, respectively.
2.3. Dissolved GHG concentrations
To determine the concentrations of GHGs dissolved in peat water, two types of in-situ headspace methods were applied. We used an in-situ equilibration chamber for CO2 and a rapid equilibration method for CH4 and N2O. An in-situ equilibration chamber (i.e., a small chamber designed to equilibrate air with the surrounding dissolved gases) was developed according to previous studies (Faulkner et al. Citation1989; Yu and DeLaune Citation2006). An inverted 60-mL plastic syringe was used as the chamber and was connected aboveground to a thin tube, which was equipped with a three-way stopcock at the upper end. A chamber was installed at depths of 20, 40, 60 and 80 cm. First, 60 mL of O2-free air was injected through the tube, and the stopcock was closed to keep the injected air from escaping. Two to four weeks following the injection, the air was extracted from the chamber and collected into an aluminum sample bag. CO2 concentrations were determined on the day of sampling according to the same method used to measure CO2 flux described above. The dissolved gas concentration in pore water (Cl, mol mol−1) was given by Henry’s law:
where Cg is the concentration of the sample air (ppmv), P is the total pressure inside the chamber (atm), and KH is Henry’s constant (mol mol−1 atm−1). The effect of water pressure above the chamber is reflected in P. The value of KH, a function of soil temperature, was cited from a chemical engineering handbook (Society of Chemical Engineers and Japan Citation1999). Soil temperature was preliminarily observed at depths of 5, 10, 30 and 50 cm (see below). The average temperatures between 10 and 30 cm and between 30 and 50 cm were used as the soil temperatures at 20 and 40 cm, respectively. Soil temperatures at 60 and 80 cm were assumed to be equivalent to that at 50 cm.
The Cl in the molar ratio was converted to Cl (mol L−1) according to the following equation:
where ρw is the water density (1000 g L−1), and mw is the molecular weight of water (H2O, 18.01 g mol−1). The time required to reach CO2 equilibrium was investigated beforehand at the same study sites as follows. The CO2 concentrations in the air samples collected following different equilibration durations (5–100 d) were compared, and no statistically significant differences were observed. Therefore, CO2 in the collected samples was assumed to be in equilibrium with CO2 dissolved in the surrounding water.
Gas samples for the analyses of dissolved CH4 and N2O were obtained by rapidly equilibrating a fixed volume of air with pore water in a syringe. Water samples were collected from the same depths as those used for dissolved CO2 measurements (i.e., 20, 40, 60 and 80 cm). At the beginning of field observations, a water sampler consisting of a stainless steel pipe (0.7 cm in diameter), a plastic tube and a three-way stopcock was installed at each depth. During the observation intervals, the sampler was filled with water, and the stopcock was kept closed. Before sampling, the water remaining in the sampler was removed. First, 30 mL was collected from the sampler directly into a 60-mL plastic syringe. Next, the same volume of O2-free air was injected into the same syringe. The syringe was then shaken by hand for 2 min (100 times). After that, 20 mL of the air that was equilibrated with the water sample was stored in a pre-evacuated vial. The concentrations of CH4 and N2O in the bottles were determined in the same manner used for flux measurements for these gases. According to this rapid equilibration method, the dissolved gas concentrations in pore water could be calculated using the following equation (Alberto et al. Citation2000):
where Cbg is the initial concentration of the gas in the O2-free air, α is the water:air partition coefficient and Vg and Vl are the volume of the gaseous and liquid phases, respectively. Eq. 5 can be simplified provided that Cg >> Cbg and Vg = Vl in the following equation:
Here, α is defined as C′l/Cg, where C′l is the dissolved gas concentration in the liquid phase inside the syringe. The variable C′l was calculated in the same manner as Cl in Eqs. 3 and 4, assuming that the temperature inside the syringe was equal to T. The variable Cg was also converted to the same unit of C′l as follows:
where V (L mol−1) is the volume of 1-mol air at total pressure P (= 1.0 atm), and temperature T (°C) can be calculated as:
2.4. DO concentration
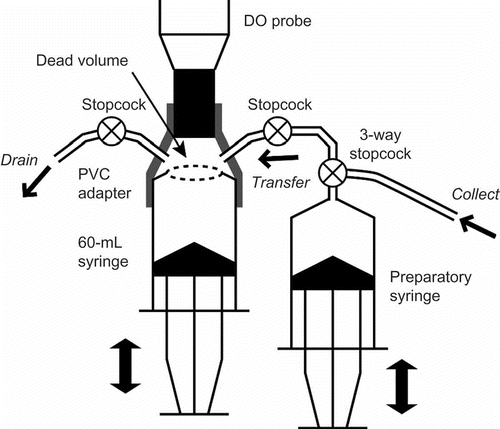
DO concentrations were measured in situ by extracting pore water directly into a closed cell equipped with a DO sensor to avoid contact between the water sample and the atmosphere. A luminescent DO probe (LDO10101; Hach, Loveland, USA) was used because, in principle, it neither consumed oxygen nor required water flow toward the sensor during measurement. The closed cell consisted of the tip of the probe, a 60-mL plastic syringe and a connecting polyvinyl chloride (PVC) adapter (). Pore water was extracted from the same type of water sampler used for the measurement of dissolved GHGs, separately installed at depths of 10, 20, 40 and 80 cm. The water was first stored in the preparatory syringe and then pushed into the closed cell. As the water entered, the cell plunger was synchronously pushed out. After measurement, the water was drained from another cell port. DO was measured in December 2011 and March, April and May 2012.
