ABSTRACT
Soil organic phosphorus (P) is an important P source for biota especially in P-limited forests. Organic P has various chemical formations which differ in bioavailability and these organic P can be degraded by phosphatase enzymes. Here, we report soil P fractions inferred from solution 31P-NMR spectroscopy and soil phosphatase activities of two tropical rain forests on contrasting parent materials; sedimentary and ultramafic igneous (serpentinite) rocks. Compared to the sedimentary soils and previous studies, P fractions of the serpentinite soils have distinctly high proportions of pyrophosphate and scyllo-inositol hexakisphosphate (scyllo-IP6). The accumulation of pyrophosphate and scyllo-IP6 may be related to strong sorptive capacity of iron oxides present in the serpentinite soils, which implies a consequent low P availability in the serpentinite soils. Mean value of soil phosphatase activities was higher in the serpentinite soils than in the sedimentary soils, suggesting that biota in these serpentinite forests depend more on soil organic P as a P source.
1. Introduction
In tropical rain forests on highly weathered soils, biological processes are considered to be limited by phosphorus (P) (Vitousek Citation1984) because soil P occurs as biologically unavailable forms such as organic and occluded P (Walker and Syers Citation1976). P-limited plant roots and microbes actively release phosphatase into soils, degrading soil organic P into inorganic P (orthophosphate) to acquire soil P. Indeed, phosphatase activities for plant roots and soils are generally higher in P-limited forests (Allison et al. Citation2007; Kitayama Citation2013; Ushio et al. Citation2015).
Soil organic P occurs in various chemical formations which differ in bioavailability (Turner Citation2008). Thus, the detection of chemical formations of soil P is important to evaluate P availability. Solution 31P-NMR techniques have been applied to soil P fractionation, dividing soil P into several groups, for example, orthophosphate, monoester-P (glucose phosphate, mononucleotide, and inositol phosphate) and diester-P (nucleic acid and phospholipid), with a smaller amount of phosphonates, pyrophosphates, and polyphosphates (Turner and Engelbrecht Citation2011). 31P-NMR has elucidated soil P fractions in various ecosystems, yet only a few studies were conducted in tropical rain forests, where organic P can be an important P source for biota (Vincent et al. Citation2010). Here, we report soil P fractions with soil phosphatase activities in two tropical rain forests which differ in P availability.
2. Materials and methods
In September 2013, soil sampling was conducted in two lowland rain forests on sedimentary rock and ultramafic igneous (serpentinite) rock around Mount Kinabalu (6° 5ʹ N, 116° 33ʹ E), Sabah, Malaysia. Site data are shown in . The two forests are located at 700 m and have the same climate, but differ in nutrient status (Kitayama et al. Citation2000; Kitayama and Aiba Citation2002). Wagai and Mayer (Citation2007) and Wagai et al. (Citation2009) investigated the soils in our forests and found a much higher content of iron oxides, hydroxides, and oxyhydroxides (collectively called iron oxides hereafter) and specific surface area in the serpentinite soils (). Their mineralogical characteristics must promote strong P sorption onto soil minerals. Consequently, available P (Bray-I extracted) is much lower in the serpentinite soils (Mori et al. Citation2016). We laid five transects of 40 m at 10-m intervals and collected 20 soil cores (0–15 cm) at 2-m intervals along each line in each forest. These sampling points cover 40 m × 40 m area in each 1-ha plot. A total of 20 cores per line were homogenized (i.e., five replicates in each site) and taken to laboratory, then sieved with 2-mm mesh.
Table 1. General description on climate, ecosystem, and soil properties of the two forests on sedimentary rock and serpentinite rock on Mount Kinabalu, Borneo.
