ABSTRACT
Previous meta-analyses revealed that the ratio of activities of carbon (C)-acquiring enzyme to nitrogen (N)-acquiring enzymes in tropical forest ecosystems was nearly identical to those in other ecosystems, despite of the N-rich condition in tropical forests. This could be explained by microbes in tropical forest soils, which require a large amount of N to produce N-rich acid phosphatase (AP) for catalyzation of the organic form of phosphorus (P) and compensation for poor P availability in soils. Based on this idea, we hypothesized that experimental P fertilization would reduce the allocation to N-acquiring enzymes compared with that of C-acquiring enzymes, i.e. that it would increase the ratios of activities of β-1,4-glucosidase (BG) to β-1,4-acetylglucosaminidase (NAG) and leucine aminopeptidase (LAP). We tested this hypothesis using an experimental fertilization site with factorial N (100 kg ha−1 yr−1) and P (50 kg ha−1 yr−1) addition in a primary tropical lowland forest in Bornean Malaysia, where our earlier work demonstrated that P fertilization reduced AP activity. Contrary to our hypothesis, the BG:NAG and BG:(NAG + LAP) ratios were not altered by either N or P fertilizations. This result indicated that AP production was not a reason for the maintenance of a relatively high investment in N-acquiring enzyme at our study site. Rather, NAG and LAP production was likely driven by C acquisition, rather than N acquisition, as the target substrates contained C as well as N. This idea was supported by the fact that neither the BG:NAG ratio nor the BG:(NAG + LAP) ratio was elevated by N addition. We propose that the ratios of activities of BG to NAG and LAP do not necessarily indicate the ratio of C:N acquisition, at least in our N-rich tropical forest ecosystem.
1. Introduction
Tropical lowland forests are generally described as ‘P-depleted ecosystems’ (Camenzind et al. Citation2018; Crews et al., Citation1995; Elser et al. Citation2007; Kitayama and Aiba Citation2002; Vitousek Citation1984; Walker and Syers Citation1976) because soils are deeply weathered and most remaining phosphorous (P) is occluded on aluminum and iron (Miller et al. Citation2001) (note that not necessarily P-limited (Mori et al. Citation2018)). In such ecosystems, plants and microbes adapt to the P-poor condition by catalyzing organic forms of P using acid phosphatase (AP) (Allison et al. Citation2007; Kitayama Citation2013; Turner and Engelbrecht Citation2011; Yokoyama et al. Citation2017).
The high dependence of biota on organic forms of P for P acquisition in tropical forest ecosystems is well described by extracellular enzymatic stoichiometry, such as the ratios of activities of β-1,4-glucosidase (BG, hydrolyzing glucose from cellobiose), β-1,4-acetylglucosaminidase (NAG, hydrolyzing glucosamine from chitobiose and amino sugar oligomers), leucine aminopeptidase (LAP, hydrolyzing leucine and other hydrophobic amino acids from the N terminus of polypeptide), and AP (hydrolyzing phosphate from phosphosaccharides and phospholipids). These ratios have been used to assess microbial resource allocation and nutrient demand (Sinsabaugh et al. Citation2009, Citation2008; Waring et al. Citation2014) because extracellular enzymes are produced to mediate the microbial nutrient acquisition from organic matter (Caldwell Citation2005; Olander and Vitousek Citation2000). A meta-analysis by Sinsabaugh et al. (Citation2008) demonstrated that the BG:AP ratio was lower in tropical soils than in higher-latitude soils, indicating higher microbial investment to enzymes targeting P than in other ecosystems. Waring et al. (Citation2014) also reported lower BG:AP and NAG:AP ratios in tropical soils compared with global averages, and suggested that microbial activities are limited mainly by P availability in tropical ecosystems.
On the other hand, BG:NAG ratios in tropical ecosystems were nearly identical to the global average (Waring et al. Citation2014). If the BG:NAG ratio indicates the ratio of carbon (C):nitrogen (N) acquisition (Sinsabaugh et al. Citation2008), and tropical forest ecosystems are rich in N resources (Vitousek Citation1984), the similar BG:NAG values in tropical forest ecosystems and other ecosystems indicate that microbes require more N relative to C (i.e. N:C acquisition ratio is higher) in tropical forests compared with other ecosystems. This suggestion is reasonable if microbes in tropical forests produce a larger amount of AP to compensate for P shortage, as a large amount of N relative to C is likely required to produce enzymes including AP, which have higher N content (C:N ratio ~ 4) compared with other organic matter (Houlton et al. Citation2008; Marklein and Houlton Citation2012; Olander and Vitousek Citation2000). If this is the case, experimental P fertilization reduces the allocation to N-acquiring enzymes compared with C-acquiring enzymes, i.e. elevates the BG:NAG and BG:(NAG + LAP) ratios, because microbes no longer need to produce AP at the expense of N.
