Abstract
This study was undertaken to investigate the factors explaining the results of our previous study revealing that selenium (Se) may enhance the nodulation of alfalfa (Medicago sativa L.) via improved carbohydrate metabolism. Symbiotic and non-symbiotic alfalfa plants were cultivated without or with low (1 µM) Se addition at low (2 mM) and adequate (10 mM) N supply levels. The plants were analyzed for root nodulation, photosynthesis and growth parameters. The antioxidative defense and glutathione (GSH) redox state were also investigated. In the inoculated low-nitrogen (N) plants, Se markedly increased the number and fresh weight (FW) of the nodules without any effect on nitrogenase activity. Particularly in the nodulated low-N plants, it also significantly increased the net assimilation rate and soluble sugars, and enhanced protein synthesis. Though Se generally diminished the activity of antioxidative enzymes, the concentrations of hydrogen peroxide (H2O2) and superoxide radical (O2• –) remained constant or even significantly diminished. This gives evidence that Se enhanced the protection of plants against oxidative damage. Se tended to increase GSH particularly in the leaves, and significantly increased the reduced glutathione/oxidized glutathione (GSH/GSSG) particularly in the low-N plants. We suggest that Se exerts its positive effect on the carbohydrate metabolism via modulation in the glutathione redox state.
INTRODUCTION
Selenium (Se) has not yet been classified as an essential element for higher plants, while at low concentrations it is an essential micronutrient for humans and animals (Terry et al. Citation2000; Pilon-Smits et al. Citation2014). This nutrient enters the food chain mainly through the diet and its level in food depends on the bioavailable reserves in the soil, and plant uptake and accumulation capacity (Hartikainen Citation2005). In several countries where dietary Se intake is low or deficient, Se-deficiency disorders have been reported in both livestock and humans (Coppinger and Diamond Citation2001). An immediate solution to overcome the low dietary Se intake is to enrich food and feed crops by using Se fertilizers, i.e., by means of agronomic biofortification (Hartikainen Citation2005).
As a perennial legume, alfalfa (Medicago sativa L.) is one of the most widely cultivated crops used as feed in dairy and meat farming. With an estimated global production of over 400 million tons, alfalfa is a key component in sustainable agriculture (Jensen et al. Citation2012). In addition to nitrogen (N2) fixation and related energy saving, the other advantages of this forage plant in crop rotation and soil conservation are its beneficial effects on chemical, biological and physical properties of soils (Jensen et al. Citation2012). Biofortification of alfalfa with Se fertilizers could be used as a strategy to enhance dietary Se supply.
Alfalfa forms symbiosis with Sinorhizobium meliloti, and its N2 fixation potential is considerably higher than that of other legume species. The amount of N2 fixed by legume species is in the range of 24–250 kg N ha−1 year−1, alfalfa and clover (Trifolium repens L.) having the highest potential (Cooper and Scherer Citation2012). Although an adequate N supply, e.g. so-called starter-N, at the earlier growth stages is necessary for the peak nodulation and N2 fixation, application of N fertilizers (particularly as nitrate) during further growth stages strongly decreases the numbers of nodules and N2 fixation (Cooper and Scherer Citation2012).
Numerous papers reporting beneficial effects of Se supplementation on growth and productivity of crop plants (for reviews see Broadley et al. Citation2012) discuss the mechanisms involved in the growth-promoting effects of Se. The beneficial effects have been attributed to an improved ability of plants to counteract the toxicity of reactive oxygen species (ROS) generated under stressful conditions (Feng et al. Citation2013). Se activates antioxidative enzymes such as glutathione peroxidase (GPX) and superoxide dismutase (SOD; Hartikainen et al. Citation2000; Djanaguiraman et al. Citation2010), increases the concentration of antioxidants such as α-tocopherol and reduces lipid peroxidation (Hartikainen et al. Citation2000).
In nodulated legumes, the respiration rate and energy requirement are high (Udvardi and Poole Citation2013). Thus, the nodules encounter a high risk of oxidative damage caused by excess generation of ROS such as hydrogen peroxide (H2O2) and superoxide (O2•–) radicals (Minchin et al. Citation2008). This means that a high antioxidative capacity is needed to balance between generation and detoxification of ROS and to support the N2 fixation process. The antioxidative systems include ascorbate and glutathione as well as the function of enzymes such as ascorbate peroxidase (APX), glutathione reductase (GR) and SOD (Matamoros et al. Citation2003). Elevated levels of antioxidants are reported to increase the N2 fixation rate by 4-fold in planta as well as in vitro (Ross et al. Citation1999).
Glutathione (γ-Glu-Cys-Gly) is the most abundant non-protein thiol, and its reduced form (GSH) is present in various plant tissues. Analysis of mutants has revealed that GSH is necessary for plant development, and the knockout mutants for one of the glutathione-synthesizing enzymes, γ-glutamyl cystein synthetase (γ-ECS), are embryo lethal (Cairns et al. Citation2006).
In addition to its role as one of the regulatory metabolites for sulfur (S)-assimilating enzyme (Kopriva Citation2006), GSH and its oxidized disulfide form (GSSG) constitute an important redox couple that transfers reducing equivalents from Nicotinamide adenine dinucleotide phosphate (NADPH) to ROS (Foyer and Noctor Citation2011). In the ascorbate–glutathione cycle, ascorbic acid is oxidized to monodehydroascorbate and/or dehydroascorbate by APX using H2O2. Re-reduction of dehydroascorbate is driven by cycling of GSH to GSSG in a reaction catalyzed by dehydroascorbate reductase. GSSG is then re-reduced by GR at the expense of NADPH (Foyer and Halliwell Citation1976; Foyer and Noctor Citation2011). Interestingly, legume root nodules are the organs with the highest GSH (and/or GSH analogue homoglutathione, hGSH) content (Matamoros et al. Citation2003). Reduction of the GSH content using pharmacological and transgenic approaches inhibits the formation of nodules, indicating that GSH plays a crucial role in nodule development (Frendo et al. Citation2005). Further detailed work on the determination of the importance of GSH in the function of nodules demonstrated that the γ-ECS-deficient nodules were smaller in size and showed lower nitrogenase activity than did the control (El Msehli et al. Citation2011).