The extraction procedure is based on Turner and Engelbrecht (Citation2011) with a minor modification. Moist soils (1.5 g) were shaken with 30 ml of 0.25 M NaOH + 50 mM EDTA for 4 h, centrifuged for 30 min at 4830 g, and 20 ml of supernatant was spiked with 1 ml of 100 μg P ml−1 methylene diphosphonic acid (MDPA) solution as an internal standard. Spiked extracts were then frozen and lyophilized. 100 mg of lyophilized extract was re-dissolved in 0.6 mL of 1.0 M NaOH and 0.1 M Na2EDTA in 10% D2O solution, and transferred to an NMR tube. 31P-NMR spectra were collected on an Alpha 600 FT NMR spectrophotometer (JEOL, Tokyo) at a 242.85 MHz frequency with the following parameters: a pulse width of 15.00 µs (90°), an acquisition time of 0.452 s, a pulse delay time of 2.0000 s, a broadband proton decoupling at 30°C, and scan number of 30,000. We confirmed that the relaxation of 31P magnetization was enough to recover (estimated by null method) and P in the standard orthophosphate solutions in the presence of 1.0 M NaOH and 0.1 M Na2EDTA in 10% D2O were quantitatively determined at least <1000 P ppm at the measurement condition (data not shown). Signals were assigned to model compounds spiked in NaOH-EDTA as reported by Turner et al. (Citation2003). The spectra were divided manually into five groups: MDPA (17.5 ppm), orthophosphate (6.2 ppm), monoester-P (6.0–4.0 ppm), diester-P (−0.1 ppm), and pyrophosphate (−4.1 ppm). Signal areas were determined by integration and concentration of each P compound was calculated based on the area of MDPA using Alice 2 for windows (JEOL, Tokyo).
Brief explanation of soil phosphatase activities assay is as follows (see Kitayama Citation2013; Yokoyama et al. Citation2017 for the details). Moist soils (0.2 g) were incubated in 5-ml MUB buffer solution (pH 5.0) containing 0.04 M p-nitrophenyl phosphate (pNPP) for 1 h. Reaction was terminated with 4 ml of 0.5 M NaOH and 1 ml of 0.5 M CaCl2. Concentration of p-nitrophenol (pNP) hydrolyzed from pNPP during assay was measured on a spectrometer at 410 nm. The amount of pNP produced during incubation per sample dry weight per incubation time was defined as phosphatase activities.
3. Results and discussions
Obtained NMR spectra are shown in . Proportion of each P fraction in the sedimentary soils was similar to the results of other earlier studies (Turner and Engelbrecht Citation2011). By contrast, we found distinct characteristics of P fractions in the serpentinite soils (). Of particular interest was an abundance of pyrophosphate in the serpentinite soils (23.3 µg P g−1 soil, 22.5% in NaOH-EDTA P) despite previous studies which reported a small proportion of pyrophosphate in soils (mostly less than 5% in NaOH-EDTA P). Although pyrophosphate is rapidly degraded by enzymatic hydrolysis in soils (McBeath et al. Citation2006), pyrophosphate might have accumulated in these serpentinite soils due to an extremely higher iron oxides concentration (Wagai and Mayer Citation2007) and a greater specific surface area (Wagai et al. Citation2009). Pyrophosphate can be protected against enzymatic hydrolysis because it is strongly sorbed onto the minerals in the serpentinite soils. In fact, pyrophosphate has a much stronger affinity with soil minerals than orthophosphate (McBeath et al. Citation2007). As to the origin of pyrophosphate, we suggest that pyrophosphate is supplied from saprophytic as well as mycorrhizal fungal biomass (Makarov et al. Citation2005; Bunemann et al. Citation2008). Particularly, mycorrhizal fungi associated with plant roots are known to translocate P in the form of polyphosphate, which is a precursor of pyrophosphate (Bücking and Heyser Citation1999). However, interestingly, available data suggest ectomycorrhiza biomass (Okada et al. unpublished) or soil fungal biomass (Wagai et al. Citation2011; Ikeda et al. unpublished) does not differ between the two sites. This again points the possibility that pyrophosphate has preferentially accumulated and been concentrated by sorption in the serpentinite soils via fungal turnover in a long run. Another possibility in addition to the fungal origin is that pyrophosphate is derived also from serpentinite rock itself. Pyrophosphate can be formed during serpentinization under hydrothermal conditions, which might have preceded the formation of adenosine triphosphate (ATP) in a prebiotic environment because it has condensed P as well as pyrophosphate (Holm and Baltscheffsky Citation2011). Long-term weathering may concentrate pyrophosphate in our serpentinite soils by preferential sorption onto the weathering products, iron oxides. However, this possibility is less likely and needs to be substantiated by investigating other serpentinite soils in the future. Although the origin of pyrophosphate remains elusive, the high accumulation of pyrophosphate in our serpentinite soils implies low P availability to biota in this forest if it is due to strong sorption of pyrophosphate.