In this study, we hypothesized that N fertilization reduces microbial allocation to N-acquiring enzymes compared with C-acquiring enzymes because the N fertilization reduces the microbial N demands. We also hypothesized that P fertilization similarly reduces microbial allocation to N-acquiring enzymes compared with C-acquiring enzymes because microbes in tropical forest soils produce N-acquiring enzymes for N-rich AP production and P supplement halts the production of the enzymes. In 2011, a nutrient fertilization experiment with factorial N and P addition was initiated to study the ecological response to additional nutrients (N and P) in tropical lowland forests in Bornean Malaysia by Imai et al. (unpublished). We tested our hypotheses by making use of this experimental site. Our earlier work demonstrated that P fertilization significantly reduced soil and root AP activities (Yokoyama et al. Citation2017).
2. Materials and methods
A field experiment was performed in a primary lowland mixed-dipterocarp tropical rainforest in the Deramakot Forest Reserves (551 km2), Sabah, Malaysian Borneo (5° 14–30ʹ N, 117° 11–36ʹ E) (Imai et al. Citation2009, Citation2010). The climate at the study site is humid equatorial, with a mean annual temperature of 25.2°C and annual precipitation of 3,098 mm in the period 2008–2010 (Ong et al. Citation2013). Experimental nutrient manipulation was initiated in December 2011, after the establishment of twelve 0.12-ha (30 m × 40 m) plots (Imai et al. unpublished). The nutrient treatments (n = 3 each) were control (without N or P application), N application, P application, and N + P application. N in the form of urea (100 kg N ha−1) and P in the form of triple super phosphate (50 kg P ha−1) were applied by hand. To apply the fertilizer uniformly, we divided each plot into twelve 10 m × 10 m areas and scattered the fertilizer at twelve separate times. Thereafter, the nutrient applications were conducted at the same rate at approximately the same time every year. Results on the ecosystem responses to the fertilization will be reported elsewhere (Imai et al. unpublished). Yokoyama et al. (Citation2017) reported soil and root AP activities and Mori et al. (Citation2017) reported greenhouse gas fluxes from soils in this experimental forest.
In January 2016, we collected six soil cores (diameter of the soil core was 1.8 cm) at depths of 0–5 cm from each grid point of the plot. Soils were transported using an icebox with ice packs to keep the soils under the cold condition. In laboratories, soils were kept in refrigerators at 4°C. Soil samples were passed through a 2-mm sieve after the removal of roots and large organic matter. Soil chemical data have been reported in our earlier works (, Imai et al. Citation2010; Yokoyama et al. Citation2017).
Table 1. Soil chemical properties of the study site.
Hydrolytic enzyme activities were determined by fluorescence enzyme assays, generally following Saiya-Cork et al. (Citation2002). Substrates (200 μM) used to determine enzyme activities were as follows: BG, 4-methylumbelliferyl-D-glucopyranoside; NAG, 4-methylumbelliferyl N-acetyl-D-glucosaminide; and LAP, L-leucine-7-amino-4-methylcoumarin hydrochloride. Soil suspensions were prepared by adding 1–2 g fresh soil to 91 mL sodium acetate buffer, adjusted to pH 4.2 to approximate the soil pH of the field condition (Yokoyama et al. Citation2017). The suspensions were stirred continuously using a magnetic stirrer, and 200-µL aliquots were dispensed into 96-well microplates. After adding 50-µL substrate, samples in the microplates were incubated at 25°C in the dark. The changes in fluorescence were monitored six times over 8 h. The slope of the regression lines between the fluorescence values and incubated times were used to calculate the activities. Standard lines prepared for all soil samples were determined from changes in fluorescence of standard wells, where different concentrations of 50-mL solutions of 4-methylumbelliferone (MUB) or 7-amino-4-methylcoumarin (MUC) were dispensed with 200-µL aliquots of sample suspension. Enzyme activity was expressed as nanomoles MUB per gram soil per hour. Eight replicate wells were used for each assay.
Two-way (without N vs. with N, without P vs. with P) analysis of variance, followed by Tukey’s post-hoc test, was used to compare data, assuming normality. Enzyme activity data were log transformed before the analysis. All statistical analyses were performed using R version 3.2.2.