Reduced glutathione is also used by GPX as a hydrogen donor for catalyzing the reduction of H2O2, thus protecting membranes from peroxidative damage (Foyer and Noctor Citation2011). The role of GPX in ROS homeostasis in plants has been doubted by some researchers (Jung et al. Citation2002). However, Chen et al. (Citation2004) showed changes in the expression of plant GPX genes as response to abiotic stresses, as well as the role of GPX in limiting oxidative burst and programmed cell death. Furthermore, Chang et al. (Citation2009) reported GPX to have a role in protecting plants during acclimation to photo-oxidative stress.
There are some relationships between Se assimilation and GSH metabolism. Selenate (SeO4−2) is taken up through sulfate (SO42–) transporters, and both are assimilated by means of the same enzymes (Terry et al. Citation2000). During selenate assimilation, conversion of selenite to selenide is undertaken via a non-enzymatic step in the presence of GSH. Moreover, the overexpression of genes involved in GSH synthesis in transgenic Brassica juncea (L.) Czern. is shown to result in increased shoot Se accumulation (Bañuelos et al. Citation2005). However, there are no published studies on the effect of Se at non-toxic concentrations on the assimilation of S and the concentration of S-containing metabolites such as GSH, particularly under non-stress conditions.
The effect of Se on growth of legume plants under symbiotic conditions and its impact on root nodulation had not been investigated before our previous work (Owusu-Sekyere et al. Citation2013). In that work, we observed a positive effect of Se supplementation on nodulation (but not on nitrogenase activity) only under lower Se concentrations (1 and 5 μmol L−1) and under low N supply (2 mmol L−1). In addition, Se increased the carbohydrate concentration concomitantly with increased activity of F1,6 BPase. Therefore, we concluded that Se up-regulates carbohydrate metabolism via altered redox potential which may stimulate nodulation (Owusu-Sekyere et al. Citation2013).
Regarding the well-known effect of nitrate level on the nodulation and activity of nitrogenase in symbiotic legume plants (Cooper and Scherer Citation2012) and the improvement of nitrate assimilation by Se (Hajiboland and Sadeghzadeh Citation2014), this study was undertaken to examine the impact of a low selenate addition (1 μmol Se L−1) on the growth and physiological parameters of symbiotic and non-symbiotic alfalfa (Medicago sativa) cultivated at low and adequate nitrate supply levels. As for plant physiological parameters, a special emphasis was made on the concentration of ROS and the activity of ROS-scavenging enzymes, as well as on the redox status of glutathione as the possible regulatory and signaling metabolite. We hypothesized that the redox status of glutathione may have been an important factor that mediated the effect of Se on carbohydrate metabolism observed in our previous work.
MATERIAL AND METHODS
Experimental design and growth conditions
The experimental design is described in detail by Owusu-Sekyere et al. (Citation2013). Briefly, 15 surface-sterilized seeds of alfalfa (Medicago sativa L. cv. Gareh-yondjeh) were germinated in the dark in autoclaved 2.5-L pots filled with washed perlite, and moistened with double-distilled water. After germination of the plants, the pots were irrigated to field capacity with nutrient solution (Hoagland and Arnon Citation1950; without N) and thereafter with water by daily weighing throughout the 10-week growth period. One-week-old seedlings were thinned to 10 plants per pot. Thereafter, to half of the pots 1 µM sodium selenate (Na2SeO4) (Fluka; +Se) was gradually added directly to perlite within 4 weeks, the total addition being 200 µg pot−1. The rest of the plants were cultivated without added Se (–Se).
One week after the first Se application, half of the +Se and –Se plants were inoculated with Sinorhizobium meliloti by adding 0.5 mL of the inoculum suspension (~7 × 108 mL−1) per plant. The non-inoculated plants served as controls. Four weeks after sowing, the pots with and without Se received either 150 mL of 1 mM (low N level) or 5 mM calcium nitrate (Ca(NO3)2) (adequate N level) pot−1 week−1. To equalize the calcium (Ca) concentrations, calcium chloride (CaCl2) was added to the low-N solution. The volume of the nutrient solution was gradually increased, being 650 mL pot−1 in the last cultivation week. Plants were grown under controlled conditions with a temperature regime of 25/18°C day/night, light conditions of 14/10 h light/dark period, relative humidity of 60/70% and photon flux density of about 300 µmol m−2 s−1. All treatments were performed in four replicates.
Measurement of physiological parameters and harvesting
Nine weeks after the onset of Se supplementation, the assimilation of carbon dioxide (CO2) and the transpiration rate were measured with a calibrated infrared gas analyzer of the portable photosynthesis system (LCA-4, ADC Bioscientific Ltd., UK) between 10:00 and 13:00. The photosynthetically active radiation (PAR) intensity of 300 µmol m−2 s−1 at the leaf surface was measured with a quantum sensor attached to the leaf chamber of the gas exchange unit. The net photosynthesis rate by unit of leaf area (A, µmol CO2 m−2 s−1), the transpiration rate (E, mmol H2O m−2 s−1) and the stomatal conductance to water vapor (gs, mol m−2 s−1) were calculated on the basis of the variation of CO2 and humidity inside the chamber. After the gas exchange analyses, the plants were harvested. The fresh weight (FW) of the separated shoots and roots were recorded, and a portion of the plant material was dried at 70°C for 2 d to determine the dry weight (DW). Nodules were separated from the roots and their number and weight were recorded.
