Abstract
Nitrate-ammonium (N-A) and ammonium-nitrate (A-N) transition experiments were performed with tobacco (Nicotiana tabacum, cv Gatersleben) plants to study whether the increased expression of ammonium assimilation-related genes found under short-term boron (B) deficiency is maintained when the nitrogen source changes. Asparagine synthetase (AS), glutamate dehydrogenase (GDH) and glutamine synthetase (GS) gene overexpressions in roots under B deprivation were detected in both nitrogen transitions, although GS and, especially, AS up-regulations were observed earlier in the ammonium-nitrate transition. Transcript levels were inversely correlated with hexose contents after 24 h of B deprivation. Our results suggest that tobacco roots respond to short-term B deficiency by increasing the expression of AS, GDH and GS genes irrespectively of the nitrogen source. A hypothetical model to explain these results is proposed and discussed. Thus, the combined action of these enzymes would avoid the accumulation of ammonia and help maintain the activity of the tricarboxylic acid cycle when tobacco roots are affected by B deficiency, the plant organ that first senses this stress. Therefore, under B deficiency, they would act as an ammonium detoxifying mechanism, being a complement to the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway. We propose that the protective role of these enzymes could be extended to changing nitrogen-supply conditions.
INTRODUCTION
Nitrogen (N) is the fourth most abundant element in plants, after carbon, oxygen and hydrogen. The largest source of nitrogen in nature is the atmospheric N2, but most vascular plants are unable to fix it; only legumes and other plants are able to assimilate it by the symbiotic association with microorganisms. Most plants take N directly from soil as nitrate or ammonium, while nitrate is the main nitrogenous source (Maldonado et al. Citation2010); this anion, besides being a nutrient, acts as a signal in the regulation of carbon (C) and N metabolism (Scheible et al. Citation1997a), and biomass allocation between the shoot and the root in tobacco (Nicotiana tabacum, cv Gatersleben) plants (Scheible et al. Citation1997b). In any case, ammonium, from either soil or reduction of nitrate, must be quickly incorporated into organic compounds for the synthesis of amino acids, since the accumulation of ammonia is very toxic to plants.
Ammonia assimilation is carried out through two ways: by the glutamate dehydrogenase (GDH) enzyme and the glutamine synthetase-glutamate synthase (GS-GOGAT) cycle. Before the discovery of the GS-GOGAT cycle (Lea and Miflin Citation1974), it was thought that GDH was the major enzyme involved in ammonium assimilation in vascular plants. Glutamate dehydrogenase catalyzes the synthesis of glutamate (Glu) by reversible reductive amination of 2-oxoglutarate (OG) with ammonium (Forde and Lea Citation2007). As this enzyme catalyzes a reversible reaction, it is difficult to determine in what direction this reaction occurs preferentially in vivo. If the reaction occurs in the direction of releasing ammonia, OG could be supplied to the tricarboxylic acid cycle under C limiting conditions and offset the C:N balance in the cells (Miflin and Habash Citation2002; Dubois et al. Citation2003; Fontaine et al. Citation2012). Despite the existence of GDH, the major pathway for assimilating ammonia is the GS-GOGAT cycle (Lea et al. Citation1990).
In addition to these two routes, it is well known that asparagine synthetase (AS) enzyme can use ammonium directly as a substrate to yield asparagine (Asn) when the ammonium concentration is high enough (Oaks and Ross Citation1984). Asn synthesis is favored when there is a deficit of C, and this amino acid predominates in the phloem of many species especially during darkness, owing that its higher N:C ratio as compared with that of glutamine (Gln) and its high chemical stability (Urquhart and Joy Citation1981; Sieciechowicz et al. Citation1988). In fact, the N:C ratio is the main factor regulating the synthesis of Asn (Lam et al. Citation1996; Herrera-Rodríguez et al. Citation2004). In addition, Asn also accumulates under stress conditions (Lea et al. Citation2007), and it is proposed that this biological response would help maintain the cell osmotic pressure (Gilbert et al. Citation1998; Garthwaite et al. Citation2005). Several genes encoding AS have been identified, whose number can vary between species (Lea et al. Citation2007; Todd et al. Citation2008). Although there are variations in the regulatory mechanisms of these genes, in general one of the AS genes is induced by the decrease in soluble carbohydrate levels and in some cases by dark, while the expression of the other AS gene is stimulated by light and by the presence of carbohydrates (Lea et al. Citation2007).
Boron (B) is a micronutrient for vascular plants and its deficiency or toxicity may cause adverse effects in various physiological and metabolic processes (Nable et al. Citation1997; Blevins and Lukaszewski Citation1998; Bolaños et al. Citation2004; Reid Citation2007; Camacho-Cristóbal et al. Citation2008; Herrera-Rodríguez et al. Citation2010). B, both as boric acid and as borate, can form complexes with a wide variety of biological compounds containing two hydroxyl groups in cis configuration. Thus, the main known function of B in plants is the establishment of ester bonds between the borate anion and apiose residues of rhamnogalacturonan II (RGII) (Kobayashi et al. Citation1996), such that the formation of this complex is essential for the function and structure of cell wall (O’Neill et al. Citation2004).