Dead space in the closed cell was initially filled with ambient air or pore water at different depths. To approximate the true value of the DO concentration in the cell, 50 mL of pore water was repeatedly extracted until the measured value was stabilized. The stroke of the extraction was limited to 10 times to reduce the influence of the inflow of pore water from other depths. Finally, DO was determined based on the sequential data of measured DO by solving a numerical convergence model with Excel® Solver.
2.5. Environmental parameters
Water table levels were measured and recorded with a pressure logger (Hobo U20, Onset, Bourne, USA) settled near the bottom of a perforated PVC pipe (ca. 1.5 m from the ground surface). The influence of the change in atmospheric pressure was canceled based on the pressure data observed at the ground surface level inside the well of FD, where the water table level was lower than −20 cm during the observation period (see Results). The water table level in the well was converted to that at the position of the flux chambers by canceling the small differences in the altitude of the ground surface. Precipitation was measured in the vicinity of the study sites with a tipping bucket rain gauge linked to a HOBO Pendant® event data logger.
Values for pH and electric conductivity (EC) were measured at depths of 10, 20, 40 and 80 cm. Water samples were extracted from the same samplers used for DO measurements. The values of pH and EC were determined in situ with portable sensors (pH: B-212, Horiba, Japan; EC: B-173, Horiba, Japan).
For the measurement of dissolved nitrogenous ions (nitrate (NO3−) and ammonium (NH4+)), 50 mL of water were collected into a plastic bottle at depths of 10, 20, 40 and 80 cm using the same samplers used for DO measurements. These water samples were stored in a refrigerator until analysis. Concentrations of NO3− were measured by ion chromatography (Dionex QIC Analyzer, Thermo Fisher Scientific, Waltham, USA). Concentrations of NH4+ were determined by colorimetry based on the indophenol-blue method and an ultraviolet-visible spectrophotometer (UV mini 1240, Shimadzu, Kyoto, Japan).
Prior to the flux observations made in this study, hourly monitoring of soil temperatures was conducted in February, June and December 2011 at the flooded sites and in July 2011 for all study sites. Soil temperatures were measured at depths of 5, 10, 30 and 50 cm with a T-type thermocouple and recorded to a 4-ch data logger (47SD Thermo Recorder, Sato Shoji, Japan). One monitoring effort was usually continued over 4 to 10 consecutive days.
2.6. Statistics
A one-way analysis of variance (ANOVA) was conducted to test for differences in the environmental parameters among the six study sites. In addition, the effects of land use (forest or burnt area) and drainage (flooded or drained) on GHG fluxes were tested by two-way ANOVA, and the effects of land use, drainage and depth on dissolved gas concentrations were tested using three-way ANOVA. Multiple comparison tests among the sites and depths were conducted using the Bonferroni method. A probability level less than 0.05 (P < 0.05) was considered statistically significant. All analyses were performed in IBM SPSS Statistics Ver. 21.
3. RESULTS
3.1. Environmental parameters
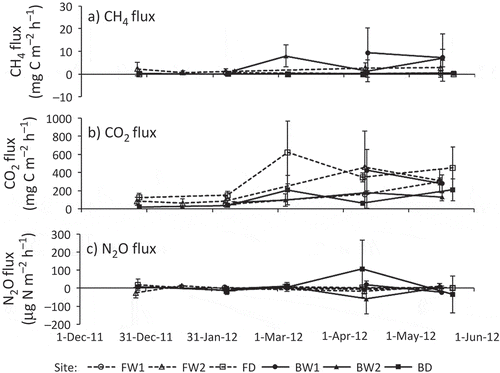
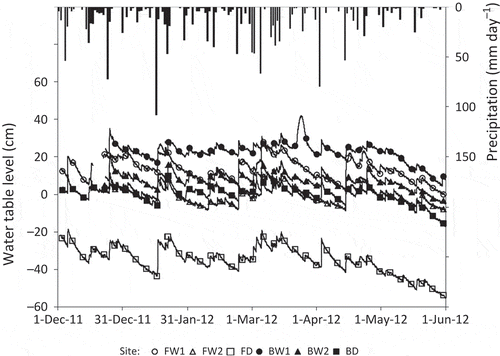
shows the temporal changes in precipitation and water table level that occurred during the observation period. The water table levels occasionally increased at all sites following large amounts of rainfall, but they were generally stable from December 2011 to April 2012. In late May, the water table levels were at their lowest due to lower monthly precipitation amounts (94 mm compared to 205–371 mm in other months). Sites FW1, BW1 and BW2 generally remained flooded during the study period. At BW1, a temporary rise in water table level was recorded in late March despite the fact that heavy rainfall was not recorded and similar increases at other sites were not observed. The rise in water table at BW1 may have been caused by an increase in the Kahayan River’s water level. Sites FW2 and BD remained flooded for 45 and 60% of the observation period, respectively. With a water table level at below −20 cm during the study period, site FD was never flooded.
Figure 2 Seasonal changes in precipitation (vertical bars) and water table level at each site (lines with symbols).