Determination of the activity of antioxidative enzymes
The fresh leaf and root samples were ground in liquid N2 to determine the activity of antioxidative enzymes. The measurements were carried out using a spectrophotometer (Specord 200, Analytical Jena, Germany). Total superoxide dismutase (SOD, EC 1.15.1.1) activity was determined by a monoformazan formation test. One unit of SOD was defined as the amount of enzyme required to induce a 50% inhibition of nitroblue tetrazolium (NBT) reduction measured at 560 nm and compared with control samples without enzyme aliquot (Giannopolitis and Ries Citation1977). The activity of ascorbate peroxidase (APX, EC 1.11.1.11) was determined by measuring ascorbic acid oxidation at 25°C (Boominathan and Doran Citation2002). Peroxidase (POD, EC 1.11.1.7) activity was assayed using the guaiacol test where the enzyme activity is calculated as enzyme protein required for the formation of 1 µmol tetraguaiacol min−1 (Chance and Maehly Citation1955). Glutathione peroxidase (GPD, EC 1.11.1.9) activity was measured by the method described by Hartikainen et al. (Citation2000) using H2O2 as substrate. The enzyme activity was calculated as a decrease in GSH within the reaction time as compared to that in the non-enzyme reaction. The activity of glutathione reductase (GR, EC 1.6.4.2) was assayed following the oxidation of NADPH at 340 nm and calculated as enzyme protein required for oxidation of one μM NADPH in 1 min (Foyer and Halliwell Citation1976).
Determination of oxidants and reduced and oxidized glutathione
Lipid peroxidation was estimated from the amount of malondialdehyde (MDA) formed in a reaction mixture containing thiobarbituric acid (Sigma) at 532 nm. Levels of MDA were calculated from a 1,1,3,3-tetraethoxypropane (Sigma) standard curve (Boominathan and Doran Citation2002). The concentration of H2O2 was determined using potassium titanium-oxalate at 508 nm (Patterson et al. Citation1984). Generation of superoxide radicals (O2•–) was determined by reduction of NBT using the method described by Djanaguiraman et al. (Citation2010). Reduced (GSH) and oxidized (GSSG) glutathione were determined according to the method described by Shi et al. (Citation2006), using o-phthalaldehyde as fluorescent reagent at excitation and emission wavelength of 350 and 450 nm, respectively (Shimadzu RF 5301). Standard curves were made for GSH and GSSG using purchased chemicals (Sigma).
Determination of total free amino acids, proteins and carbohydrates
Soluble proteins were determined as described by Bradford (Citation1976) using a commercial reagent (Sigma) and bovine serum albumin (BSA) (Merck) as standard. The content of total free α-amino acids was assayed using a ninhydrin colorimetric method (Hwang and Ederer Citation1975). Glycine was used for production of a standard curve. For determination of carbohydrates, leaves were homogenized in 100 mM phosphate buffer (pH 7.5) at 4°C and, after centrifugation at 12,000 g for 15 min, the supernatant was used for the determination of total soluble sugars. The pellets were used for starch analysis carried out using the method described in Magné et al. (Citation2006).
Statistical analysis
The experiment was undertaken in a complete randomized block design with 2 Se levels × 2 N levels × 2 inoculation treatments and with four replications. Comparisons of means were performed by the Tukey test (p < 0.05). The Sigma stat (3.02) package was used for statistical analysis.
RESULTS
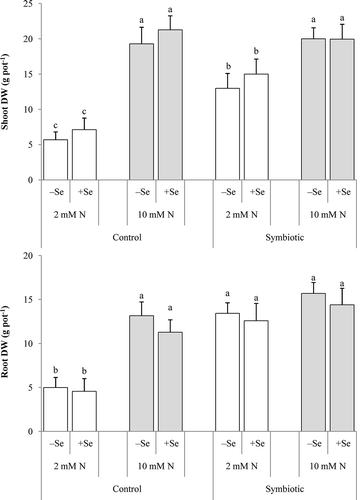
In all plants, the adequate N addition decisively increased DW in the shoots, but in the roots only in the control plants (). The inoculation, in turn, increased both the shoot and root biomass but only when the N supply was low. The Se addition did not affect the plant growth in any treatment. However, in the inoculated low-N plants, it markedly increased the number and FW of the nodules but did not affect their DW or the nitrogenase activity (). As expected, the adequate N supply diminished all nodulation parameters (data not shown).
Table 1 Fresh (FW) and dry weight (DW) (mg pot−1) and number (pot−1) of nodules, the ratio of nodule DW to root DW (× 1000), and nitrogenase activity [pmol acetylene (C2H2) g−1 nodule DW min−1] in inoculated alfalfa (Medicago sativa L.) grown without and with added selenium (Se) at low (2 mM) nitrogen (N) supply level. Values followed by the same letter in each column do not differ significantly (P < 0.05)
Figure 1 Dry weight (DW) of (a) shoot and (b) root in alfalfa (Medicago sativa L.) grown without and with selenium (Se) at low (2 mM, open bar) or adequate (10 mM, dark bar) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Bars indicated by the same letter are not significantly different (P < 0.05).
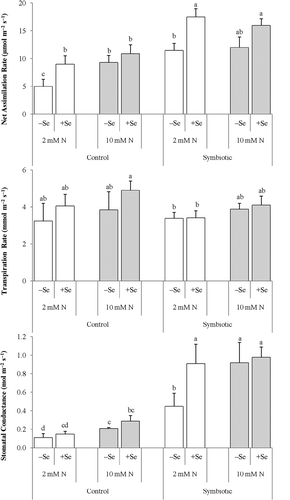
As for gas exchange, the transpiration rate was not affected by any of the experimental factors (). However, at the low N level, the Se supplementation significantly increased in all plants the net assimilation rate, and in the symbiotic ones also the stomatal conductance. Inoculation, in turn, enhanced the stomatal conductance irrespective of the N supply.
Figure 2 (a) Net assimilation rate, (b) transpiration rate and (c) stomatal conductance in alfalfa (Medicago sativa L.) grown without and with selenium (Se) at low (2 mM, open bar) or adequate (10 mM, dark bar) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Bars indicated by the same letter are not significantly different (P < 0.05).