Regarding N assimilation in vascular plants, there are reports involving B in nitrate assimilation. A decreased nitrate reductase (NR) activity and an increased nitrate concentration in plants (sunflower (Helianthus annuus), tomato (Solanum lycopersicum), oilseed rape (Brassica napus)) subjected to B deficiency have been described (Kastori and Petrovic Citation1989; Ramón et al. Citation1989; Shen et al. Citation1993). These effects were attributed to the possible role of B in NR synthesis or increasing nitrate uptake (Ruiz et al. Citation1998). Instead, it has been observed in tobacco plants that a severe B deficiency for six weeks led to a decreased leaf NR activity as well as a lower leaf concentration of nitrate compared with those of control plants (Camacho-Cristóbal and González-Fontes Citation1999). In addition, it has been shown that short-term B deficiency carries a decrease in nitrate concentration in tobacco plants, in both leaves and roots, without causing a decrease in NR activity; this drop in nitrate content was attributed to the slower uptake of this anion probably due to a fall in root mRNA levels of H+-ATPase membrane gene (PMA2) under B deficiency (Camacho-Cristóbal and González-Fontes Citation2007).
More recently, it has been reported that AS would play a significant role in ammonium assimilation in tobacco roots subjected to B deprivation (Beato et al. Citation2010), GDH being also involved in this process (Beato et al. Citation2011). Given the importance of N sources in ammonium assimilation, the aim of this work was to study the effects of short-term B deficiency on the expression of ammonium-related genes under changing N-supply conditions. For this purpose, an experimental approach to obtain roots with gradually lower concentrations of nitrate and higher concentrations of ammonium, and vice versa, was performed. In this way, we checked how B deficiency affects ammonium-related gene expressions as plants are being subjected to N-source transitions and, therefore, changing the internal nitrate/ammonium ratio.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Nicotiana tabacum (cv Gatersleben, a gift from Prof. Dr. Mark Stitt, Max Planck Institute of Molecular Plant Physiology, Potsdam, Germany) were surface-sterilized as previously described by Beato et al. (Citation2010). Subsequently, seeds were placed on moistened paper in Petri dishes. After 1 week, germinated seeds were sown in seedbeds filled with a mixture of perlite and vermiculite (1/1, volume/volume). During a 3-week period, plants were watered every 2 d with a culture medium solution (nitrate culture medium, NCM) containing 4 mM calcium nitrate (Ca(NO3)2), 4 mM potassium nitrate (KNO3), 2 mM magnesium sulfate (MgSO4), 2.5 mM potassium dihydrogen phosphate (KH2PO4), 0.5 mM dipotassium hydrogen phosphate (K2HPO4), 50 μM ethylenediaminetetraacetic acid ferric sodium salt (FeNa-EDTA), 50 μM sodium chloride (NaCl), 10 μM manganese(II) chloride (MnCl2), 2 μM zinc sulfate (ZnSO4), 1 μM copper(II) sulfate (CuSO4), 0.5 μM disodium molybdate(VI) (Na2MoO4), and 0.2 μM cobalt(II) chloride (CoCl2). This NCM solution, with nitrate as the sole N source, was supplemented with 8 μM boric acid (H3BO3). Immediately afterwards, plants were transferred to hydroponic cultures during 1 week using 0.25 × NCM supplemented with 2 μM H3BO3, renewing the medium every 3–4 d. After this week, sets of plants were transferred to an ammonium culture medium (ACM) without adding B and other sets were grown with the respective identical medium but supplemented with 2 μM H3BO3 (control plants for each experiment). The ACM solution, with ammonium as the sole nitrogen source, contained 3 mM ammonium chloride (NH4Cl), 1 mM calcium chloride (CaCl2), 0.5 mM potassium sulfate (K2SO4), 0.5 mM MgSO4, 0.5 mM KH2PO4, 0.25 mM K2HPO4, 12.5 μM FeNa-EDTA, 12.5 μM NaCl, 2.5 μM MnCl2, 0.5 μM ZnSO4, 0.25 μM CuSO4, 0.125 μM Na2MoO4, and 0.05 μM CoCl2. Tobacco plants were harvested randomly 0, 4, 8 and 24 h after the onset of this last transference (3 mM nitrate to 3 mM ammonium, N-A transition), where time zero corresponded to 1 h after the beginning of the photoperiod. Roots were quickly separated, dried with paper towel, frozen in liquid N2 and stored at –80°C until further analysis.
In parallel, another set of tobacco plants, grown in seedbeds for 3 weeks and watered with NCM solution as previously described, were transferred to hydroponic cultures during 3 d using 0.25 × NCM supplemented with 2 μM H3BO3 in order to acclimate them to hydroponic conditions using the same N source and also to avoid a too-long exposure to ammonium. Immediately afterwards, plants were transferred during 4 d to the ACM solution. Subsequently, sets of plants were transferred to 0.25 × NCM solution without adding B and other sets were grown with the respective identical medium but supplemented with 2 μM H3BO3 (control plants for each experiment). Tobacco plants were harvested randomly 0, 4, 8 and 24 h after the onset of this last transference (3 mM ammonium-3 mM nitrate, A-N transition), where time zero corresponded to 1 h after the beginning of the photoperiod. Roots were harvested and stored as before.