The air temperatures observed following flux chamber measurements and other environmental parameters are summarized in . Air temperatures at the burnt sites ranged from 32 to 34°C and were higher than those at the forest sites (27–28°C); however, a significant difference in air temperature was not detected between the same land use types. Forest sites likely had lower temperatures because they were more shaded by dense vegetation. Diurnal soil temperatures varied by as much as 6°C at a depth of 5 cm, but variations were less than 1°C at 50 cm. Although we observed large differences in diurnal variation in soil temperatures, differences in daily mean soil temperatures were small at all depths and study intervals. The largest difference in daily mean soil temperature was 3.3°C which occurred at BW1 at a depth of 5 cm (February to December 2011); temperatures differed by < 2°C for all other depths and sites. The average soil temperatures were 24.5–25.5°C at the forest sites and 27.6–28.1°C at the burnt sites, reflecting differences in air temperature between the different land use types. The vertical distributions in average soil temperatures are shown in .
Table 1 Environmental factors at all study sites averaged for the observation period
The pH of peat water ranged from 3.5 to 4.0 among the study sites, indicating acidic conditions. The EC of peat water ranged from 35 to 125 μS cm−1 among the study sites and was higher in the forest sites. Variations in pH and EC between measurement depths and months were small. NO3–-N concentrations ranged from 0.0519 to 0.260 mg L−1 and were relatively higher in the burnt sites. NH4+-N concentrations ranged from 0.061 to 0.205 mg L−1, with no significant differences among the study sites.
3.2. GHG fluxes
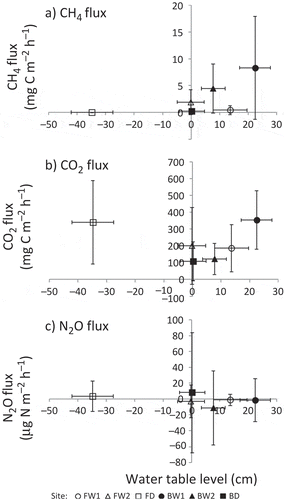
Temporal changes in GHG (i.e., CH4, CO2 and N2O) fluxes are shown in . Fluxes in GHGs and CO2-equivalent GWP were averaged for each group (classified by land use and drainage) and are summarized in , with the results of two-way ANOVA. The relationships between water table levels and these fluxes are shown in .
Table 2 Comparison of greenhouse gas (GHG) fluxes among different land use and drainage conditions.
The site-specific averages of CH4 fluxes ranged from 0.0084 to 5.75 mg C m−2 h−1 (). We periodically observed high CH4 emissions at BW1 and BW2 () and negative CH4 fluxes (= CH4 absorption) at the forest sites. At FD, 7 of 15 measured fluxes were negative. However, on average, all sites were a net CH4 source during the observation period. In the burnt sites, higher average CH4 fluxes were associated with higher average water table levels (). Relative to the burnt sites, the CH4 fluxes at the forest sites were consistently lower regardless of water table levels. The results of two-way ANOVA indicated that both main effects (land use and drainage) and their interaction were significant, and CH4 flux at the flooded burnt sites was significantly higher than those of the other sites ().
The site-specific averages of CO2 fluxes ranged from 108 to 340 mg C m−2 h−1 (). CO2 fluxes tended to be relatively low (< 150 mg C m−2 h−1) and stable until February 2012, with higher values (> 350 mg C m−2 h−1) occurring after March 2012 at FW2, FD and BD (). Among the study sites, CO2 flux was highest at FD, except for in April 2012. In the forest sites, slightly lower average CO2 fluxes were associated with higher average water table levels (). In contrast, the largest CO2 flux was associated with the highest water table level at BW1. However, this trend may have been an artifact of sampling effort; CO2 flux was not measured at BW1 from December 2011 to February 2012, when this flux was generally low at all other sites. According to the results of two-way ANOVA, the effects of land use and the interaction of land use and drainage were significant, and CO2 flux at the drained forest site was significantly higher than that at the drained burnt sites ().
The site-specific averages of N2O flux ranged from −8.7 to 8.1 μg N m−2 h−1 (). Although we observed variation in N2O fluxes in early April at BD and BW2, little variation was observed in this flux at other sites during the observation period (). On average, the N2O fluxes at the drained sites tended to be positive, whereas they tended to be negative at flooded sites. This difference suggests that drainage influenced whether a site acted as a sink or a source of N2O in tropical peatlands. However, the relationship between N2O flux and water table level was unclear (). The results of two-way ANOVA suggest that there were no significant differences in N2O fluxes between sites based on either land use or drainage ().
GWP was mostly affected by CO2 flux, followed by CH4 flux. At BW1 and BW2, 18–25% of the GWP were attributed to CH4, while most of the GWP (> 92%) was due to CO2 at other sites. As a result, average GWP at the flooded burnt sites was 20% larger compared to the flooded forest sites, although the CO2 fluxes at both sites were similar (). The results of two-way ANOVA were similar to those for the analysis of CO2 flux, except that the simple main effect of drainage in the burnt area was significant (). This exception was probably due to the fact that CH4 emissions in the flooded burnt sites were larger than those in the drained burnt site. According to the post hoc test, GWP in the drained forest site was significantly higher than the GWPs of the drained burnt sites, as was observed for CO2 flux.