As for free amino acids, in the control plants the adequate N supply increased them in the leaves but not in the roots (). The opposite response was seen in the inoculated plants, where the adequate N tended to increase amino acids in the root but had no effect in the leaves. Se, in turn, diminished amino acids in both plant parts of the low-N controls, whereas in the high-N inoculated plants this occurred only in the roots. The adequate N supply approximately duplicated proteins in the leaves of the control plants, but no effect was seen in the inoculated plants (). Interestingly, Se significantly enhanced the protein synthesis in the leaves of the high-N control plants, but in the inoculated ones only when the N level was low. As for roots, at the low N level, Se increased proteins irrespective of inoculation.
Table 2 Total free α-amino acids [μmol g−1 fresh weight (FW)] and soluble proteins (mg g−1 FW), soluble sugars (mg eq. glucose g−1 FW) and starch (mg g−1 FW) in the leaves and roots of alfalfa (Medicago sativa L.) grown without or with added selenium (Se) at low (2 mM) or adequate (10 mM) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Values followed by the same letter in each column within each plant organ do not differ significantly (P < 0.05)
In the control plants, the N level did not affect the soluble sugars, whereas the starch diminished in the roots of the high-N plants (). In the inoculated plants, on the contrary, the adequate N markedly increased soluble sugars in the leaves but did not affect starch in the roots. Se, in turn, markedly elevated soluble sugars in the leaves and, at adequate N levels, also in the roots. In the inoculated plants, this positive response to Se was seen in the leaves at the adequate N level, whereas it occurred in the roots at the low N level. As for starch, no Se-induced responses were detected.
Generally, the enzyme activities were decisively lower in the leaves than in the roots, except for GR that showed an opposite reaction pattern (). In the leaves of the low-N plants, inoculation regularly reduced the activity of SOD, APX, POD and GPD, but reduced that of GR only when no Se was added. On the contrary, when the high-N plants were cultivated without Se, inoculation tended to diminish APX, POD, GPD and GR. When the high-N plants were supplied with Se, inoculation increased POD and GPD but diminished APX. As for the roots, the inoculation-induced changes in the enzyme activities showed less distinct and regular trends than in the leaves. In the low-N plants without added Se, inoculation diminished APX and GPD but increased SOD. In the high-N plants, on the contrary, it increased APX and GPD irrespective of the Se treatment, but drastically lowered SOD in the Se-supplied plants.
Table 3 Activity of superoxide dismutase (SOD, U mg−1 protein), ascorbate peroxidase (APX, μmol mg−1 pro. min−1), peroxidase (POD, μmol tetraguaiacol mg−1 protein min−1), glutathione peroxidase (GPD, μmol mg−1 pro. min−1) and glutathione reductase (GR, μmol mg−1 pro. min−1) in the leaves and roots of alfalfa (Medicago sativa L.) grown without or with selenium (Se) at low (2 mM) or adequate (10 mM) nitrogen (N) supply level either without (control) or with inoculation (symbiotic). Values in each column within each plant organ followed by the same letter do not differ significantly (P < 0.05)
The MDA concentration and the production of O2•– species in the leaves did not differ among the treatments (). As for other oxidants, in the control plants the adequate N supply diminished H2O2, whereas in the inoculated plants this occurred only in combination with Se. On the contrary, in the roots of the inoculated plants the oxidants showed some contrasting responses to the N supply and the Se addition. At the adequate N level, the concentration of MDA drastically diminished, whereas that of O2•– species increased. An Se-induced decrease in the oxidant concentration became evident as a decrease in the H2O2 concentration and production of O2•– species.
Table 4 Concentration of malondialdehyde [MDA, pmol g−1 fresh weight (FW)], acetylene (H2O2; μmol g−1 FW) and superoxide radical (O2• –) (Δ abs g−1 FW min−1) in the leaves and roots of alfalfa (Medicago sativa L.) grown without and with selenium (Se) at low (2 mM) or adequate (10 mM) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Values in each column followed by the same letter do not differ significantly (P < 0.05)
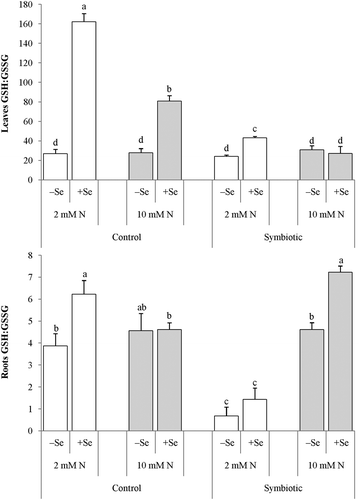
Glutathione species showed a distinct pattern in that the roots were much higher in GSH but decisively lower in GSSG than the leaves (). There was a clear trend that Se increased the GSH concentration in both plant parts, the relative increase in the leaves being more marked. In the control plants, the adequate N supply tended to elevate both glutathione species in the leaves but not in the roots, whereas the inoculated plants showed no clear trends in their responses to adequate N. Similarly, the Se-evoked increase in GSSG showed a more pronounced trend in the leaves than in the roots. The statistically non-significant difference in GSSG between the –Se and +Se treatments in the low-N control plants was due to a wide range of data in this column. When using t-test, the differences were statistically significant. Interestingly, the added Se increased the ratio of GSH to GSSG in the leaves in all treatment combinations, with the exception of inoculated plants (). In the roots, on the other hand, Se increased this ratio significantly only in the non-inoculated low-N plants and in the inoculated high-N plants.
Table 5 Concentration of reduced glutathione [GSH, μmol g−1 fresh weight (FW)] and oxidized glutathione (GSSG, nmol g−1 FW or μmol g−1 FW) in the leaves and roots of alfalfa (Medicago sativa L.) grown without and with selenium (Se) at low (2 mM) or adequate (10 mM) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Values in each column followed by the same letter do not differ significantly (P < 0.05)
Figure 3 The ratio of the reduced glutathione/oxidized glutathione ratio GSH:GSSG in the (a) leaves and (b) roots of control and symbiotic alfalfa (Medicago sativa L.) grown under low (2 mM, open bar) or adequate (10 mM, dark bar) nitrogen (N) supply either without (control) or with inoculation (symbiotic). Bars indicated by the same letter are not significantly different (P < 0.05). Se: selenium.
