Abstract
In order to determine the mechanism of lettuce root rot suppression in topsoil of andosols we investigated the effect of removing humus from a topsoil of andosol and adding aluminum substances to a disease-conducive subsoil of andosol on the incidence of lettuce root rot. Both topsoil of andosols heated at 250°C and treatment with hydrogen peroxide sustained disease suppressive effect, and exhibited similar levels of disease suppression as the untreated topsoil of andosol. The disease suppressive effect was lost by heating topsoil of andosol at 400°C. Next, both subsoil of andosol amendment with aluminum-containing humic acid (Al-humic acid) and aluminum hydroxide gel (Al(OH)3) added the disease suppressive effect, but not with humic acid or crystalline structure aluminum hydroxide [crystalline Al(OH)3]. The addition of Al-humic acid and Al(OH)3 to potato dextrose agar medium significantly inhibited growth of the causal agent of lettuce root rot, Fusarium oxysporum f. sp. lactucae, compared with the control. Based on the above results, we speculate that noncrystalline aluminum hydroxide, which forms complexes with humus, inhibits growth of pathogenic fungus and thereby suppresses incidence of lettuce root rot.
1. INTRODUCTION
Lettuce root rot is a soil-borne disease caused by Fusarium oxysporum f. sp. lactucae. In Japan, the disease was first reported in 1955 in butterhead lettuce (Lactuca sativa var. capitata L.)-producing areas in Tokyo (Matsuo and Motohashi Citation1967) and has spread rapidly since the 1980s. Besides Japan, the disease has been confirmed in the United States, Brazil, Iran and Italy. The disease can be controlled by soil solarization, fumigation or switching to resistant varieties. But soil solarization is not effective in the open culture of high-altitude cool regions like the major lettuce-producing areas in Japan. Soil disinfection by fumigation and the switch to resistant varieties have notable production costs. In addition, in terms of environmental conservation, there has been an increasing need to develop comprehensive control measures which do not depend on soil sterilization.
As a typical crop disease, there is a long history of research on the life cycle of and control measures for diseases caused by Fusarium species. From this research, it is known that soils exist that are not conducive to the occurrence of soil-borne diseases (i.e., suppressive soils). Topsoils of andosols (hereafter, topsoils), which are typically used for vegetable production in Japan, have been demonstrated to be suppressive to radish yellows (causal agent: F. oxysporum f. sp. raphani) (Komada Citation1976). It has been reported that the suppression of bean root rot (causal agent: F. solani f. sp. phaseoli) (Furuya and Ui Citation1981) and radish yellows (Toyota et al. Citation1994) by topsoils is related to soil biological activity, although the disease suppression mechanism remains poorly understood.
Our previous study investigated the causal relationship between the incidence of soil-borne diseases and soil chemistry (Murakami et al. Citation2004) and we have previously demonstrated that topsoils are suppressive to lettuce root rot (Kitaguchi et al. Citation2004), that this disease suppression involves aluminum and that this disease-suppressive effect of topsoils is lost with excessive application of phosphoric acid (Kitaguchi et al. Citation2004). Meanwhile, it has been shown that although both topsoils and subsoil of andosols (hereafter, subsoils) contain large amounts of aluminum, there is a striking difference in their disease-suppressive effect (Kitaguchi et al. Citation2004). In topsoils, active aluminum primarily forms complexes with polymerized hydroxyaluminum or humus, and is involved in phosphoric acid adsorption. In contrast, in subsoils, active aluminum is primarily in the forms of allophane (Gunjigake and Wada Citation1981). From this, it is speculated that the difference in disease-suppressive effects of topsoils and subsoils is attributed to one or more of the following: the humus found in topsoils, interactive effects of aluminum forming complexes with this humus or effects from other species of aluminum. However, the details of this mechanism remain unclear.
Therefore, in order to clarify the disease-suppression mechanisms of topsoils, in this study, we conducted experiments to investigate the effect of humus removal from topsoils and the addition of aluminum to subsoils on incidence of lettuce root rot.