Tobacco plants were grown under a 12 h light/12 h dark regime (350 μmol · m−2 · s−1 of photosynthetically active radiation at plant height), a day/night regime of 25/20°C temperature, and 70/80% relative humidity.
Analytical-grade compounds were always used to prepare nutrient solutions and reagents. Purified water was obtained by a system consisting of three units (active charcoal, ion exchanger and reverse osmosis) connected in series to an ELGA water purification system (PURELAB ultra), which supplied water with an electrical resistivity of 18.2 MΩ cm.
RNA isolation, cDNA synthesis and quantitative real-time PCR analysis
Total RNA extraction, cDNA synthesis and quantitative real-time polymerase chain reaction (PCR) reactions were carried out according to Beato et al. (Citation2010). The amplicon of tobacco elongation factor 1-α gene (accession number AF120093) (forward primer: GCGTCAAACTGTTGCTGTTG; reverse primer: CTGCAACGTTCATTTCTTCTTCT) was used as an internal control to normalize all data.
The AS and GDH (Ntgdh-NAD;A1 and Ntgdh-NAD;B2) specific primers used for the quantitative real-time PCR were the same described in Beato et al. (Citation2010) and Beato et al. (Citation2011), respectively. The specific primers for the quantification of tobacco GLN1.5 RNA transcripts were as follows: forward primer GGAACAACCCTTTCCTCACA and reverse primer ACAGGCAATGACCTGAGCTT.
Quantitative real-time PCR efficiency for all these tobacco genes was higher than 94%.
Metabolite analyses
All concentrations were determined on a dry weight basis. Nitrate, ammonium and amino acids were analyzed in the same ethanolic extract as described by Beato et al. (Citation2010). Glucose, fructose and sucrose were measured in the same ethanol/water extracts used for nitrate determinations, according to Stitt et al. (Citation1989). The sum of these sugar contents was considered to be the total soluble carbohydrate concentration.
Statistical analysis
The data shown are representative results of two experiments (N-A and A-N transitions) that were repeated twice. All analytical determinations were carried out on roots from five or six separate plants harvested randomly. Results were statistically analyzed using the Student’s t-test. The data shown are mean values ± standard deviation (SD).
RESULTS
Boron deficiency increases the expression levels of GDH, GLN1.5 and AS genes in tobacco roots
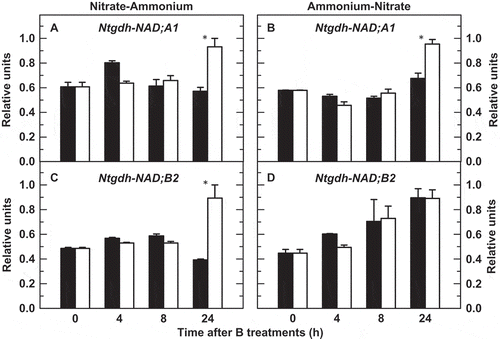
Ntgdh-NAD;A1 and Ntgdh-NAD;B2 (encoding, respectively, α and β subunits of GDH; Purnell et al. Citation2005) gene expressions were analyzed in tobacco roots from plants subjected or not subjected to a 24-h period of B deficiency in two N-source transitions ( and : 3 mM nitrate-3 mM ammonium; and : 3 mM ammonium-3 mM nitrate). In B-sufficient roots, the expression levels of Ntgdh-NAD;A1 remained virtually steady in both N-A and A-N transitions ( and ); however, the transcript levels of this gene increased significantly after 24 h of B deficiency. The same behavior was observed in the Ntgdh-NAD;B2 gene in the N-A transition (), even though its expression increased with time in the A-N transition irrespective of the B treatment ().
Figure 1 Quantitative real-time polymerase chain reaction (PCR) analyses of root transcript levels for (A, B) Ntgdh-NAD;A1 and (C, D) Ntgdh-NAD;B2 genes of tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions. Plants were subjected (open bars) or not (filled bars) to a 24-h period of boron (B) deprivation from zero time. For more details see Materials and Methods. The results are given as means ± standard deviation (SD) (n = 6 separate plants). Asterisks indicate statistically significant differences between plants treated or not with B (Student’s t-test, *P < 0.05).
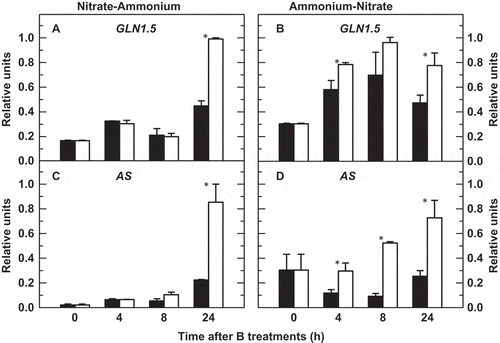
The effect of B deficiency on the expression levels of GLN1.5, which encodes a cytosolic GS isoform in tobacco plants (Dubois et al. Citation1996), and AS genes was analyzed in roots under both N-source transitions (). The expression of these two genes increased along the N-A transition, this effect being more marked after 24 h of B deprivation ( and ). The up-regulation of GLN1.5 and AS genes under B deficiency was observed even earlier, i.e. 4 h after the onset of B treatments, in the A-N transition experiment ( and ).