DISCUSSION
Se did not affect dry matter production, but the inoculation of the low-N plants significantly increased the DW of the shoots (). This outcome is likely attributable to the light conditions in the growth chamber (300 µmol m−2 s−1) being less favorable to maintain a sufficient assimilation rate than those under field conditions. Symbiotic N2 fixation relies on the supply of photosynthates, particularly those derived from current assimilation (Voisin et al. Citation2003). Under low light conditions, the roots compete more efficiently for photosynthates than the shoots do, leading to a higher export of photosynthates to the roots. This reaction pattern explains the finding that the external N supply and inoculation exerted a similar stimulatory effect on root growth.
In our study, in the plants without Se the adequate N supply duplicated the stomatal conductance (). This response is in harmony with findings reported by Iqbal et al. (Citation2011), that plants supplied with adequate N have more open stomata than the N-deficient ones. However, the impact of inoculation was incomparable irrespective of Se. The beneficial effect of inoculation is in harmony with the earlier results obtained with the association of roots with Rhizobiaceae (Franzini et al. Citation2010) and arbuscular mycorrhizal fungi (Augé Citation2000). Interestingly, enhanced stomatal opening is reported also for Rhizobia species that form natural endophytic associations with the roots of cereal plants such as rice (Oryza sativa L.) (Chi et al. Citation2005). It is likely that endophytic associations increase the sink strength of roots needed for optimization of the stomatal CO2 fluxes to maximize the assimilation rate.
In the low-N control and inoculated plants, the added Se decisively enhanced the net CO2 assimilation rate (). In the N-sufficient plants, on the contrary, a high rate of net CO2 assimilation rate was achieved (12–16 µmol m−2 s−1) without Se, and probably any further increase was not possible because of biochemical limitations, i.e. limited enzyme activities. The mechanism explaining the Se-induced increase in the stomatal conductance remained obscure. Enhanced stomatal opening by Se is likely attributable to activated proton pumping with an unknown mechanism that promotes potassium ion (K+) inward currents. Ethylene is a plant growth regulator found to stimulate the stomatal opening (Iqbal et al. Citation2011) and also to be involved in the signaling pathway of plants’ response to Se (Tamaoki et al. Citation2008). Thus, further studies are needed to unravel whether the role of Se in the stomatal mechanisms is connected to ethylene production.
Se significantly increased soluble sugars in both the shoots and roots as well as starch in the roots (). In the Se-supplied plants, the increase in non-structural carbohydrates may be attributable to a higher CO2 fixation as result of enhanced stomatal conductance. Another possible mechanism is the activation of the key enzymes involved in the CO2 assimilation. Owusu-Sekyere et al. (Citation2013) reported a significant increase of fructose 1,6 BPase activity in the Se-treated alfalfa plants. In our study, the increased number and FW of the nodules in the Se-supplied low-N plants might be attributable to increase in carbohydrates produced by photosynthesis in the shoot. These compounds provide energy and carbon skeletons required for development and maintenance of nodules (Voisin et al. Citation2003).
Though a significant enhancement of net photosynthesis rate, unexpectedly, Se-treated alfalfa plants did not produce higher biomass. This may indicate that extra CO2 fixed upon Se treatment was not allocated to dry matter production but resulted in the accumulation of carbohydrates and/or was recruited to protein synthesis following the elevated N assimilation. Our previous works on the effect of low concentrations of Se showed that leaf photosynthesis rate, N assimilation and protein contents increase consistently with Se supplementation (Hajiboland and Sadeghzadeh Citation2014; Hajiboland et al. Citation2014, Citation2015) while growth response is highly dependent on plant species and genotypes (Hajiboland and Amjad Citation2007; Hajiboland et al. Citation2014, Citation2015).
Irrespective of inoculation, in the low-N plants, Se increased the soluble proteins in the roots (). As for the leaves, on the contrary, the positive effect was seen in the low-N symbiotic plants and in the control plants supplied adequately with N. Possible factors explaining the increased protein content in non-symbiotic plants were the activation of nitrate reductase by Se, as reported previously (Nowak et al. Citation2004; Hajiboland and Sadeghzadeh Citation2014), and an increased supply of carbon (C) skeletons for amino acid synthesis. Although we did not analyze the activity of nitrate reductase (NR) or key enzymes in N assimilation such as glutamine synthetase, several potential mechanisms could be suggested for Se-mediated enhancement of protein content via changes in NR activity. Minor amino acids such as histidine, asparagines and cystein as regulators of NR activity (Stitt et al. Citation2002) are subjected to changes by Se (Lee et al. Citation2005). Regarding relationships between Se assimilation and glutathione synthesis pathways (Bañuelos et al. Citation2005) and the central role of glutathione in nitrate assimilation and N metabolism (Kopriva and Rennenberg Citation2004), it could be also speculated that Se might influence NR activity by changes in the glutathione redox state (see below). It is noteworthy that in our study, the nitrogenase activity in the symbiotic plants did not respond to added Se. Thus, the N2 fixation per se was not affected by Se application. In addition, lower amino acid contents associated with higher amounts of protein suggest that elevated protein synthesis was responsible for depletion of amino acids in Se-treated plants. Similar results were obtained previously in wheat (Triticum aestivum L.) (Hajiboland and Sadeghzadeh Citation2014).
The added Se lowered the activity of antioxidative enzymes (), which is in contrast to earlier reports with other plant species (Hartikainen et al. Citation2000; Xue and Hartikainen Citation2000; Djanaguiraman et al. Citation2010). Nevertheless, there are also reports in which no Se-induced enhancement of antioxidative enzyme activities was observed (Hajiboland and Amjad Citation2007; Filek et al. Citation2008). It is noteworthy that in the present study, the added Se elevated the content of soluble proteins. Actually, when the enzyme activities were calculated on the FW basis instead of the protein weight basis, some enzymes showed increased activity as a response to Se. The most prominent effect of Se was observed in SOD activity, which was reduced irrespective of the calculation basis. Interestingly, even if the Se addition apparently reduced the activity of antioxidative enzymes, it tended to diminish the concentrations of H2O2 and O2•–. This can be taken to indicate that in the Se-treated plants, the lower production of ROS and/or their enhanced scavenging diminished the requirement of antioxidative enzymes. In the roots of the Se-supplied plants, lipid peroxidation (MDA concentration) was stable or was even significantly lowered. This supports the conclusion that the plants had a high capacity to defend against ROS despite the reduction of the activity antioxidative enzymes.