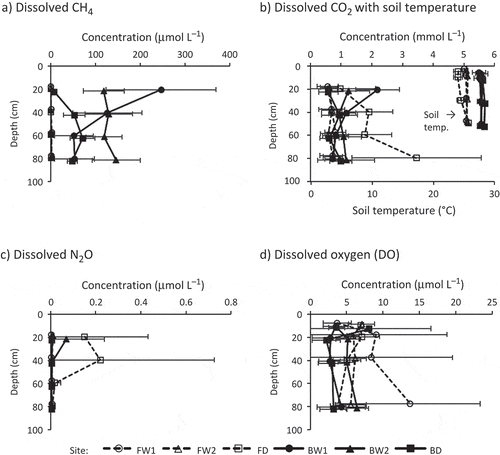
Figure 5 Vertical distribution in dissolved concentrations of (a) methane (CH4), (b) carbon dioxide (CO2), (c) nitrous oxide (N2O) and (d) dissolved oxygen (DO), averaged for each depth. Soil temperature preliminarily observed in 2011 was plotted with dissolved CO2 (b). Error bars show standard deviations. For better identifiability, the depths in the profiles were slightly shifted from their actual depths (20, 40, 60 and 80 cm for CH4, CO2 and N2O; 10, 20, 40 and 80 cm for DO; 5, 10, 30 and 50 cm for soil temperature).
3.3. Dissolved concentrations of GHGs and DO
The vertical distributions of the dissolved concentrations of GHGs (i.e., CH4, CO2 and N2O) and oxygen are shown in . Soil temperatures preliminarily observed in 2011 are also plotted in . The results of three-way ANOVA on these dissolved gases based on land use, drainage and depth are summarized in .
Table 3 Results of three-way analysis of variance (ANOVA) on dissolved gas concentrations among different land use, drainage and depth
Dissolved CH4 concentrations in the burnt sites ranged from 50 to 250 μmol L−1 (except at 20 cm in BD), 1–4 orders of magnitude higher than concentrations measured in the forest sites (where dissolved CH4 was < 2 μmol L−1; ). From 20 to 60 cm depth, dissolved CH4 concentrations increased with increasing depth at BD.
Dissolved CO2 ranged from 0.5 to 3.5 mmol L−1 (). Dissolved CO2 concentrations at FD were highest at depths of 40, 60 and 80 cm out of all the study sites, showing an increasing trend with depth. In contrast, BW1 showed a decreasing trend with depth, with dissolved CO2 being highest at 20 cm out of all the study sites. Other sites did not show clear vertical trends. Similar to CO2 flux, dissolved CO2 showed an increasing trend during the observation period at all sites, especially after March 2012 (time series data are not shown).
We applied the daily mean soil temperature averaged for each depth and site () to the calculation for dissolved CO2 concentration (Eq. 3). The largest diurnal variation in soil temperature at a depth of 20 cm was 3.2°C, recorded at FD in July 2011. This means that the difference between the daily average and the actual hourly soil temperature was as much as ± 1.6°C. According to the sensitivity analysis for the dissolved CO2 calculation, errors of ± 1.6°C in soil temperature produced differences of ± 4.2% in dissolved CO2. Because diurnal variations in soil temperatures were less than 3.2°C for all other sites and at the lower depths, errors associated with the use of daily mean soil temperatures in calculations were less than ± 4.2% for all other sites.
The dissolved N2O concentrations were typically < 0.01 μmol L−1 (142 data/167 in total; ). Only five data points for dissolved N2O were > 0.1 μmol L−1; four of these points were obtained at depths of 20 and 40 cm in FD, and the fifth point was at 20 cm in BW2. As a result, dissolved N2O concentrations in the drained forest site were higher compared to other sites at 20 and 40 cm.
Dissolved oxygen concentrations were generally < 10 μmol L−1, much lower than DO in a solution which is in equilibrium with the atmospheric oxygen level (220–280 μmol L−1; ). In the burnt sites, DO concentrations decreased between depths of 10 and 20 cm and became stable below 20 cm, suggesting the presence of an oxygen supply from the ground surface. According to the results of three-way ANOVA, only the main effect of land use was significant (forest; 6.9 ± 5.6 μmol L−1 > burnt; 4.0 ± 2.9 μmol L−1).
4. DISCUSSION
4.1. CH4 fluxes and dissolved concentrations
In this study, CH4 fluxes were mainly influenced by drainage conditions (; ). Previous studies have reported positive relationships between mean water table level and seasonal CH4 emissions in non-flooded northern peatlands (e.g., Bubier and Moore Citation1993; Pelletier et al. Citation2007). In tropical peatlands, Jauhiainen et al. (Citation2005) found that CH4 fluxes were between −75 and 260 μg C m−2 h−1 and generally increased with increasing water table level (−70 to +20 cm). Melling et al. (Citation2005a) showed that CH4 fluxes ranged from −4.53 to 8.40 μg C m−2 h−1 in a mixed peat swamp forest (water table level: −60 to −20 cm), from −7.44 to 102 μg C m−2 h−1 in a sago (Metroxylon sagu) plantation (−40 to 0 cm), and from −32.8 to 4.17 μg C m−2 h−1 in an oil palm (Elaeis guineensis) plantation (−80 to −30 cm) and also increased with increasing water table levels. We observed similar CH4 fluxes and trends in the drained sites to those reported in these previous studies ().
Based on a comprehensive review on CH4 flux observation in tropical peatlands, Couwenberg et al. (Citation2010) recently reported that CH4 fluxes are generally low and often distinctly negative for water levels below −20 cm, while tend to be higher and more variable at higher water levels. Jauhiainen et al. (Citation2005) found that CH4 fluxes were positive at water table levels > −50 cm and were negative at levels < −60 cm at locations that were in the vicinity of our study area. Jauhiainen et al. (Citation2008) also reported that CH4 fluxes began to increase as the water table levels rose higher than −40 to −20 cm in a drained forest or higher than −30 to 0 cm in a deforested, burned site. Considering the relationship between CH4 flux and water table level, there is an empirical depth of water table level at which CH4 production and oxidation are balanced. In this study, the drained forest site was both a weak sink and source of CH4 (; ) with a water table level that ranged from −39.7 to −7.0 cm at the time of flux measurement, similar to the water levels observed in the previous studies.