Figure 2 Quantitative real-time polymerase chain reaction (PCR) analyses of root transcript levels for (A, B) GLN1.5 and (C, D) AS genes of tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions. Plants were subjected (open bars) or not (filled bars) to a 24-h period of boron (B) deprivation from zero time. For more details see Materials and Methods. The results are given as means ± standard deviation (SD) (n = 6 separate plants). Asterisks indicate statistically significant differences between plants treated or not with B (Student’s t-test, *P < 0.05).
Boron deficiency increases asparagine content in tobacco roots irrespective of nitrogen source
Experiments were conducted to analyze the levels of several metabolites related to N metabolism in roots ( and ). As expected for the N-A transition, root nitrate and ammonium concentrations followed opposite trends with time ( and ). The same can be described for the A-N transition ( and ). No significant differences were found in the concentration of these ions between the two short-term B treatments ().
Figure 3 Effects of boron (B) deficiency on root nitrate and ammonium concentrations in tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions. Root concentrations of (A, B) nitrate and (C, D) ammonium were determined in plants subjected (open bars) or not (filled bars) to a 24-h period of B deprivation from zero time. The results are given as means ± standard deviation (SD) (n = 5 separate plants). Asterisks indicate statistically significant differences between plants treated or not with B (Student’s t-test, *P < 0.05). DW, dry weight.
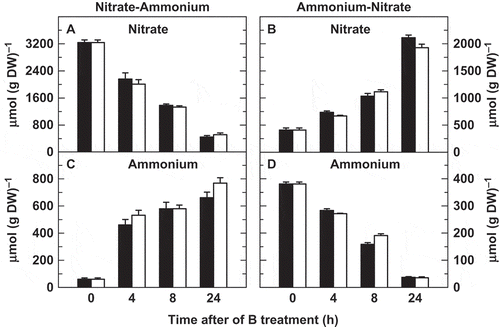
Figure 4 Effects of boron (B) deficiency on several root amino acid concentrations in tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions. Root concentrations of (A, B) asparagine, (C, D) glutamine, (E, F) aspartate and (G, H) glutamate were determined in plants subjected (open bars) or not (filled bars) to a 24-h period of B deprivation from zero time. The results are given as means ± standard deviation (SD) (n = 5 separate plants). Asterisks indicate statistically significant differences between plants treated or not with B (Student’s t-test, *P < 0.05). DW, dry weight.
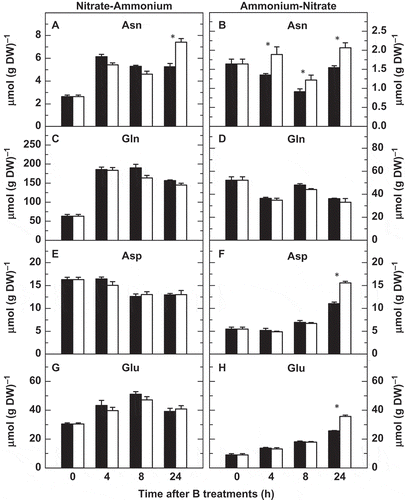
In the N-A transition, root Asn concentrations increased rapidly within the first 4 h in both B treatments, then their levels remained nearly steady and, finally, the Asn levels of B-deficient roots rose up to be significantly higher than those of control plants (). A similar effect of B deficiency on the Asn concentrations was found in the A-N transition at the end of the experiment, but with lower values (). In contrast to Gln contents in the A-N transition (), root Gln concentrations were higher and increased clearly 4 h after the onset of the N-A experiment in both control and B-deprived plants ().
Root aspartate (Asp) concentrations were virtually steady throughout the experiment irrespective of the B treatment in the N-A transition (). However, in the A-N transition, Asp contents were low during the first hours and significantly higher in B-deficient roots after 24 h of B treatments (). Finally, in the A-N transition, root Glu concentration increased from the beginning until the end of the experiment, Glu levels being significantly higher in B-deficient roots (). By contrast, in the N-A transition, Glu concentrations in both B treatments followed a pattern similar to those shown by Gln, no significant differences being observed in root Glu levels between the two B treatments (cf. and ).
Boron deficiency decreases glucose and fructose contents in tobacco roots
The effect of B deficiency on soluble carbohydrate levels was determined in the two N-source transitions. In the N-A transition, glucose and fructose concentrations increased in the first 4 h and afterwards their contents remained nearly constant, even though contents of both sugars turned to decrease with time, being significantly lower in B-deficient roots ( and ). Concentrations of fructose and especially glucose were higher in the A-N transition but, in contrast to the N-A transition, they decreased during the experiment, although again the contents of glucose and fructose were significantly lower in B-deficient roots ( and ). On the other hand, no significant differences were found in root sucrose concentrations between B treatments in the two N-source transitions ( and ).
Figure 5 Effects of boron (B) deficiency on several root carbohydrates in tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions. Root concentrations of (A, B) glucose, (C, D) fructose, (E, F) sucrose and (G, H) total soluble carbohydrates (glucose, fructose and sucrose) were determined in plants subjected (open bars) or not (filled bars) to a 24-h period of B deprivation along two nitrogen-source transitions. The results are given as means ± standard deviation (SD) (n = 5 separate plants). Asterisks indicate statistically significant differences between plants treated or not with B (Student’s t-test, *P < 0.05). DW, dry weight.