2. MATERIALS AND METHODS
2.1 Soil samples
Samples of topsoil and subsoil were collected in Kanuma City, Tochigi Prefecture, Japan. The soils were classified as Andosols (Classification Committee of Cultivated Soils Citation1996). The soils were air-dried and passed through a 2-mm sieve prior to being used in the experiments below.
2.2 Treatments to remove humus in topsoils
Three samples were prepared. The first was heated at 250°C, the second was heated at 400°C and the third was given the hydrogen peroxide treatment to topsoil.
The topsoil was heated using an electric furnace. Soils were heated at 250°C for 1 h, the temperature at which humus decomposition starts, and at 400°C for 1 h, the temperature at which humus decomposition ends (Shindo and Urabe Citation1993).
The topsoil was added to 10% hydrogen peroxide solution (soil:solution = 1:20) and heated in a water bath at 80°C for 2 days. This procedure was repeated until the addition of new hydrogen peroxide solution no longer yielded gas bubbles indicating the decomposition of organic matter. In addition, in order to remove ammonia nitrogen, zeolite (5-mm diameter) was added to the soil in a 1:1 ratio. The soil–zeolite mix was then passed through a 3-mm sieve to remove the zeolite, and the resulting sample (hereafter, hydrogen peroxide-treated soil) was used for experiments. Soil chemical properties are shown in .
Table 1 Chemical properties of heating and hydrogen peroxide treatment in topsoil of andosols.
2.3 Preparation of humic acid and aluminum substances
Humic acid, aluminum-containing humic acid (hereafter, Al-humic acid) and aluminum hydroxide gel [hereafter, Al(OH)3] substances were prepared in this study.
The aluminum-containing substances were generated with reference to the method by Higashi (Citation1983). A 1:4 (soil:solution) slurry was created by adding 0.1 M sodium hydroxide (NaOH) to the topsoil, which was placed in an 80°C water bath for 30 minutes to allow decomposition of the humus. After centrifuging (6000 rpm, 20 min), the supernatant was collected and used as humic extract. Humic acid was prepared by adding 2.5 M sulfuric acid (H2SO4) to the humic extract, adjusting the pH to 1.0, and recovering the precipitated fraction by centrifuging. The Al-humic acid was prepared by adding polyaluminum chloride solution (PAC; Sankei Kasei Co., Inc.) to the humic extract, adjusting the solution pH to 3.5, and recovering the precipitated fraction by centrifuging. The Al(OH)3 was prepared by adding 0.1 M NaOH to the PAC, adjusting the pH to 7.0, and recovering the precipitated fraction by centrifuging.
Each of the three substances above was rinsed with pure water until the electrical conductivity was 1.0 dS m−1 or less. The chemical properties of these three substances are presented in .
Table 2 Chemical properties of humus, Al-humus, and Al(OH)3 substances.
2.4 Application of humus and aluminum substances to subsoils
Each of the humic acid, Al(OH)3 or Al-humic acid substances was added to the disease-conducive subsoil. Al(OH)3 and Al-humic acid were added in quantities such that the adding aluminum content would be equivalent to the soluble aluminum content of the topsoil (6.0 g kg−1). Humic acid was added such that the added carbon content would be 5.0 g kg−1. X-ray diffraction analysis of the Al(OH)3 substance did not yield a clear peak and indicated that it was completely noncrystalline (data not shown). Accordingly, to investigate the effect of Al(OH)3 form on disease incidence, another soil was prepared by adding aluminum hydroxide that has a crystalline structure [hereafter, crystalline Al(OH)3, Kanto Chemical Co., Inc.] so that the adding aluminum content was 6.0 g kg−1.
Each mixture was left at room temperature for 7 d and with a moisture content equivalent to 50% of maximum water-holding capacity. After air-drying, disease incidence was investigated in the manner described below.
2.5 Pathogen and potted plant experiment
The pathogen used in this study is F. oxysporum f. sp. lactuce F-9501 (hereafter, F-9501). It was isolated from diseased crisp head lettuce plants (Lactuca sativa var. capitata L.) (Fujinaga et al. Citation2001), and was provided by the Nagano Vegetable and Ornamental Crops Experiment (Nagano, Japan).