In contrast to the CH4 fluxes, dissolved CH4 concentrations were mainly affected by land use (). Ueda et al. (Citation2000) found that dissolved CH4 concentrations in groundwater in a coastal peat swamp in Thailand varied from 0.01 to 417 μmol L−1, with average values of 48 and 226 μmol L−1 in wet seasons spanning 4 years at two sampling sites. Koschorreck (Citation2000) reported that CH4 in pore water at the top 8 cm of a silty loam sediment on an island in the Amazon River ranged from 0 to 900 μmol L−1. Terazawa et al. (Citation2007) found that dissolved CH4 concentrations in groundwater ranged from 5.6 to 28.4 μmol L−1 in a floodplain forest located in northern Japan. Pangala et al. (Citation2013) recently reported that dissolved CH4 concentrations observed in the vicinity of the flooded forest sites in this study ranged from 113–1539 μmol L−1, much higher than our results. Several differences between the two studies, including sampling depths (50–150 cm in Pangala et al. Citation2013 compared to 20–80 cm in this study) and procedures, may have contributed to this discrepancy in dissolved CH4 concentrations. However, the primary reason for this difference is unclear.
The CH4 flux in the drained forest site was the lowest out of all sites (), likely because this site had the lowest water table level (20–60 cm below the ground surface; ). Under such aerobic conditions, methanotrophic activity would have been promoted over methanogenesis. Hanson and Hanson (Citation1996) indicated that anoxic soils produce CH4, while well-drained soils act as a sink for atmospheric CH4 due to CH4 oxidation. In the drained burnt site, CH4 fluxes were lower than in the flooded forest sites, although dissolved CH4 concentrations were much higher in the burnt sites compared to the forest sites (). Previous studies suggested that CH4 diffusing toward the atmosphere is oxidized to CO2 by methanotrophic bacteria when oxic conditions are present in the upper peat profile (Inubushi et al. Citation2003; Jauhiainen et al. Citation2005, Citation2008; Couwenberg et al. Citation2010). In the drained burnt site, the occasionally non-flooded condition () would create an aerobic layer near the ground surface. This would allow CH4 produced in deeper peat layers to be oxidized and CH4 emissions and dissolved CH4 concentrations at a depth of 20 cm to decrease. However, in the flooded burnt sites, the lack of an aerobic surface layer prohibited CH4 oxidation, resulting in a higher CH4 flux compared to other sites and a higher dissolved CH4 concentration at 20 cm compared to deeper layers.
4.2. CO2 fluxes and dissolved concentrations
In tropical peatlands, Melling et al. (Citation2005b) reported that soil CO2 fluxes ranged from 100–533 mg C m−2 h−1 in a mixed peat swamp forest, from 63–245 mg C m−2 h−1 in a sago plantation, and from 46–335 mg C m−2 h−1 in an oil palm plantation in Sarawak, Malaysia. Jauhiainen et al. (Citation2005) also reported that CO2 fluxes were 132–166 mg C m−2 h−1 in hummocks and 37.9–188 mg C m−2 h−1 in hollows in a tropical peat swamp forest in Central Kalimantan. The CO2 fluxes obtained in this study were comparable to those found in previous studies. Ueda et al. (Citation2000) reported that the dissolved CO2 concentrations in groundwater in a coastal peat swamp in Thailand varied from 0.240 to 3.29 mmol L−1 during the wet season, similar to the range observed in this study.
The high CO2 fluxes ( and ) and dissolved CO2 concentrations at depths of 40–80 cm () in the drained forest site suggested that CO2 production was enhanced due to root respiration at this site. In addition, the water table level at this site was consistently at 20–60 cm below the ground surface, much lower than the other sites (). The aerobic conditions that resulted from the low water table level in the drained forest site should promote peat decomposition and contribute to high CO2 emissions at this site. Dissolved CO2 concentrations at 20 cm were lower than those at deeper layers, which could be attributed to diffusive CO2 loss into the atmosphere through the unsaturated top layer in the drained forest site.
The CO2 fluxes and dissolved concentrations in the drained burnt site, which were significantly lower than those in the drained forest site (; and ), suggested a low root respiration rate from poor vegetation cover. Insufficient drainage relative to the drained forest site () may have also inhibited CO2 production through peat decomposition in the drained burnt site. In the flooded forest sites, the inhibition of aerobic peat decomposition would suppress CO2 emissions and result in lower dissolved CO2 concentrations. In the flooded burnt sites, a continuously flooded condition () would inhibit CO2 diffusion into the atmosphere and cause the high dissolved CO2 concentrations at 20 cm.
The increasing trends in CO2 flux and dissolved concentration during the observation period (), which differed from the trends in CH4, N2O and other environmental factors, could be attributed to the increase in plant root respiration as opposed to microbial peat decomposition. Although we could not determine the cause of this trend from data collected in this study, the acclimation of plant roots to saturated conditions may be one possible explanation (Drew et al. Citation1994; Mommer et al. Citation2004).