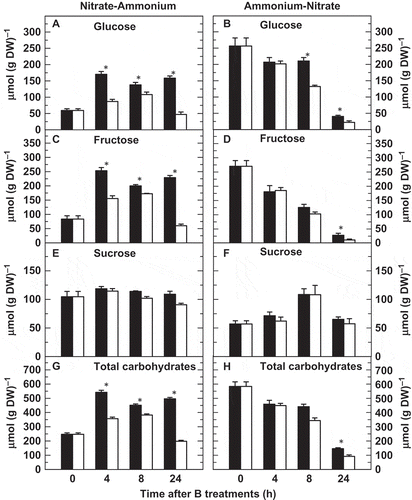
In both N-source transitions, total root soluble carbohydrates (the sum of glucose, fructose and sucrose) followed approximately similar patterns to glucose and fructose concentrations irrespective of B treatments ( and ).
We determined concentration ratios between ammonium, nitrate, or ammonium plus nitrate and total carbohydrates or hexoses (glucose plus fructose). Among all of them, we only found a correlation between ammonium/hexose ratio and the gene expression levels of AS, GDH and GS in B-deficient roots (). Remarkably, the ratio increased in B-deficient roots along the experiments, this rise being more notable in the N-A transition.
DISCUSSION
Plants assimilate N in the form of nitrate or ammonium; however, due to its toxicity, ammonium has to be rapidly assimilated to prevent this cation from reaching toxic concentrations. Since most of the ammonium taken up by plants is assimilated in the roots (Britto and Kronzucker Citation2002; Maldonado et al. Citation2010), a large enough supply of C skeletons is required for maximum growth when ammonium concentrations are high (Schortemeyer et al. Citation1997; Siddiqi et al. Citation2002). Consistently, hexose levels clearly increased in B-sufficient roots during the first 4 h in the nitrate-ammonium (N-A) transition (cf. , and ). In addition, GLN1.5 and AS transcript levels augmented with time in the N-A transition ( and ), which could explain the increase in Asn and Gln contents ( and ). By contrast, and in agreement with the above explanation, both hexose and ammonium concentrations of B-sufficient roots decreased from the beginning of the ammonium-nitrate (A-N) transition experiment (cf. , and ).
Interestingly, root concentrations of glucose and fructose were notably lower after 24 h of B deficiency in both transition experiments when compared to those from B-sufficient roots (–). This decrease could be explained as, under this nutrient deprivation, the hexoses are converted to erythrose-4-phosphate, through the oxidative pentose phosphate pathway, and to phosphoenolpyruvate, by glycolysis (Marschner Citation1995). Both compounds are precursors to the shikimic acid pathway for the synthesis of phenolic compounds. Consistently with this, it has been described that root phenolics and shikimate concentrations increase in B-deficient plants (Camacho-Cristóbal et al. Citation2002; Beato et al. Citation2011).
It is noteworthy that, in both N-A and A-N transitions, root transcript levels of at least one of the analyzed genes encoding GDH (Ntgdh-NAD;A1 and Ntgdh-NAD;B2) increased significantly when tobacco plants were grown under B deprivation for 24 h (). The decline in hexoses under B-deficient conditions might be involved in the enhancement of GDH transcript levels (), as the expression of these genes can be regulated by carbohydrates (Restivo Citation2004; Miyashita and Good Citation2008). GDH is a crucial enzyme that connects C and N metabolism, and it is becoming increasingly clear that the main physiological role of GDH enzyme is to supply OG for the tricarboxylic acid cycle (Fontaine et al. Citation2012). Therefore, under B deficiency, decreased root concentrations of glucose and fructose together with an overexpression of Ntgdh-NAD;A1 and Ntgdh-NAD;B2 genes could indicate that OG generated by the GDH enzyme is being incorporated into the tricarboxylic acid cycle as an alternative source of C when this element becomes limiting (Miyashita and Good Citation2008; Lehmann et al. Citation2010). Remarkably, various abiotic stresses such as salt stress, cadmium toxicity, extreme temperatures and hypoxia can cause overexpression or repression of GDH genes in several plant species (Restivo Citation2004; Limami et al. Citation2008; Ferraro et al. Citation2012).
Abiotic stresses, such as drought and salt stress, stimulate protein degradation and thereby increase the ammonium content (Feng and Barker Citation1993). However, in both transitions, there was no significant difference in the ammonium concentration between B-sufficient and B-deficient roots despite the remarkable and expected variation in ammonium levels measured along time ( and ). This fact could be explained as a consequence of the efficient withdrawal of ammonium through AS and GS enzymes, which is consistent with the higher expression levels of their respective genes found under B deficiency (). Hence, these enzymes would act to remove ammonium in tobacco roots from not only protein degradation but also from the deaminating activity of GDH. These data suggest that, under B deprivation, AS might act as a complement to the GS-GOGAT cycle using ammonium to prevent this cation from reaching toxic concentrations in tobacco roots (Sivasankar and Oaks Citation1996). Furthermore, it has been reported that GS gene expression is affected by salinity, drought, and N starvation (Teixeira and Pereira Citation2007; Caputo et al. Citation2009), and that an increase in AS transcript levels occurs with other abiotic stresses such as salinity, osmotic stress, heavy metal exposure and cold (Chevalier et al. Citation1996; Wong et al. Citation2004; Wang et al. Citation2005; Herrera-Rodríguez et al. Citation2007).