Elevated GSH concentrations detected in the shoots and roots in some Se-supplied plants () were not associated with higher activity of GR that reduces the oxidized glutatione (GSSG) back to GSH (). Furthermore, though Se increased also GSSG, its absolute concentration remained still three orders of magnitude lower than that of GSH. This outcome suggests that the higher GSH contents likely result from a higher GSH synthesis rate, not from a higher conversion rate of GSSG into GSH. At high concentrations (200 µM), Se is reported to lower GSH content (Schiavon et al. Citation2012) likely because of the known competition between Se and S. At lower concentrations, however, Se is not able to efficiently compete with S. Tamaoki et al. (Citation2008) demonstrated that Se enhances the expression of some ethylene- and jasmonic acid-responsive genes, and in S assimilation it activates one of the downstream pathways enhancing sulfate reduction. Actually, this mechanism prevents Se from replacing S in proteins and other S-containing compounds (Tamaoki et al. Citation2008). In the present study, it may also explain the finding that the Se supply increased GSH concentrations. However, further studies are needed to confirm the effect of low Se concentrations on S-assimilating enzymes and subsequent GSH synthesis.
Irrespective of the mechanisms explaining the increased glutathione synthesis, a higher GSH:GSSG ratio in the Se-supplied plants may play an important role in the Se-mediated responses in plants, and particularly in their carbohydrate metabolism. Glutathione is a multifunctional metabolite that indirectly influences many fundamental cellular processes (Cairns et al. Citation2006). It is involved in maintaining the redox homeostasis that permits regulation of the cellular metabolism under both non-stress and stressful conditions, and is a link between the regulation of gene expression and the redox state of cells or specific subcelluar compartments (Foyer and Noctor Citation2011). Thiolation of proteins catalyzed by glutathione-S-transferase is a reversible posttranslational modification that acts as a redox-driven regulator of signal transduction cascades and metabolic pathways (Dixon et al. Citation2005). A high GSH concentration is required to prevent the oxidation of thiol groups in the target proteins (Oelze et al. Citation2008). Elevated GSH levels and the altered GSH/GSSG ratio have been evidenced in different plant species under stress conditions (Gill et al. Citation2013).
A shift in the glutathione redox potential can be sensed by various downstream proteins including glutaredoxins and thioredoxins (TRXs). The latter are small disulfide proteins responsible for oxido-reduction of disulfide bonds in different target proteins (Jacquot et al. Citation1997). Various members of the TRX family exist in all cellular compartments. In chloroplasts, TRXs have important roles in the regulation of photosynthesis. As a consequence, even minor deviations in the glutathione redox potential due to either depletion of GSH or increasing oxidation can be exploited for fine-tuning of the activity of target proteins (Meyer et al. Citation2012). Fructose 1,6 BPase is known to be one of the target proteins for TRXs (Pagano et al. Citation2000), and some members of the TRX family are found to be activated by GSH (Chibani et al. Citation2012). Thus, we assume that Se exerts its effect on the carbohydrate metabolism in general and on the activity of F1,6 BPase in particular via increased GSH/GSSG ratio.
Apart from metabolic changes brought about by GSH in the leaves, the metabolism of nodules could be also greatly modulated by elevated levels of GSH. Modulation of the level of GSH by RNA interference approach indicated the link between GSH content and the expression of some development and functioning nodule markers (El Msehli et al. Citation2011). The γ-ECS-overexpressing plants showed higher level of sucrose synthase, leghemoglobin and thioredoxin. Higher leghemoglobin expression in γ-ECS-overexpressing plants suggests that energy metabolism is modified by GSH content, and the higher sucrose synthase expression level fulfills the higher C requirement in these conditions (El Msehli et al. Citation2011). Accordingly, the lack of any significant rise of shoot biomass and nodule dry weight despite higher photosynthesis rate in Se-treated plants could be explained by higher energy metabolism under these conditions. In addition, the higher GSH/GSSG ratio in Se-treated plants may be responsible for the higher protein content observed here as a result of coordination between C and N metabolism (Kopriva and Rennenberg Citation2004). More detailed studies are needed to unravel the changes mediated by Se in the GSH/GSSG redox signaling pathway.
In addition to its role in the metabolism of established nodules, an increased number of nodules observed here in Se-treated low N plants () being associated with higher root GSH content () may also be explained by the role of the plant GSH pool in nodule formation. In GSH-depleted plants, a strong reduction of the number of nodules observed by Pucciariello et al. (Citation2009) suggests that GSH plays a critical role during the first steps of nodule organogenesis.
In contrast to FW, nodule DW was only slightly higher in Se-treated plants. Higher water content in the nodules of Se-treated plants may be due to higher water and photosynthate import through phloem, leading in turn to higher soluble carbohydrates as osmoticums and greater water-retention capacity in the nodules.
In the present study, Se did not considerably increase the dry matter production of alfalfa. However, it increased the number of nodules and their fresh weight, enhanced the net photosynthetic rate, increased soluble sugars and protein concentrations and improved the protection against ROS. These positive responses indicate that the impact of the Se supplementation on plant growth may become apparent under stressful conditions. The Se-treated plants can be taken to be more resistant against drought and salinity stress due to their higher ability for osmoregulation as well as to their greater capability to counteract ROS, as was observed in wheat and rapeseed (Brassica napus L.) plants (Hasanuzzaman et al. Citation2011; Hajiboland et al. Citation2014), and to maintain oxidative status by means of glutathione regarding the role of GSH in plants stress tolerance (Gill et al. Citation2013).