Table 2. Mean (±SD) concentration (µg P g−1 soil) and proportion in NaOH-EDTA extracts (%) of each soil P fraction of the sedimentary soils and the serpentinite soils.
Figure 1. Examples of solution 31P-NMR spectra of NaOH-EDTA extracts of the sedimentary soils and the serpentinite soils. The boxes show the detailed spectra in an orthophosphate and monoester region (6.5–3.0 ppm). Each signal shows A, MDPA (17.5 ppm); B, orthophosphate (6.2 ppm); C, monoester-P (6.0–4.0 ppm); D, diester-P (−0.1 ppm); E, pyrophosphate (−4.1 ppm); and F, scyllo-IP6 (4.2 ppm). Four vertical lines in each box show the chemical shifts of myo-IP6 (5.97 ppm: C-2 phosphate on the inositol ring, 5.00 ppm: C-4 and C-6, 4.61 ppm: C-1 and C-3, and 4.50 ppm: C-5). The chemical shifts of myo-IP6 were estimated from the relative positions of myo-IP6 and scyllo-IP6 reported on the literature reference (Cade-Menun Citation2015) and the chemical shift of scyllo-IP6 (4.19 ppm) in this study as a criterion. Black vertical lines in each box describe just the ratio of each height of four peaks of myo-IP6 (1:2:2:1) as a reference but do not represent the observed height of the peaks.
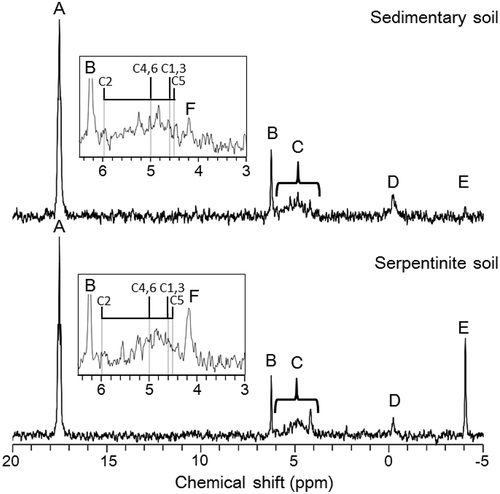
While it is difficult to distinguish each compound in the monoester region of 31P-NMR spectra due to dense chemical shifts, we recognized a higher peak at 4.2 ppm in the serpentinite soils (), which can be assumed to be scyllo-Inositol hexakisphosphate (IP6) based on literature reference of chemical shifts (Turner and Richardson Citation2004). The peak at 4.2 ppm occurs at the right side of monoester region in these spectra, which has been empirically identified as scyllo-IP6 or choline phosphate. Choline phosphate, a compound of labile monoester P, appears close to that of scyllo-IP6, yet choline phosphate is unlikely to be quantitatively important in soils taking into account rapid turnover of labile monoester P (Turner and Richardson Citation2004). Additionally, choline phosphate can be generated after degradation of phosphatidyl choline during alkali extraction, yet phosphatidyl choline is transformed into α-glycerophosphate or β-glycerophosphate rather than choline phosphate (Doolette et al. Citation2009). Therefore, the amount of choline phosphate in extraction is expected to be marginal. Scyllo-IP6 is one of the four stereoisomers of IP6 found in soils, which are myo-IP6, scyllo-IP6, neo-IP6, and D-chiro-IP6, and myo-IP6 and scyllo-IP6 account for most IP6 in soils (Turner Citation2007). Contrary to scyllo-IP6, we could not recognize the clear peaks of myo-IP6 since they were obscured by noises (); therefore, it is impossible to discuss the amount of myo-IP6 or the total amount of IP6. Yet at least scyllo-IP6 is more abundant in serpentinite soils than sedimentary soils. The abundance of scyllo-IP6 in the serpentinite soils may be explained by the same mechanisms as pyrophosphate accumulation because IP6 strongly adsorbs to soil minerals and forms IP6-mineral complexes (Celi and Barberis Citation2007). Thus, the predominance of scyllo-IP6 also suggests reduced P availability in the serpentinite soils compared to the sedimentary soils.