3. Results and discussion
Waring et al.’s (Citation2014) meta-analysis showed that BG:NAG ratios in tropical ecosystems (1.83 ± 0.31) were nearly identical to the global average, despite the N-rich condition. In the present study, the BG:NAG and BG:(NAG + LAP) ratios () were similar or even lower than the global averages (1.81 ± 0.47 and 1.43 ± 0.22, respectively) (Sinsabaugh et al. Citation2009; Waring et al. Citation2014)). If BG:NAG ratio indicates the ratio of C:N acquisition (Sinsabaugh et al. Citation2008), these results indicate that microbes in tropical soils invest a similar amount of or even more resources (such as C and N to synthesize enzymes) in N acquisition (compared with those for C acquisition), despite the N-rich condition. Initially, we hypothesized that this similarity occurred because microbes in tropical forest soils require large amounts of N to produce N-rich AP for catalyzation of organic forms of P and compensation for poor P availability in soils. Indeed, our earlier work demonstrated that P fertilization substantially suppressed AP activity (Yokoyama et al. Citation2017), indicating a large investment in the acquisition of P resources at our study site. However, our results did not support this hypothesis. P fertilization had no influence on BG, NAG, or LAP activities (). Accordingly, the ratios of BG:NAG and BG:(NAG + LAP) were not altered by the experimental P fertilization (). We also found that N fertilization did not influence the activities of BG, NAG, or LAP, or ratios of these activities (BG:NAG and BG:(NAG + LAP)). These results indicate that the relatively high allocations to NAG and LAP (compared with BG) were not caused by a large N demand (and large AP production which utilizes the acquired N). Although P fertilization elevated the BG:NAG ratio in a tropical forest in Panama (Turner and Wright Citation2014), their results also showed that N fertilization did not elevate the BG:NAG ratio, which does not support our hypotheses (note that our hypotheses are supported only when both N and P have stimulatory effects on BG:NAG or BG:(NAG+ LAP)). Therefore we consider that a large N demand in order to produce AP was not a reason for the relatively high allocations to NAG and LAP compared with BG. However, cautions are required to interpret the present results, because according to a power analysis (G*power was used for the analysis, Faul et al. Citation2007, Citation2009) sample sizes were not enough to falsify our hypotheses.
Figure 1. Effects of N and P fertilization on (a) BG, (b) NAG, and (c) LAP activities a primary lowland tropical rainforest in Borneo. Data are illustrated as box plots (n = 3 per treatment). BG, β-1,4-glucosidase; NAG, β-1,4-acetylglucosaminidase; LAP, leucine aminopeptidase. Results of two-way analysis of variance are shown. The band near the middle of the box indicates the median value. The top and bottom of the box are the first and third quartiles, respectively. The whiskers show the maximum and minimum values.
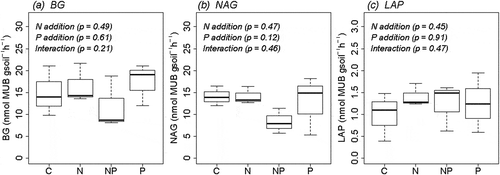
Figure 2. Effects of N and P fertilization on ratios of (a) BG to NAG and (b) BG to NAG + LAP. Data are illustrated as box plots (n = 3 per treatment). BG, β-1,4-glucosidase; NAG, β-1,4-acetylglucosaminidase; LAP, leucine aminopeptidase. Results of two-way analysis of variance are shown. The band near the middle of the box indicates the median value. The top and bottom of the box are the first and third quartiles, respectively. The whiskers show the maximum and minimum values.
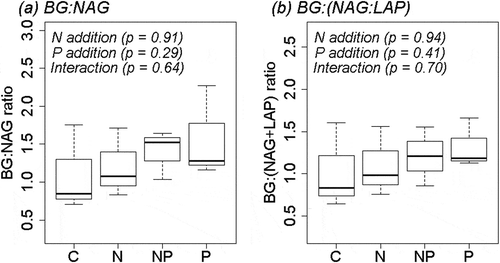
If the fertilizations had no impacts on ratios of BG to NAG and LAP activities, the results beg the question of why the N-rich tropical ecosystem had similar or even lower ratios of BG to NAG and LAP activities? At our study site, NAG and LAP activities were likely driven by C acquisition, rather than N acquisition. As chitin (NAG catalyzes the terminal reaction in chitin degradation) and protein (LAP hydrolyzes leucine and other hydrophobic amino acids from the N terminus of polypeptides) contain C as well as N, microbes can catalyze these organic compounds for C acquisition. This idea was supported by the fact that N fertilization did not suppress N-acquiring enzymes (NAG and LAP) or elevate the ratios of BG:NAG and BG:(NAG + LAP) (). The lack of a NAG activity response to experimental N addition was also observed in other tropical forests (Turner and Wright Citation2014; Wang et al. Citation2015). Furthermore, a recent meta-analysis revealed that N fertilization did not alter the ratio of activities of C-acquiring to N-acquiring enzymes (Jian et al. Citation2016). We propose that the ratios of activities of BG to NAG and LAP do not necessarily indicate the ratio of C to N requirements, at least in our N-rich tropical forest ecosystem.