REFERENCES
- Augé RM 2000: Stomatal behavior of arbuscular mycorrhizal plants. In Arbuscular Mycorrhizas: physiology and Function, Eds. Kapulnik Y, Douds DD, pp. 201–237. Kluwer Academic Publishers, Dordrecht.
- Bañuelos G, Terry N, Leduc DL, Pilon-Smits EAH, Mackey B 2005: Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol., 39, 1771–1777. doi:10.1021/es049035f
- Boominathan R, Doran PM 2002: Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol., 156, 205–215. doi:10.1046/j.1469-8137.2002.00506.x
- Bradford MM 1976: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Broadley M, Brown P, Cakmak I, Ma JF, Rengel Z, Zhao F 2012: Function of nutrients: micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, Ed. Marschner P, pp. 249–269. Academic Press, London.
- Cairns NG, Pasternak M, Wachter A, Cobbett CS, Meyer AJ 2006: Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol., 141, 446–455. doi:10.1104/pp.106.077982
- Chance B, Maehly AC 1955: Assay of catalases and peroxidases. Meth. Enzymol., 2, 764–775.
- Chang CCC, Ślesak I, Jordá L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpińska B, Karpiński S 2009: Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol., 150, 670–683. doi:10.1104/pp.109.135566
- Chen S, Vaghchhipawala Z, Li W, Han Asard H, Dickman MB 2004: Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by bax and oxidative stresses in yeast and plants. Plant Physiol., 135, 1630–1641. doi:10.1104/pp.103.038091
- Chi F, Shen S-H, Cheng H-P, Jing Y-X, Yanni YG, Dazzo FB 2005: Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol., 71, 7271–7278. doi:10.1128/AEM.71.11.7271-7278.2005
- Chibani K, Tarrago L, Gualberto JM, Wingsle G, Rey P, Jacquot J-P, Rouhier N 2012: Atypical thioredoxins in poplar: the glutathione-dependent thioredoxin-like 2.1 supports the activity of target enzymes possessing a single redox active cysteine. Plant Physiol., 159, 592–605. doi:10.1104/pp.112.197723
- Cooper JE, Scherer HW 2012: Nitrogen fixation. In Marschner’s Mineral Nutrition of Higher Plants, Ed. Marschner P, pp. 389–408. Academic Press, London.
- Coppinger RJ, Diamond AM 2001: Selenium deficiency and human disease. In Selenium: its Molecular Biology and Role in Human Health, Ed. Hatfield DL, pp. 219–233. Kluwer Academic Publishers, MA.
- Dixon DP, Skipsey M, Grundy NM, Edwards R 2005: Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol., 138, 2233–2244. doi:10.1104/pp.104.058917
- Djanaguiraman M, Prasad PVV, Seppänen M 2010: Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem., 48, 999–1007. doi:10.1016/j.plaphy.2010.09.009
- El Msehli S, Lambert A, Baldacci-Cresp F, Hopkins J, Boncompagni E, Aschi Smiti S, Hérouart D, Frendo P 2011: Crucial role of (homo)glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol., 192, 496–506.
- Feng R, Wei C, Tu S 2013: The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot., 87, 58–68. doi:10.1016/j.envexpbot.2012.09.002
- Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A 2008: The protective role of selenium in rape seedlings subjected to cadmium stress. J. Plant Physiol., 165, 833–844. doi:10.1016/j.jplph.2007.06.006
- Foyer CH, Halliwell B 1976: The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta, 133, 21–25. doi:10.1007/BF00386001
- Foyer CH, Noctor G 2011: Ascorbate and glutathione: the heart of the redox hub. Plant Physiol., 155, 2–18. doi:10.1104/pp.110.167569
- Franzini VI, Azcón R, Latanze-Mendes F, Aroca R 2010: Interactions between Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. J. Plant Physiol., 167, 614–619. doi:10.1016/j.jplph.2009.11.010
- Frendo P, Harrison J, Norman C, Hernandez Jimenez MJ, Van de Sype G, Gilabert A, Puppo A 2005: Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol. Plant Microbe Interact., 18, 254–259. doi:10.1094/MPMI-18-0254
- Giannopolitis CN, Ries SK 1977: Superoxide dismutases. Occurrence in higher plants. Plant Physiol., 59, 309–314. doi:10.1104/pp.59.2.309
- Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N 2013: Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem., 70, 204–212. doi:10.1016/j.plaphy.2013.05.032
- Hajiboland R, Amjad L 2007: Does antioxidant capacity of leaves play a role in growth response to selenium at different sulfur nutritional status? Plant Soil Environ., 53, 207–215.
- Hajiboland R, Ebrahimi N, Sadeghzadeh N, Sadeghzadeh B, Mohammadi SA 2015: Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric. Slov., In press.
- Hajiboland R, Sadeghzadeh N 2014: Effect of selenium on CO2 and NO3 − assimilation under low and adequate nitrogen supply in wheat (Triticum aestivum L.). Photosynthetica, 52, 501–510. doi:10.1007/s11099-014-0058-1
- Hajiboland R, Sadeghzadeh N, Sadeghzadeh B 2014: Effect of Se application on photosynthesis, osmolytes and water relations in two durum wheat (Triticum durum L.) genotypes under drought stress. Acta Agric. Slov., 103, 167–179.
- Hartikainen H 2005: Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol., 18, 309–318. doi:10.1016/j.jtemb.2005.02.009
- Hartikainen H, Xue T, Piironen V 2000: Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil, 225, 193–200. doi:10.1023/A:1026512921026
- Hasanuzzaman M, Hossain MA, Fujita M 2011: Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res., 143, 1704–1721. doi:10.1007/s12011-011-8958-4
- Hoagland DR, Arnon DI 1950: The Water Culture Method for Growing Plants without Soil. California Agr. Exp. St. Circ, Berkeley, CA.
- Hwang M, Ederer GM 1975: Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J. Clin. Microbiol., 1, 114.