4.3. N2O fluxes and dissolved concentrations
Like the fluxes in CH4, the N2O fluxes observed in this study were generally related to drainage condition. Melling et al. (Citation2007) reported that the N2O flux ranged from −3.4 to 19.7 μg N m−2 h−1 in a mixed swamp forest, from 1.0 to 176.3 μg N m−2 h−1 in a sago plantation, and from 0.9 to 58.4 μg N m−2 h−1 in an oil palm plantation. The N2O fluxes in the mixed peat swamp forest in that study were comparable to those observed in the drained sites of this study. Takakai et al. (Citation2006) also measured N2O flux at locations that were identical with several of those observed in this study. According to their results, the average N2O fluxes in the wet season (2002 to 2004) were 49 ± 63 μg N m−2 h−1 in the drained forest site and 55 ± 100 μg N m−2 h−1 in the drained burnt site, comparable to the observations made in the wet season (2011–2012) in this study. Ueda et al. (Citation2000) found that dissolved N2O concentrations in the groundwater of a coastal peat swamp in Thailand varied from 0 to 0.012 μmol L−1, with average values of 0.006 and 0.008 μmol L−1 (in the wet seasons of 4 consecutive years) at two sampling sites. These results were similar to those observed in this study.
Given that methanogenesis is an anaerobic process that tends to occur after all other electron donors have been consumed (Burgin and Groffman Citation2012), the flooded sites in this study were likely anaerobic. Under anaerobic conditions, even atmospheric N2O can be absorbed into peat water and consumed by denitrification, which oxidizes organic carbon by reducing NO3− (Burgin and Groffman Citation2012). A recent review indicated that net negative N2O fluxes have been reported in numerous previous studies, showing that low mineral N and high moisture content are favorable for N2O consumption (Chapuis-Lardy et al. Citation2007). In this study, the flooded burnt sites consumed more N2O than the flooded forest sites (). This could be attributed to differences in DO concentrations, with significantly higher concentrations in the forest sites compared to the burnt sites (; ).
NO3− concentration is one of the important controlling factors for soil N processes, including nitrification and denitrification. In this study, the effect of NO3− on N2O flux was unclear, although there were significant differences in dissolved NO3− concentrations in peat water among the study sites (). Takakai et al. (Citation2006) found that the N2O flux in a cropland in the vicinity of our study area increased with increasing NO3−-N content in the top 10 cm of soil. In that study, however, the NO3−-N content was significantly higher in cropland (200–300 mg kg−1 dry soil) than in the drained forest or in the drained burnt sites (0.43–91 and 0.79–5.9 mg kg−1 dry soil, respectively). In this study, the total NO3−-N content was likely too small to influence N2O emissions.
4.4. Effect of plant roots on dissolved GHGs and DO
Ueda et al. (Citation2000) measured DO concentrations in the groundwater of a coastal peat swamp in Thailand, finding that most concentrations were below the detection limit and had a maximum value of 14 μmol L−1. Liebner et al. (Citation2012) observed a rapid decrease (from > 80% to 0% of air saturation) in DO in the top 20 cm of soil in an alpine wetland and a constant DO profile below the top layer. Given these observations, it is likely that DO in saturated peat soils remains very low even near the ground surface, similar to DO concentrations observed in this study (). Most of DO in peat water would be consumed in the top 10 cm, thus there was no significant differences among depths at each study site.
The high dissolved CH4 () and the low DO () in the burnt sites may be due to a lack of large trees, which have large and deep root systems that can supply oxygen into the rhizosphere. The increase in the concentration of dissolved CH4 with depth in the flooded forest sites suggested that CH4 oxidation rate by plant-derived O2 may depend on the amount of plant root biomass, which usually decreases with increasing depth. Previous studies have also reported increased dissolved CH4 as well as decreased DO (Liebner et al. Citation2012), redox potential (Koschorreck Citation2000; Fritz et al. Citation2011), and root density (Fritz et al. Citation2011) in saturated peat profiles. Thus, O2 supply via plant roots would reduce net CH4 production in forest sites even under flooded conditions. The decline in DO by CH4 oxidation could be compensated by this plant-mediated oxygen supply.
In addition, dissolved N2O concentrations at depths of 20 and 40 cm in the drained forest site () could also be explained by the effect of drainage and soil O2 on denitrification. Namely, a water table level that was consistently 20–60 cm below the ground surface () and high DO concentrations () created slightly oxic conditions in the peat profile, which is favorable for N2O production. Burgin and Groffman (Citation2012) found that N2O production in intact soil cores collected from a riparian wetland in the northeastern US increased with increasing O2 concentration. However, nitrification is also an important microbial N process that produces N2O. Bollmann and Conrad (Citation1998) suggested that the main source of N2O was through nitrification when soil moisture is low and through denitrification when soil moisture is high. Therefore, the high concentrations of dissolved N2O in the drained forest site may have been due to nitrification in the drained surface layer.
5. CONCLUSIONS
Our first hypothesis was supported given that (1) CH4 emissions in the flooded burnt sites were significantly larger than those in the flooded forest sites, (2) dissolved CH4 concentrations in the burnt sites were much higher than those in the forest sites and (3) DO concentrations in the forest sites were significantly higher than those in the burnt sites. In this study, however, CH4 fluxes were affected by drainage conditions rather than land use. The CH4 flux and the dissolved CH4 concentration at a depth of 20 cm in the drained burnt site were similar to values observed in the forest sites, suggesting that CH4 oxidation in the surface soil layer occurred in the drained burnt site. Given the high dissolved CH4 concentrations observed in the deep layers, CH4 emissions in the drained burnt site will likely rapidly increase when this area is flooded again.