F-9501 was shaken (120 rpm) in potato dextrose liquid medium (Difco Laboratories Inc.) for 6 d at 28°C, and the culture was filtered through cheesecloth to remove mycelial fragments. The resulting budcells were then harvested by centrifugation at 6000 rpm for 10 min, washed twice with sterilized distilled water and then suspended in sterilized distilled water to achieve a concentration of 2.0 × 105 budcells mL−1. Twenty milliliters of the prepared budcell suspension was added to 200 g of soil after the pH (H2O) had been adjusted to 6.0 ± 0.1 using dolomite. The infested soil was placed in vinyl pots (diameter = 7 cm, height = 8 cm) and planted with 3-week-old lettuce seedlings (Lactuca sativa L.) cv. Patriot (Nittohnousan Co., Inc.). The lettuce was cultivated for 30 d in a growth chamber (daylight period: 12 h, 30°C, 12,000 l×; dark period: 12 h, 25°C) and assessed for disease severity. The disease incidence experiment was repeated twice with a 5-pot replicate per treatment.
2.6 Disease severity
The disease severity of these plantings was evaluated based on the following test index. The test index was: Rank 0, no symptoms; Rank 1, brown roots; Rank 2, plants showing leaf necrosis; Rank 3, plants wilted and dead.
Disease severity = (3A + 2B + C) × 100/(3N); “A” was the number of Rank 3 plants, “B” was the number of Rank 2 plants, “C” was the number of Rank 1 plants, “N” was the number of examined plants.
2.7 Growth rate of the isolate on PDA containing humus and aluminum-containing materials
Hyphal tips (4-mm diameter) were taken from the margins of F-9501 colonies on PDA (Oxoid Ltd.) plates. The hyphal tips were transferred to fresh potato dextrose agar (PDA) media amended with Al-humic acid, Al(OH)3 or crystalline Al(OH)3 equivalent to 265 mg L–1 aluminum, or humic acid equivalent to 200 mg L−1 carbon. Their pH was adjusted to 6.0 with 0.1 M sodium hydroxide. After incubating for 5 d at 25°C, colony diameter was evaluated. The experiment was repeated twice with five replicates per treatment.
2.8 Soil analysis
Total carbon was measured using an automated nitrogen and carbon analyzer (Sumigraph NC-220F, Sumika Chemical Analysis Service, Ltd.). Soil pH (H2O) and electrical conductivity was evaluated using standard methods. Cation exchange capacity (CEC) was determined by the method proposed by Muramoto et al. (Citation1992). Phosphate absorption coefficient was assessed using the orthophosphate method (Nanzyo et al. Citation1992). Soluble aluminum was assessed using the pH 4 sodium acetate extraction method (Takahashi Citation1997).
3. RESULTS
3.1 Effect of heating and hydrogen peroxide on incidence of Fusarium wilt in topsoils
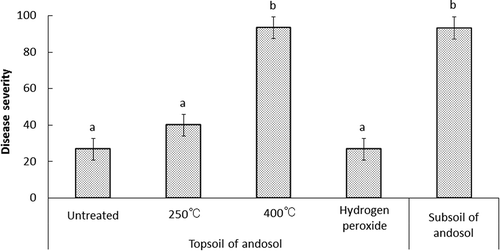
The chemical properties of heat- and hydrogen peroxide-treated soil are shown in . Heating reduced the total carbon content, with the 400°C treatment resulting in lower total carbon content than the subsoil. The hydrogen peroxide treatment dramatically reduced total carbon content to 10.5 g kg−1, below that of the subsoil. CEC fell from 30.1 cmolc kg−1 to 14.2, 7.94 and 24.0 cmolc kg−1 after the 250°C, 400°C and hydrogen peroxide treatments, respectively. Soluble aluminum increased from 6.0 g kg−1 to 11.2, 26.6 and 13.9 g kg−1 after the 250°C, 400°C and hydrogen peroxide treatments, respectively.
In terms of disease-suppressive effect, the hydrogen peroxide and 250°C treated soils resulted in disease severity of 20 and 40, respectively, and exhibited similar levels of disease suppression as the untreated topsoil. Disease severity for the 400°C treatment was 93, indicating a loss of disease-suppressive effect ().