In addition, up-regulation of tobacco AS gene in roots under C-limiting conditions is supported in the literature. In asparagus (Asparagus officinalis) plants, it has been shown that low levels of soluble carbohydrates act as a signal for induction of AS gene expression (Winichayakul et al. Citation2004). In A. thaliana ASN1, expressed primarily in shoots, is a Gln-dependent gene and its transcription is subjected to repression by light (Lam et al. Citation1994). Furthermore, the accumulation of ASN1 transcripts in darkness is repressed by addition of carbohydrates such as glucose and sucrose (Lam et al. Citation1994). In sunflower, the expression of HAS1 and HAS1.1 genes is repressed by light and sucrose, while their transcript levels increase in the dark (Herrera-Rodríguez et al. Citation2002, Citation2004).
Regarding GLN1.5 gene, in both transition experiments there was an increased GLN1.5 gene expression after 24 h of B deprivation, which was more notable in the N-A transition ( and ); in this last, it could be explained by the fact that B-deficient roots had slightly higher ammonium concentrations at this time ( and ). Addition of ammonium to corn and Arabidopsis plants caused the overexpression of some of the genes encoding the root cytosolic GS (Sakakibara et al. Citation1996; Ishiyama et al. Citation2004). Moreover, the higher GLN1.5 gene expression in B-deficient roots at the end of the transitions was concomitant with the lowest hexose contents (cf. , and –).
Interestingly, ammonium/hexose ratio correlated with the expression levels of AS, GDH and GS of B-deficient roots (), that is, their ratio values were increasing along the experiments, this correlation being especially good at 24 h of B deficiency in both N-source transitions. These results suggest that the higher expression of these ammonium-related genes found in B-deficient roots depends rather of the ammonium/hexose ratio that just a decrease in hexoses (; –). This could explain why the drastic decrease in hexoses in B-sufficient roots was not enough to result in higher gene expressions when compared to those from B-deficient roots (cf. , and and ).
According to the results of this work and others recently published by our group, we propose a possible model to explain how tobacco roots respond to short-term B deficiency (). Two early effects of this nutrient deficiency would be the decline in glucose and fructose concentrations and an increase in the levels of soluble amino acids, probably by proteolysis due to this stress. Decreased hexoses would induce the expression of genes encoding GDH, AS and GS, this decrease being probably sensed as a higher ammonium/hexose ratio (). Deaminating activity of GDH would provide OG and ammonium. Thus, under C-limiting conditions, OG would contribute to maintain the tricarboxylic acid cycle, and the released ammonium would be recycled through the GS-GOGAT pathway and AS enzyme. Recycling of OG through these routes would avoid both the ammonium reaching toxic levels and the depletion of amino acids (). It has been reported that, when nitrate is the sole N source, AS and GDH would play a key role in the mechanism of ammonium detoxification in B-deficiency conditions (Beato et al. Citation2011). Herein, we propose that this protective role can be extended even to changing N-supply conditions. More research is needed to establish the molecular mechanisms by which B-deprivation signals elicit these responses at a gene expression level.
Figure 6 Proposed model for the short-term response of roots of tobacco (Nicotiana tabacum, cv Gatersleben) plants to boron (B) deficiency. Dashed lines indicate processes that are directly or indirectly favored by B deficiency. For more details see text. AS, asparagine synthetase; GDH, glutamate dehydrogenase; GOGAT, glutamate synthase; GS, glutamine synthetase; NH4+, ammonium.
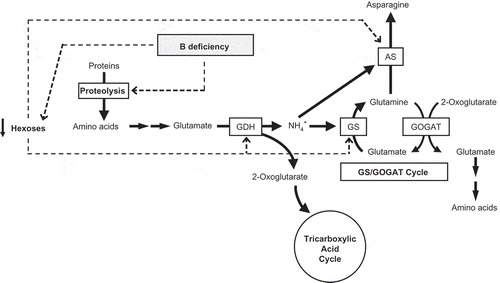
Table 1 Effects of boron (B) deficiency on the concentration ratios between ammonium and hexoses (glucose plus fructose) in roots of tobacco (Nicotiana tabacum, cv Gatersleben) plants grown under two nitrogen-source transitions
ACKNOWLEDGMENTS
This work was supported by the Ministerio de Economía y Competitividad (BFU2009-08397 and BFU2012-37445) and Junta de Andalucía (BIO-266 and P09-CVI-4721), Spain. The authors also thank Marta Fernández García for skilful technical assistance.
REFERENCES
- Beato VM, Navarro-Gochicoa MT, Rexach J, Herrera-Rodríguez MB, Camacho-Cristóbal JJ, Kempa S, Weckwerth W, González-Fontes A 2011: Expression of root glutamate dehydrogenase genes in tobacco plants subjected to boron deprivation. Plant Physiol. Biochem., 49, 1350–1354.