Our second hypothesis was weakly supported by the observed GWPs in the flooded burnt sites, which were 20% higher than those in the flooded forest sites (no significant difference; P = 0.493). CO2 fluxes in both sites were almost equivalent, and high CH4 emissions in the flooded burnt sites actually increased GWPs at these sites. In this study, however, GWP was mainly determined by CO2 flux. Consequently, GWP and CO2 flux in the drained forest site were the highest for all study sites, and N2O flux made little contribution to GWP.
Acknowledgments
The authors would like to thank the staff members of The Center for International Cooperation in Sustainable Management of Tropical Peatland (CIMTROP), University of Palangka Raya (Ube Tito, Patih Rumbih, Trianson Rogath, and Jeni Ricardo) for their support during the field observation. Also, the authors’ appreciation goes to Prof. Takashi Inoue (Research Faculty of Agriculture, Hokkaido University) and Haiki Mart Yupi (Graduate School of Agriculture, Hokkaido University) for providing climate data. This study was financially supported by the study and research achievement for short visit program in FY2012 and the institutional program for young researcher overseas visits (Soshikiteki) in FY2012, The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 23710001, and Ministry of Education, Culture, Sports, Science and Technology (MEXT) Global Center of Excellence (COE) Program “Establishment of Center for Integrated Field Environmental Science (IFES-GCOE)”.
REFERENCES
- Alberto MCR, Arah JRM, Neue HU, Wassmann R, Lantin RS, Aduna JB, Bronson KF 2000: A sampling technique for the determination of dissolved methane in soil solution. Chemosphere: Global Change Sci., 2, 57–63.
- Bollmann A, Conrad R 1998: Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biol., 4, 387–396. doi: 10.1046/j.1365-2486.1998.00161.x
- Bubier JL, Moore TR 1993: Methane emissions from wetlands in the mid boreal region of northern Ontario, Canada. Ecology., 74, 2240–2254.
- Burgin AJ, Groffman PM 2012: Soil O2 controls denitrification rates and N2O yield in a riparian wetland. J. Geophys. Res., 117, G01010. doi:10.1029/2011JG001799
- Chapuis-Lardy L, Wrage N, Metay A, Chottes JL, Bernouxs M 2007: Soils, a sink for N2O? A review. Global Change Biol., 13, 1–17.
- Couwenberg J, Domain R, Joosten H 2010: Greenhouse gas fluxes from tropical peatlands in South-East Asia. Global Change Biol., 16, 1715–1732.
- Drew MC, Greg Cobb B, Johnson JR, Andrews D, Morgan PW, Jordan W, He CJ 1994: Metabolic acclimation of root tips to oxygen deficiency. Ann. Bot., 74, 281–286. doi: 10.1006/anbo.1994.1119
- Faulkner SP, Patrick Jr WH, Gambrell RP 1989: Field techniques for measuring wetland soil parameters. Soil Sci. Soc. Am. J., 53, 883–890.
- Fritz C, Pancotto VA, Elzenga JTM, Visser EJW, Grootjans AP, Pol A, Iturraspe R, Roelofs JGM, Smolders AJP 2011: Zero methane emission bogs: extreme rhizosphere oxygenation by cushion plants in Patagonia. New Phytologist, 190, 398–408. doi: 10.1111/j.1469-8137.2010.03604.x
- Hanson RS, Hanson TE 1996: Methanotrophic bacteria. Microbiol. Rev., 60, 439–471.
- Hirano T, Segah H, Harada T, Limin S, June T, Hirata R, Osaki M 2007: Carbon dioxide balance of a tropical peat swamp forest in Kalimantan, Indonesia. Global Change Biol., 13, 412–425.
- Hirano T, Segah H, Kusin K, Limin S, Takahashi H, Osaki M 2012: Effects ofdisturbances on the carbon balance of tropical peat swamp forests. Global Change Biol., 18, 3410–3422.
- IPCC 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL Jr (eds.)]. Cambridge University Press, Cambridge, UK and New York, USA, 996 pp.
- Inubushi K, Furukawa Y, Hadi A, Purnomo E, Tsuruta H 2003: Seasonal changes of CO2, CH4 and N2O fluxes in relation toland-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere, 52, 603–608.
- Jauhiainen J, Limin S, Silvennoinen H, Vasander H 2008: Carbon dioxide and methane fluxes in drained tropical peat before and after hydrological restoration. Ecology, 89, 3503–3514.
- Jauhiainen J, Takahashi H, Heikkinen JEP, Martikainen PJ, Vasander H 2005: Carbon fluxes from a tropical peat swamp forest floor. Global Change Biol., 11, 1788–1797. doi:10.1111/j.1365-2486.2005.01031.x
- Joabsson A, Christensen TR, Wallen B 1999: Vascular plant controls on methane emissions from northern peat forming wetlands. Tree, 14, 385–388.
- Koschorreck M 2000: Methane turnover in exposed sediments of an Amazon floodplain lake. Biogeochemistry, 50, 195–206.
- Kozlowski TT 1997: Responses of woody plants to flooding and salinity. Tree Physiology On-Line Monograph., 1, 1–29.
- Laanbroek HJ 2010: Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Annals of Botany, 105, 141–153. doi:10.1093/aob/mcp201
- Liebner S, Schwarzenbach SP, Zeyer J 2012: Methane emissions from an alpine fen in central Switzerland. Biogeochemistry, 109, 287–299. doi:10.1007/s10533-011-9629-4
- Megonigal JP, Day FP 1992: Effects of flooding on root and shoot production of bald cypress in large experimental enclosures. Ecology, 73, 1182–1193.
- Melling L, Hatano R, Goh KJ 2005a: Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biol. Biochem., 37, 1445–1453.