Acknowledgements
The authors thank Sabah Forestry Department and Forest Research Centre, Sabah for their support. The authors also thank Mr. P. Lagan for the general support in the field.
Additional information
Funding
References
- Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL 2007: Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol. Biochem., 39, 1770–1781.
- Caldwell BA 2005: Enzyme activities as a component of soil biodiversity: A review. Pedobiologia (Jena), 49, 637–644. doi:10.1016/j.pedobi.2005.06.003
- Camenzind T, Hattenschwiler S, Treseder K, Lehmann A, Rillig MC 2018: Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr., 88, 4–21. 10.1002/ecm.1279
- Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM 1995: Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology, 76, 1407–1424. 10.2307/1938144
- Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE 2007: Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett., 10, 1135–1142. 10.1111/ele.2007.10.issue-12
- Faul F, Erdfelder E, Buchner A, Lang AG 2009: Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods, 41, 1149–1160. 10.3758/BRM.41.4.1149
- Faul F, Erdfelder E, Lang AG, Buchner A 2007: G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods, 39, 175–191.
- Houlton BZ, Wang Y-P, Vitousek PM, Field CB 2008: A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature, 454, 327–330. doi:10.1038/nature07028
- Imai N, Kitayama K, Titin J 2010: Distribution of phosphorus in an above-to-below-ground profile in a Bornean tropical rain forest. J. Trop. Ecol., 26, 627–636. Available from: http://www.journals.cambridge.org/abstract_S0266467410000350 (November 2013).
- Imai N, Samejima H, Langner A, Ong RC, Kita S, Titin J, Chung AYC, Lagan P, Lee YF, Kitayama K 2009: Co-benefits of sustainable forest management in biodiversity conservation and carbon sequestration. PLoS One, 4, e8267. doi:10.1371/journal.pone.0008267
- Jian S, Li J, Chen J, Wang G, Mayes MA, Dzantor KE, Hui D, Luo Y 2016: Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem., 101, 32–43. doi:10.1016/j.soilbio.2016.07.003.
- Kitayama K 2013: The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu, Borneo. Plant Soil, 367, 215–224.http://link.springer.com/10.1007/s11104-013-1624-1 (December 2013)
- Kitayama K, Aiba SI 2002: Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu,Borneo. J. Ecol., 90, 37–51.
- Marklein AR, Houlton BZ 2012: Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol., 193, 696–704.
- Miller A, Schuur E, Chadwick O 2001: Redox control of phosphorus pools in Hawaiianmontane forest soils. Geoderma, 102, 219–237.
- Mori T, Imai N, Yokoyama D, Mukai M, Kitayama K. 2017: Effects of selective logging and application of phosphorus and nitrogen on fluxes of CO2, CH4 and N2O in lowland tropical rainforests of Borneo. J Trop. For. Sci. 29, 248–256.
- Mori T, Lu X, Aoyagi R, Mo J 2018: Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Funct. Ecol., 32, 1145–1154.
- Olander LP, Vitousek PM 2000: Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry, 49, 175–190.
- Ong RC, Langner A, Imai N, Kitayama K 2013: Management History of the Study Sites: the Deramakot and Tangkulap Forest Reserves. In Kitayama K, ed. Co-benefits of Sustainable Forestry: ecological Studies of a Certified Bornean Tropical Rain Forest, Ecological Research Monographs. Management History of the Study Sites: the Deramakot and Tangkulap Forest Reserves. Springer, Berlin, Heidelberg.
- Saiya-Cork KR, Sinsabaugh RL, Zak DR 2002: The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem., 34, 1309–1315.
- Sinsabaugh RL, Hill BH, Follstad Shah JJ 2009: Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature, 462, 795–798.http://www.ncbi.nlm.nih.gov/pubmed/20010687(January, 2014)
- Sinsabaugh RL, Lauber CL, Weintraub MN, et al. 2008: Stoichiometry of soil enzyme activity at global scale. Ecol. Lett., 11, 1252–1264.
- Turner BL, Engelbrecht BMJ 2011: Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry, 103, 297–315.
- Turner BL, Wright SJ 2014: The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry, 117, 115–130.
- Vitousek PM 1984: Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology, 65, 285–298.
- Walker TW, Syers JK 1976: The fate of phosphorus during pedogenesis. Geoderma, 15, 1–19.
- Wang Y, Cheng S, Yu G, Fang H, Mo J, Xu M 2015: Response of carbon utilization and enzymatic activities to nitrogen deposition in three forests of subtropical China. Can. J. For. Res., 45, 394–401.
- Waring BG, Weintraub SR, Sinsabaugh RL 2014: Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry, 117, 101–113.
- Yokoyama D, Imai N, Kitayama K 2017: Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil, 416 463–476. http://link.springer.com/10.1007/s11104-017-3225-x.