- Beato VM, Rexach J, Navarro-Gochicoa MT, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Maldonado JM, González-Fontes A 2010: A tobacco asparagine synthetase gene responds to carbon and nitrogen status and its root expression is affected under boron stress. Plant Sci., 178, 289–298.
- Blevins DG, Lukaszewski KM 1998: Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49, 481–500.
- Bolaños L, Lukaszewski K, Bonilla I, Blevins D 2004: Why boron?. Plant Physiol. Biochem., 42, 907–912.
- Britto DT, Kronzucker HJ 2002: NH4+ toxicity in higher plants: a critical review. J. Plant Physiol., 159, 567–584.
- Camacho-Cristóbal JJ, Anzelotti D, González-Fontes A 2002: Changes in phenolic metabolism of tobacco plants during short-term boron deficiency. Plant Physiol. Biochem., 40, 997–1002.
- Camacho-Cristóbal JJ, González-Fontes A 1999: Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants. Planta, 209, 528–536.
- Camacho-Cristóbal JJ, González-Fontes A 2007: Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta., 226, 443–451.
- Camacho-Cristóbal JJ, Rexach J, González-Fontes A 2008: Boron in plants: deficiency and toxicity. J. Integr. Plant Biol., 50, 1247–1255.
- Caputo C, Criado MV, Roberts IN, Gelso MA, Barneix AJ 2009: Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants. Plant Physiol. Biochem., 47, 335–342.
- Chevalier C, Bourgeois E, Just D, Raymond P 1996: Metabolic regulation of asparagine synthetase gene expression in maize (Zea mays L.) root tips. Plant J., 9, 1–11.
- Dubois F, Brugière N, Sangwan RS, Hirel B 1996: Localization of tobacco cytosolic glutamine synthetase enzymes and the corresponding transcripts shows organ- and cell-specific patterns of protein synthesis and gene expression. Plant Mol. Biol., 31, 803–817.
- Dubois F, Tercé-Laforgue T, González-Moro MB, Estavillo JM, Sangwan R, Gallais A, Hirel B 2003: Glutamate dehydrogenase in plants: is there a new story for an old enzyme?. Plant Physiol. Biochem., 41, 565–576.
- Feng J, Barker AV 1993: Ethylene evolution and ammonium accumulation by tomato plants under water and salinity stresses. J. Plant Nutr., 15, 2471–2490.
- Ferraro G, Bortolotti S, Mortera P, Schlereth A, Stitt M, Carrari F, Kamenetzky L, Valle EM 2012: Novel glutamate dehydrogenase genes show increased transcript and protein abundances in mature tomato fruits. J. Plant Physiol., 169, 899–907.
- Fontaine JX, Tercé-Laforgue T, Armengaud P et al. 2012: Characterization of a NADH-dependent glutamate dehydrogenase mutant of Arabidopsis demonstrates the key role of this enzyme in root carbon and nitrogen metabolism. Plant Cell, 24, 4044–4065.
- Forde BG, Lea PJ 2007: Glutamate in plants: metabolism, regulation, and signalling. J. Exp. Bot., 58, 2339–2358.
- Garthwaite AJ, von Bothmer R, Colmer TD 2005: Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl- into the shoots. J. Exp. Bot., 56, 2365–2378.
- Gilbert GA, Gadush MV, Wilson C, Madore MA 1998: Amino acid accumulation in sink and source tissues of Coleus blumei Benth. during salinity stress. J. Exp. Bot., 49, 107–114.
- Herrera-Rodríguez MB, Carrasco-Ballesteros S, Maldonado JM, Pineda M, Aguilar M, Pérez-Vicente R 2002: Three genes showing distinct regulatory patterns encode the asparagine synthetase of sunflower (Helianthus annuus). New Phytol., 155, 33–45.
- Herrera-Rodríguez MB, González-Fontes A, Rexach J, Camacho-Cristóbal JJ, Maldonado JM, Navarro-Gochicoa MT 2010: Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress, 4, 115–122.
- Herrera-Rodríguez MB, Maldonado JM, Pérez-Vicente R 2004: Light and metabolic regulation of HAS1, HAS1.1 and HAS2, three asparagine synthetase genes in Helianthus annuus. Plant Physiol. Biochem., 42, 511–518.
- Herrera-Rodríguez MB, Pérez-Vicente R, Maldonado JM 2007: Expression of asparagine synthetase genes in sunflower (Helianthus annuus) under various environmental stresses. Plant Physiol. Biochem., 45, 33–38.
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H 2004: Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J. Biol. Chem., 279, 16598–16605.
- Kastori R, Petrovic N 1989: Effect of boron on nitrate reductase activity in young sunflower plants. J. Plant Nutr., 12, 621–632.
- Kobayashi M, Matoh T, Azuma J 1996: Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol., 110, 1017–1020.
- Lam HM, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM 1996: The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 47, 569–593.
- Lam HM, Peng SSY, Coruzzi GM 1994: Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol., 106, 1347–1357.
- Lea PJ, Miflin BJ 1974: Alternative route for nitrogen assimilation in higher plants. Nature, 251, 614–616.
- Lea PJ, Robinson SA, Stewart GR 1990: The enzymology and metabolism of glutamine, glutamate and asparagine. In The Biochemistry of Plants, Eds. Miflin BJ, Lea PJ, vol. 16, pp. 121–159. Academic Press, San Diego, CA.