- Iqbal N, Nazar R, Syeed S, Masood A, Khan NA 2011: Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot., 62, 4955–4963. doi:10.1093/jxb/err204
- Jacquot JP, Lancelin J, Meyer Y 1997: Thioredoxins: structure and function in plant cells. New Phytol., 136, 543–570. doi:10.1046/j.1469-8137.1997.00784.x
- Jensen ES, Peoples MB, Boddey RM, Gresshoff PM, Hauggaard-Nielsen H, Alves BJR, Morrison MJ 2012: Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A Review. Agron. Sustain. Dev., 32, 329–364. doi:10.1007/s13593-011-0056-7
- Jung BG, Lee KO, Lee SS, et al. 2002: A Chinese cabbage cDNA with high sequence identity to phospholipid hydroperoxide glutathione peroxidases encodes a novel isoform of thioredoxin-dependent peroxidase. J. Biol. Chem., 277, 12572–12578. doi:10.1074/jbc.M110791200
- Kopriva S 2006: Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot., 97, 479–495. doi:10.1093/aob/mcl006
- Kopriva S, Rennenberg H 2004: Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. J. Exp. Bot., 55, 1831–1842. doi:10.1093/jxb/erh203
- Lee J, Finley JW, Harnly JM 2005: Effect of selenium fertilizer on free amino acid composition of broccoli (Brassica oleracea Cv. Majestic) determined by gas chromatography with flame ionization and mass selective detection. J. Agric. Food Chem., 53, 9105–9111. doi:10.1021/jf051221x
- Magné C, Saladin G, Clément C 2006: Transient effect of the herbicide flazasulfuron on carbohydrate physiology in Vitis Vinifera. Chemosphere, 62, 650–657. doi:10.1016/j.chemosphere.2005.04.119
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M 2003: Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol., 133, 499–509. doi:10.1104/pp.103.025619
- Meyer Y, Belin C, Delorme-Hinoux V, Reichheld J-P, Riondet C 2012: Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid. Redox Signal., 17, 1–37.
- Minchin FR, James EK, Becana M 2008: Oxygen diffusion, production of reactive oxygen and nitrogen species, and antioxidants in legume nodules. In Nitrogen-Fixing Symbioses, Eds. Dilworth MJ, James EK, Sprent JI, Newton WE, pp. 321–362. Springer, New York.
- Nowak J, Kaklewski K, Ligocki M 2004: Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem., 36, 1553–1558. doi:10.1016/j.soilbio.2004.07.002
- Oelze M-L, Kandlbinder A, Dietz K-J 2008: Redox regulation and overreduction control in the photosynthesizing cell: complexity in redox regulatory networks. Biochim. Biophys. Acta, 1780, 1261–1272. doi:10.1016/j.bbagen.2008.03.015
- Owusu-Sekyere A, Kontturi J, Hajiboland R, Rahmat S, Aliasgharzad N, Hartikainen H, Seppänen MM 2013: Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil, 373, 541–552. doi:10.1007/s11104-013-1815-9
- Pagano EA, Chueca A, López-Gorgé J 2000: Expression of thioredoxins f and m, and of their targets fructose-1,6-bisphosphatase and NADP malate dehydrogenase, in pea plants grown under normal and light/temperature stress conditions. J. Exp. Bot., 51, 1299–1307. doi:10.1093/jexbot/51.348.1299
- Patterson BD, Mac Rae EA, Ferguson IB 1984: Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem., 139, 487–492. doi:10.1016/0003-2697(84)90039-3
- Pilon-Smits EAH, Bañuelos GS, Parker DR 2014: Uptake, metabolism, and volatilization of selenium by terrestrial plants. In Salinity and Drainage in San Joaquin Valley, California, Eds. Change AC, Silva DB, pp. 147–164. Springer, New York.
- Pucciariello C, Innocenti G, Van de Velde W, et al. 2009: (Homo)glutathione depletion modulates host gene expression during the symbiotic interaction between Medicago truncatula and Sinorhizobium meliloti. Plant Physiol., 151, 1186–1196. doi:10.1104/pp.109.142034
- Ross EJH, Kramer SB, Dalton DA 1999: Effectiveness of ascorbate and ascorbate peroxidase in promoting nitrogen fixation in model systems. Phytochemistry, 52, 1203–1210. doi:10.1016/S0031-9422(99)00407-0
- Schiavon M, Pittarello M, Pilon-Smits EAH, Wirtz M, Hell R, Malagoli M 2012: Selenate and molybdate alter sulfate transport and assimilation in Brassica juncea L. Czern.: implications for phytoremediation. Environ. Exp. Bot., 75, 41–51. doi:10.1016/j.envexpbot.2011.08.016
- Shi Q, Zhu Z, Xu M, Qian Q, Yu J 2006: Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environ. Exp. Bot., 58, 197–205. doi:10.1016/j.envexpbot.2005.08.005
- Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible WR, Krapp A 2002: Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot., 53, 959–970. doi:10.1093/jexbot/53.370.959
- Tamaoki M, Freeman JL, Marquès L, Pilon-Smits EAH 2008: New insights into the roles of ethylene and jasmonic acid in the acquisition of selenium resistance in plants. Plant Signal. Behav., 3, 865–867. doi:10.4161/psb.3.10.6050
- Terry N, Zayed A, De Souza MP, Tarun AS 2000: Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 51, 401–432. doi:10.1146/annurev.arplant.51.1.401
- Udvardi MK, Poole PS 2013: Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol., 64, 781–805. doi:10.1146/annurev-arplant-050312-120235
- Voisin AS, Salon C, Jeudy C, Warembourg FR 2003: Root and nodule growth in Pisum sativum L. in relation to photosynthesis, analysis using 13C-labeling. Ann. Bot., 92, 557–563. doi:10.1093/aob/mcg174
- Xue TL, Hartikainen H 2000: Association of antioxidative enzymes with the synergistic effect of selenium and UV irradiation in enhancing plant growth. Agric. Food Sci. Finland, 9, 177–186.