3.2 Effect of humus and aluminum-containing substances on incidence of Fusarium wilt in subsoils
The chemical properties of the soils amended with various substances are presented in . CEC, which was 24.2 cmolc kg−1 in the untreated subsoil, decreased to 21.8 and 21.2 cmolc kg−1 in the Al-humic acid and Al(OH)3-amended soils, respectively. CEC of the crystalline Al(OH)3-amended soil did not decrease to the same degree as that in the Al(OH)3-amended soil. Soluble aluminum increased substantially in the Al-humic acid and Al(OH)3-amended soils. Again, soluble aluminum in the crystalline Al(OH)3-amended soil increased, but not to the same degree as in the Al(OH)3-amended soil.
Table 3 Chemical properties of the subsoil of andosols amended with humus and aluminum-containing substances.
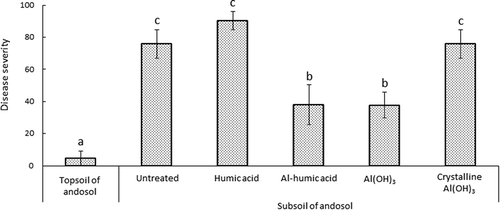
Disease suppressive effect was very low in the untreated subsoil, with a disease severity of 80, as compared to 9 in the topsoil. Disease severity in the humic acid-amended soil was 90. In contrast, disease suppression was observed in the aluminum-amended treatments, with disease severity being reduced to 40 in both the Al-humic acid and Al(OH)3-amended soils. In contrast, no disease suppression was observed in the crystalline Al(OH)3-amended soil, and disease severity did not differ from that of the untreated subsoil ().
Figure 2 Effect of humus and aluminum-containing substances on incidence of Fusarium wilt of lettuce in subsoil of andosol. Al-humic acid, aluminum-containing humic acid; Al(OH)3, aluminum hydroxide gel; Crystalline Al(OH)3, crystalline structure aluminum hydroxide. The lettuce plant (Lactuca sativa L.) cv. Patriot was used in this experiment.
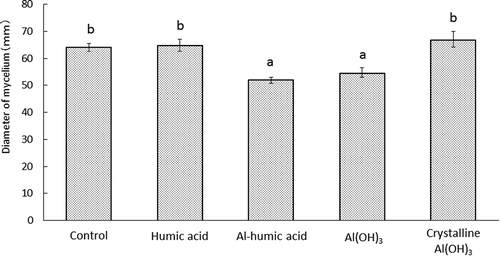
3.3 Effect of humus and aluminum-containing substances on growth rate of F-9501 on PDA
The growth of F-9501 on PDA medium amended with each substance was significantly reduced when the medium was mixed with aluminum-containing substances, reaching a colony diameter of 51 and 52 mm for Al-humic acid and Al(OH)3 treatments, respectively, as compared to 64 mm in the untreated control (). No suppression of the growth rate was observed for the humic acid and crystalline Al(OH)3 treatments.
4. DISCUSSION
Topsoils suppressed lettuce root rot better than subsoils. Therefore, in order to clarify the disease suppression mechanism of topsoils, we conducted experiments in which humus was removed from a topsoil and humic acid substances were added to a subsoil. Although topsoils heated at 250°C continued to exhibit high disease suppression, disease suppressive effect was lost in the same soil heated at 400°C. It is known that most of the humus is retained when soil is heated at 250°C but is lost when heated at 400°C (Shindo and Urabe Citation1993). In addition, dehydration is believed to occur when soil is heated at 400°C, changing the chemical structure of allophane and other substances (Henmi et al. Citation1981). In the present study, in soil heated at 400°C, total carbon fell to a level similar to that of the subsoil, while the quantity of soluble aluminum increased and CEC decreased. These results suggest that the loss of disease-suppressive effect upon heating at 400°C is attributable to changes in the chemical form of aluminum hydroxide in the topsoil resulting from dehydration.