- Lea PJ, Sodek L, Parry MAJ, Shewry PR, Halford NG 2007: Asparagine in plants. Ann. Appl. Biol., 150, 1–26.
- Lehmann T, Skrok A, Dabert M 2010: Stress-induced changes in glutamate dehydrogenase activity imply its role in adaptation to C and N metabolism in lupine embryos. Physiol. Plant., 138, 35–47.
- Limami AM, Glévarec G, Ricoult C, Cliquet JB, Planchet E 2008: Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedling under hypoxic stress. J. Exp. Bot., 59, 2325–2335.
- Maldonado JM, González-Fontes A, Rexach J 2010: Assimilation of nitrate and sulfate. In Agricultural Sciences: Topics in Modern Agriculture, Ed. González-Fontes A, Gárate A, Bonilla I, pp. 207–232. Studium Press, Houston, TX.
- Marschner H 1995: Mineral Nutrition of Higher Plants, 2nd edn, 379–396. Academic Press, San Diego, CA.
- Miflin BJ, Habash DZ 2002: The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot., 53, 979–987.
- Miyashita Y, Good AG 2008: NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J. Exp. Bot., 59, 667–680.
- Nable RO, Bañuelos GS, Paull JG 1997: Boron toxicity. Plant Soil, 193, 181–198.
- Oaks A, Ross DW 1984: Asparagine synthetase in Zea mays. Can. J. Bot., 62, 68–73.
- O´Neill MA, Ishii T, Albersheim P, Darvill AG 2004: Rhamnogalacturonan II: Structure and function of borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol., 55, 109–139.
- Purnell MP, Skopelitis DS, Roubelakis-Angelakis KA, Botella JR 2005: Modulation of higher-plant NAD(H)-dependent glutamate dehydrogenase activity in transgenic tobacco via alteration of beta subunit levels. Planta, 222, 167–180.
- Ramón AM, Carpena Ruíz RO, Gárate A 1989: In vitro stabilization and distribution of nitrate reductase in tomato plants. Incidence of boron deficiency. J. Plant Physiol., 135, 126–128.
- Reid R 2007: Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol., 48, 1673–1678.
- Restivo FM 2004: Molecular cloning of glutamate dehydrogenase genes of Nicotiana plumbaginifolia: Structure analysis and regulation of their expression by physiological and stress conditions. Plant Sci., 166, 971–982.
- Ruiz JM, Baghour M, Bretones G, Belakbir A, Romero L 1998: Nitrogen metabolism in tobacco plants (Nicotiana tabacum L.): role of boron as a possible regulatory factor. Int. J. Plant Sci., 159, 121–126.
- Sakakibara H, Shimizu H, Hase T, Yamazaki Y, Takao T, Shimonoshi Y, Sugiyama T 1996: Molecular identification and characterization of cytosolic isoforms of glutamine synthetase in maize roots. J. Biol. Chem., 271, 29561–29568.
- Scheible WR, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M 1997a: Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell, 9, 783–798.
- Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M 1997b: Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J., 11, 671–691.
- Schortemeyer M, Stamp P, Feil B 1997: Ammonium tolerance and carbohydrate status in maize cultivars. Ann. Bot., 79, 25–30.
- Shen ZG, Liang YC, Shen K 1993: Effect of boron on nitrate reductase activity in oilseed rape plants. J. Plant Nutr., 16, 1229–1239.
- Siddiqi MY, Malhotra B, Min XJ, Glass ADM 2002: Effects of ammonium and inorganic carbon enrichment on growth and yield of a hydroponic tomato crop. J. Plant Nutr. Soil Sci., 165, 191–197.
- Sieciechowicz KA, Joy KW, Ireland RJ 1988: The metabolism of asparagine in plants. Phytochemistry, 27, 663–671.
- Sivasankar S, Oaks A 1996: Nitrate assimilation in higher plants: the effect of metabolites and light. Plant Physiol. Biochem., 34, 609–620.
- Stitt M, Lilley RM, Gerhardt R, Heldt HW 1989: Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol., 174, 518–552.
- Teixeira J, Pereira S 2007: High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot., 60, 121–126.
- Todd J, Screen S, Crowley J, Peng J, Andersen S, Brown T, Qi Q, Fabbri B, Duff SMG 2008: Identification and characterization of four distinct asparagine synthetase (AsnS) genes in maize (Zea mays L.). Plant Sci., 175, 799–808.
- Urquhart AA, Joy KW 1981: Use of phloem exudate technique in the study of amino acid transport in pea plants. Plant Physiol., 68, 750–754.
- Wang H, Liu D, Sun J, Zhang A 2005: Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA. J. Plant Physiol., 162, 81–89.
- Winichayakul S, Moyle RL, Coupe SA, Davies KM, Farnden KJF 2004: Analysis of the asparagus (Asparagus officinalis) asparagine synthetase gene promoter identifies evolutionarily conserved cis-regulatory elements that mediate Suc-repression. Funct. Plant Biol., 31, 63–72.
- Wong HK, Chan HK, Coruzzi GM, Lam HM 2004: Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol., 134, 332–338.