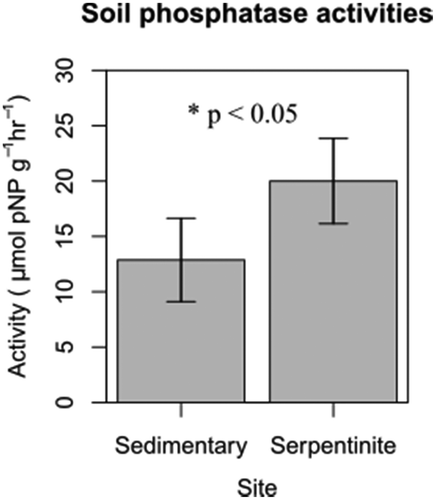
Mean value of phosphatase activities in the serpentinite soils was significantly higher than that in the sedimentary soils (t-test, p < 0.05, ). Therefore, the potential capacity to degrade soil organic P is greater in P-limited soils. Interestingly, P-use efficiency in the serpentinite forest is almost the same as that in the sedimentary forest (Kitayama and Aiba Citation2002), implying that trees in the serpentinite forest successfully acquire soil P partly due to prompt degradation of soil organic P.
Acknowledgment
We thank staffs of the Sabah Parks for their kind assistance in all aspects. We also thank local coworkers for the assistance in the fieldwork and also staffs in National Institute for Agro-Environmental Sciences, Japan, for the assistance in NMR analysis. This work was supported by JSPS KAKENHI (22255002) to K. Kitayama and by JSPS KAKENHI (JP16J11390) to D. Yokoyama.
Additional information
Funding
References
- Allison VJ , Condron LM , Peltzer DA , Richardson SJ , Turner BL 2007: Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol. Biochem . 39, 1770–1781. doi:10.1016/j.soilbio.2007.02.006
- Bücking H , Heyser W 1999: Elemental composition and function of polyphosphates in ectomycorrhizal fungi — an X-ray microanalytical study. Mycol. Res . 103, 31–39. doi:10.1017/S0953756298006935
- Bünemann EK , Smernik RJ , Doolette AL , Marschner P , Stonor R , Wakelin SA , McNeill AM 2008: Forms of phosphorus in bacteria and fungi isolated from two Australian soils. Soil Biol. Biochem . 40, 1908–1915. doi:10.1016/j.soilbio.2008.03.017
- Cade-Menun BJ 2015: Improved peak identification in 31 P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma . 257-258, 102–114. doi:10.1016/j.geoderma.2014.12.016
- Celi L , Barberis E 2007: Abiotic reactions of inositol phosphates in soil. In Inositol Phosphates: Linking Agriculture and the Environment, Eds. Turner BL , Richardson AE , Mullaney EJ , pp. 207–220. Biddles Ltd, UK.