Hydrogen peroxide treatment of the soil resulted in an increase in soluble aluminum content. It is known that most of the humus in soil forms complexes with iron and aluminum (Fujiwara and Shoji Citation1984). Accordingly, as the humus is decomposed by the hydrogen peroxide treatment, aluminum complexed with the humus is released. We inferred that no loss of disease-suppressive effect was observed because the pH of the hydrogen peroxide solution was between 6 and 7 and the released aluminum precipitated as aluminum hydroxide.
From these results, we concluded that it is not the humus but rather the aluminum complexed with humus and the form of aluminum that are important to the disease suppression.
Next, disease-conducive subsoil was found to become suppressive only after amendment with Al-humic acid and Al(OH)3. It was also revealed that the addition of Al-humic acid and Al(OH)3 inhibits the hyphal growth of F. oxysporum f. sp. luctucae on PDA medium. It is known that inhibition of the hyphal elongation of the pathogen results in the disease suppression (Takaki et al. Citation1997).
That said, in the present study, the same level of disease suppression as the topsoil could not be achieved in the subsoil, even with the addition of Al-humic acid and Al(OH)3. This may be due to the following. In the soils amended with Al-humic acid or Al(OH)3, CEC decreased and soluble aluminum increased. However, given that artificially generated aluminum hydroxide–humus complexes readily release aluminum ions when mixed into soil (Takahashi et al. Citation2007), it could be that the aluminum ions that were artificially mixed into the subsoil were adsorbed to allophane and imogolite (Wada Citation1987), thereby reducing the soil’s aluminum-mediated disease suppression.
In addition, soil amended with crystalline Al(OH)3 did not exhibit disease suppression. This is likely because no increase in soluble aluminum was observed, which indicates that it is the noncrystalline form of aluminum hydroxide that contributes to disease suppression.
It has been demonstrated that an increase in the exchangeable aluminum of a soil suppresses bean root rot (Furuya et al. Citation1996, Citation1999). In the present study, however, because disease suppression was observed even at pH 6.0, at which exchangeable aluminum is hardly detectable, we speculate that soluble aluminum and not exchangeable aluminum is involved at the pH level in the suppression of lettuce root rot. Furthermore, the observation that disease suppression and inhibition of hyphal growth occurred at pH 6.0 suggests that soluble aluminum species other than Al3+, such as Al(OH)2+ or Al(OH)2+, may also be involved in suppression of pathogen growth (Fichtner et al. Citation2001).
Based on the above results, we speculate that the disease suppression found in topsoils involves noncrystalline aluminum hydroxide, which forms complexes with humus, and that the basic aluminum ions generated by partial dissociation of noncrystalline aluminum hydroxide found in topsoils inhibit pathogen growth and thereby suppress disease.
ACKNOWLEDGMENTS
We express our thanks to Mr. H. Ogiso, Nagano Vegetable and Ornamental Crops Experiment Station, for providing isolates of F-9501.
REFERENCES
- Classification Committee of Cultivated Soils 1996: Classification of Cultivated Soils in Japan, Third Approximation. National Institute for Agro-Environmental Science, Tsukuba.
- Fichtner EJ, Hesterberg DL, Shew HD 2001: Nonphytotoxic aluminum-peat complexes suppress Phytophthora parasitica. Phytopathology, 91, 1092–1097. doi:10.1094/PHYTO.2001.91.11.1092
- Fujinaga M, Ogiso H, Tsuchiya N, Saito H 2001: Physiological specialization of Fusarium oxysporum f. sp. lactucae, a causal organism of Fusarium root rot of crisp head lettuce in Japan. J. Gen. Plant Pathol., 67, 205–206. doi:10.1007/PL00013012
- Fujiwara Y, Shoji S 1984: Active aluminum and iron in the humus horizons of andosols from northeastern Japan. Soil Sci., 40, 216–226.