- Melling L, Hatano R, Goh KJ 2005b: Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus, 57B, 1–11.
- Melling L, Hatano R, Goh KJ 2007: Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Sci. Plant Nutr., 53, 792–805.
- Mommer L, Pedersen O, Visser EJW 2004: Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant Cell Environ., 27, 1281–1287. doi:10.1111/j.1365-3040.2004.01235.x
- Morishita T, Hatano R, Desyatkin RV 2003: CH4 flux in an alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia. Soil Sci. Plant Nutr., 49, 369–377.
- Muhanmad NZ, Rieley JO 2002: Management of tropical peatlands in Indonesia: Mega reclamation project in Central Kalimantan. In: Toma Y, Takakai F, Darung U, Kuramochi K, Limin SH, Dohong S, Hatano R 2011: Nitrous oxide emission derived from soil organic matter decomposition from tropical agricultural peat soil in Central Kalimantan, Indonesia. Soil Sci. Plant Nutr., 57, 436–451.
- Notohadiprawiro T 1998: Conflict between problem-solving and optimising approach to land resources development policies-the case of Central Kalimantan wetlands. In Peatlands and climate change, Ed. Strack M, p. 223. International Peat Society (IPS), Jyväskylä, Finland.
- Page S, Hosciło A, Wösten H et al. 2009: Restoration ecology of lowland tropical peatlands in Southeast Asia: current knowledge and future research directions. Ecosystem, 12, 888–905.
- Page SE, Rieley JO, Banks CJ 2011: Global and regional importance of thetropical peatland carbon pool. Global Change Biol., 17, 798–818.
- Page SE, Rieley JO, Shotyk W, Weiss D 1999: Interdependence of peat and vegetationin a tropical peat swamp forest. Philosophical Transactions of the Royal Society of London B series, 354, 1885–1897.
- Pangala SR, Moore S, Hornibrook ERC, Gauci V 2013: Trees are major conduits for methane egress from tropical forested wetlands. New Phytologist, 197, 524–531.
- Pelletier L, Moore TR, Roulet NT, Garneau M, Beaulieu-Audy V 2007: Methane fluxes from three peatlands in the La Grande Rivière watershed, James Bay Lowland, Canada. J. Geophys. Res., 112, G01018. doi:10.1029/2006JG000216
- Shimada S, Takahashi H, Haraguchi A, Kaneko M 2001: The carboncontent characteristics of tropical peats in Central Kalimantan, Indonesia: estimating their spatial variability in density. Biogeochemistry, 53, 249–267.
- Society of Chemical Engineers, Japan 1999: Handbook of chemical engineering (in Japanese), 7th ed. Maruzen, Tokyo.
- Sundari S, Hirano T, Yamada H, Kusin K, Limin S 2012: Effect of groundwater level on soil respiration in tropical peat swamp forests. J. Agric. Meteorol., 68, 121–134.
- Takakai F, Morishita T, Hashidoko Y, Darung U, Kuramochi K, Dohong S, Limin SH, Hatano R 2006: Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, Central Kalimantan, Indonesia. Soil Sci. Plant Nutr., 52, 662–674.
- Terazawa K, Ishizuka S, Sakata T, Yamada K, Takahashi M 2007: Methane emissions from stems of Fraxinus mandshurica var. japonica trees in a floodplain forest. Soil Biol. Biochem., 39, 2689–2692.
- Toma Y, Hatano R 2007: Effect of crop residue C:N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan. Soil Sci. Plant Nutr., 53, 198–205.
- Tuah SJ, Jamal YM, Limin SH 2003: Nutritional characteristics in leaves of plants native to tropical peat swamps and heath forests of Central Kalimantan, Indonesia. Tropics, 12, 221–245.
- Tuah SJ, Osaki M, Matsubara T, Limin SH, Istomo SH, Putir PE 2001: Study on leaf elemental characteristics of native plants grown in a distinct ecosystem of tropical peatland in Central Kalimantan. In: Environmental conservation and land use management of wetland ecosystem in Southeast Asia, Annual report of JSPS-LIPI core university program for April 2000-March 2001. pp. 69–84. Graduate School of Environmental Earth Science, Hokkaido University, Sapporo.
- Ueda S, Go UCS, Yoshioka T, Yoshida N, Wada E, Miyajima T, Sugimoto A, Boontanon N, Vijarnsorn P, Boonprakub S 2000: Dynamics of dissolved O2, CO2, CH4, and N2O in a tropical coastal swamp in southern Thailand. Biogeochemistry, 49, 191–215.
- Usup A, Hashimoto Y, Takahashi H, Hayasaka H 2004: Combustion and thermal characteristics of peat fire in tropical peatland in Central Kalimantan, Indonesia. Tropics, 14, 1–19.
- van der Nat FJWA, Middelburg JJ 1998: Effects of two common macrophytes on methane dynamics in freshwater sediments. Biogeochemistry, 43, 79–104.
- van der Werf GR, Dempewolf J, Trigg SN et al. 2008: Climate regulation of fire emissions and deforestation inequatorial Asia. Proc. Natl. Acad. Sci. USA, 105, 20350–20355.
- Williams CJ, Yavitt JB 2010: Temperate wetland methanogenesis: the importance of vegetation type and root ethanol production. Soil Sci. Soc. Am. J., 74, 317–325. doi:10.2136/sssaj2008.0395
- Yu K, DeLaune RD 2006: A modified soil diffusion chamber for gas profile analysis. Soil Sci. Soc. Am. J., 70, 1237–1241.