- Doolette AL , Smernik RJ , Dougherty WJ 2009: Spiking improved solution phosphorus-31 nuclear magnetic resonance identification of soil phosphorus compounds. Soil Sci. Soc. Am. J . 73, 919. doi:10.2136/sssaj2008.0192
- Holm NG , Baltscheffsky H 2011: Links between hydrothermal environments, pyrophosphate, Na+, and early evolution. Orig. Life Evol. Biosph . 41, 483–493. doi:10.1007/s11084-011-9235-4
- Kitayama K 1992: An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetation . 102, 149–171. doi:10.1007/BF00044731
- Kitayama K 2013: The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu, Borneo. Plant Soil . 367, 215–224. doi:10.1007/s11104-013-1624-1
- Kitayama K , Aiba S 2002: Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. J. Ecol . 90, 37–51. doi:10.1046/j.0022-0477.2001.00634.x
- Kitayama K , Majalap-Lee N , Aiba S 2000: Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rainforests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia . 123, 342–349. doi:10.1007/s004420051020
- Makarov MI , Haumaier L , Zech W , Marfenina OE , Lysak LV 2005: Can 31 P NMR spectroscopy be used to indicate the origins of soil organic phosphates? Soil Biol. Biochem . 37, 15–25. doi:10.1016/j.soilbio.2004.07.022
- McBeath TM , Lombi E , McLaughlin MJ , Buenemann EK 2007: Pyrophosphate and orthophosphate addition to soils: sorption, cation concentrations, and dissolved organic carbon. Aust. J. Soil Res . 45, 237. doi:10.1071/SR07014
- McBeath TM , Smernik RJ , Lombi E , McLaughlin MJ 2006: Hydrolysis of pyrophosphate in a highly calcareous soil. Soil Sci. Soc. Am. J . 70, 856. doi:10.2136/sssaj2005.0184
- Mori T , Yokoyama D , Kitayama K 2016: Contrasting effects of exogenous phosphorus application on N2O emissions from two tropical forest soils with contrasting phosphorus availability. SpringerPlus . 5. doi:10.1186/s40064-016-2587-5
- Turner BL 2007: Inositol phosphates in soil: amounts, forms and significance of the phosphorylated inositol stereoisomers. In Inositol Phosphates: Linking Agriculture and the Environment, Eds. Turner BL , Richardson AE , Mullaney EJ , pp. 186–206. Biddles Ltd, UK.
- Turner BL 2008: Resource partitioning for soil phosphorus: a hypothesis. J. Ecol . 96, 698–702. doi:10.1111/j.1365-2745.2008.01384.x
- Turner BL , Engelbrecht BM 2011: Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry . 103, 297–315. doi:10.1007/s10533-010-9466-x
- Turner BL , Mahieu N , Condron LM 2003: Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci. Soc. Am. J . 67, 497. doi:10.2136/sssaj2003.4970
- Turner BL , Richardson AE 2004: Identification of-inositol phosphates in soil by solution phosphorus-31 nuclear magnetic resonance spectroscopy. Soil Sci. Soc. Am. J . 68, 802. doi:10.2136/sssaj2004.8020
- Ushio M , Fujiki Y , Hidaka A , Kitayama K , Poorter L 2015: Linkage of root physiology and morphology as an adaptation to soil phosphorus impoverishment in tropical montane forests. Funct. Ecol . 1235–1245. doi:10.1111/1365-2435.12424
- Vincent AG , Turner BL , Tanner EVJ 2010: Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur. J. Soil Sci . 61, 48–57. doi:10.1111/j.1365-2389.2009.01200.x
- Vitousek PM 1984: Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology . 65, 285–298. doi:10.2307/1939481
- Wagai R , Kitayama K , Satomura T , Fujinuma R , Balser T 2011: Interactive influences of climate and parent material on soil microbial community structure in Bornean tropical forest ecosystems. Ecol. Res . 26, 627–636. doi:10.1007/s11284-011-0822-7
- Wagai R , Mayer LM 2007: Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Ac . 71, 25–35. doi:10.1016/j.gca.2006.08.047
- Wagai R , Mayer LM , Kitayama K 2009: Extent and nature of organic coverage of soil mineral surfaces assessed by a gas sorption approach. Geoderma . 149, 152–160. doi:10.1016/j.geoderma.2008.11.032
- Wagai R , Mayer LM , Kitayama K , Knicker H 2008: Climate and parent material controls on organic matter storage in surface soils: A three-pool, density-separation approach. Geoderma . 147, 23–33. doi:10.1016/j.geoderma.2008.07.010
- Wagai R , Mayer LM , Kitayama K , Shirato Y 2013: Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis. Biogeochemistry . 112, 95–109. doi:10.1007/s10533-011-9652-5
- Walker TW , Syers JK 1976: The fate of phosphorus during pedogenesis. Geoderma . 15, 1–19. doi:10.1016/0016-7061(76)90066-5
- Yokoyama D , Imai N , Kitayama K 2017: Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil . 416, 463–476. doi:10.1007/s11104-017-3225-x