- Furuya H, Takahashi T, Matsumoto T 1999: Suppression of Fusarium solani f. sp. phaseoli on bean by aluminum in acid soils. Phytopathology, 89, 47–52. doi:10.1094/PHYTO.1999.89.1.47
- Furuya H, Ui T 1981: The significance of soil microorganisms on the inhibition of the macroconidial germination of Fusarium solani f. sp. phaseoli in a soil suppressive to common bean root rot. Ann. Phytopathol. Soc. Jpn., 47, 42–49 (in Japanese with English summary). doi:10.3186/jjphytopath.47.42
- Furuya H, Wakui A, the late Ui T 1996: Inhibition of macroconidial germination of Fusarium solani f. sp. phaseoli by soil aluminum. Ann. Phytopathol. Soc. Jpn., 62, 69–74 (in Japanese with English summary). doi:10.3186/jjphytopath.62.69
- Gunjigake N, Wada K 1981: Effects of phosphorus concentration and pH on phosphate retention by active aluminum and iron of ando soils. Soil Sci., 132, 347–352. doi:10.1097/00010694-198111000-00004
- Henmi T, Tange K, Minagawa T, Yoshinaga N 1981: Effect of SiO2/Al2O3 ratio on the thermal reactions of allophane. II. Infrared and X-ray powder diffraction data. Clays Clay Miner., 29, 124–128. doi:10.1346/CCMN.1981.0290206
- Higashi T 1983: Characterization of Al/Fe-humus complexes in Dystrandepts through comparison with synthetic forms. Geoderma, 31, 277–288. doi:10.1016/0016-7061(83)90041-1
- Kitaguchi H, Yokota K, Goto I 2004: Effect of soil types and pH on the incidence of Fusarium wilt of lettuce. Abstr. Annu. Meet. Jpn. Soc. Soil Sci. Plant Nutr., 50, 52 (in Japanese).
- Komada H 1976: Studies on the evaluation of activity of Fusarium oxysporum, Fusarium wilt pathogen of vegetable crops, in the soil. Bull. Tokai-Kinki Natl. Agric. Exp. Sta., 29, l32–269 (in Japanese with English summary).
- Matsuo T, Motohashi S 1967: On Fusarium oxysporum f. sp. lactucae n. f. causing root rot on lettuce. Trans. Mycol. Soc. Jpn., 32, 13–15.
- Murakami K, Nakamura F, Goto I 2004: The casual relationship between excess phosphate in the soil and incidence of clubroot disease. Jpn. J. Soil Sci. Plant Nutr., 75, 453–457 (in Japanese with English summary).
- Muramoto J, Goto I, Ninaki M 1992: Rapid analysis of exchangeable cations and cation exchange capacity of soils by a shaking extraction method. Jpn. J. Soil Sci. Plant Nutr., 63, 210–215 (in Japanese with English summary).
- Nanzyo M, Uhm TY, Shoji S 1992: Effect of exchangeable cations on the phosphate sorption coefficient of the cultivated soils. Jpn. J. Soil Sci. Plant Nutr., 63, 559–565 (in Japanese with English summary).
- Shindo H, Urabe M 1993: Changes in the humus composition of volcanic ash soils by heating at various temperatures. Soil Sci. Plant Nutr., 39, 189–192. doi:10.1080/00380768.1993.10416988
- Takahashi T 1997: Analysis of Soil Nutrients, 86–119, Youkendou, Tokyo (in Japanese).
- Takahashi T, Nanzyo M, Hiradate S 2007: Aluminum status of synthetic Al-humic substance complexes and their influence on plant root growth. Soil Sci. Plant Nutr., 53, 115–124. doi:10.1111/j.1747-0765.2007.00114.x
- Takaki S, Kitamura A, Marumoto T, Tanaka S, Nishiyama M, Ishida D 1997: Control of Fusarium diseases using antagonistic Actinomycetes: V. Mechanisms of control of radish yellows with microbial inoculum (Material A). Soil Microorg., 49, 27–33 (in Japanese with English summary).
- Toyota K, Yamamoto K, Kimura M 1994: Mechanisms of suppression of Fusarium oxysporum f. sp. raphani in soils so-called suppressive to Fusarium-wilt of radish. Soil Sci. Plant Nutr., 40, 373–380. doi:10.1080/00380768.1994.10413315
- Wada S 1987: Adsorption of Al(III) on allophane, imogolite, goethite, and noncrystalline silica and the extractability of the adsorbed Al(III) in 1 M KCl solution. Soil Sci. Plant Nutr., 33, 487–491. doi:10.1080/00380768.1987.10557594