ABSTRACT
The present investigation was based on the hypothesis that the endophytes residing in the roots of halophytes have better adaptation to saline conditions. Six halophytic herbs were collected from Khewra salt range (EC = 4.7 dS m−1 and SAR = 25.7). From these herbs, root pieces of Cenchrus ciliaris were shade dried; finely ground to powder and three plant growth promoting rhizobacteria (PGPR), Bacillus cereus, Pseudomonad moraviensis, and Stenotrophomonas maltophilia, were isolated. Root powder in sterilized and unsterilized forms was added in the saline-sodic field on wheat and mixed with soil in pot experiment with induced NaCl (150 mM). Sterilized root powder increased organic matter NO3-N and P contents of soil and leaves, fresh weight, sugar content, and yield attributes. The root powder application in unsterilized form significantly decreased EC, SAR, and Na content of field soil with concomitant increase in soil and leaves K, P, and NO3-N. The farmer’s benefit was increased by 33% at yield. Root powder-induced salt tolerance was mediated by the PGPR (residing inside the root) through increased growth and better physiological adaptations. It is inferred that root powder harboring the PGPR may be an alternative to biofertilizer with longer shelf life and may also serve as carrier for the preparation of effective biofertilizer for saline land using other PGPR bio-inoculants.
1. Introduction
Salinity is a major problem for agriculture, since 20% of irrigated land has been rendered barren globally due to salinity (King and Thomas Citation2014). In Pakistan, 6.3 million hectare land is salt-affected. The estimated land 1.9 ha is saline and 1 million hectare is impermeable saline-sodic (Shahid and Rahman Citation2011). Pakistan has a vast salt range descending from Kalabagh to Jhelum in Punjab province.
Halophytes follow different mechanisms to cope with abiotic stresses including succulence, osmotic adjustment, ion compartmentalization, maintenance of redox and energetic status and selective transport and uptake of ions, enzymatic, and nonenzymatic antioxidant response (Flowers and Colmer Citation2008). The bacteria reside in roots and rhizosphere of halophytic plants has the ability to tolerate salt stress (El-Awady et al. Citation2015).
Among the halophytes of Khewra salt range, Buffel grass (Cenchrus ciliaris L.) is important and profusely grown as natural perennial herb. The strong antibacterial activity, carbon sequestration, nitrogen cycling, soil binding, and strong antioxidant activities thrive it to tolerate higher salt concentration (Singariya et al. Citation2012).
The literature reveals that rhizobacteria of stress habitat had better tolerance than those isolated from natural habitat (Yang et al. Citation2009). Applications of such salt tolerant plant growth promoting rhizobacteria (PGPR) have been documented for improving crops productivity (Ahmed et al. Citation2011). Under stressed conditions, the efficiency of PGPR is correlated with survival efficiency, competency, and persistence (Zhu et al. Citation2011).
Synergistic relationship among plants, mycorrhizae, and PGPR appears to be a possible strategy for coping with stresses (Nadeem et al. Citation2014). Under hostile environment, this tripartite mutualistic association acts as an effective essential bioremediator of stress and improves tolerance by regulating hormonal and nutritional balances (Abd_Allah et al. Citation2015; Ruiz-Lozano et al. Citation2012).
Bacillus spp. are strong candidates for plant growth promotion due to endospore formation and multilayered cell wall composition. Secretion of peptide signals, peptide antibiotic, and extra cellular enzymes also enable them compatible for rhizosphere (Richardson et al. Citation2009). The efficiency of Pseudomonas is affected by soil pH, soil type, moisture content, and nutrient abundance (Wachowska et al. Citation2006).
Bacillus cereus and Pseudomonad moraviensis have been administrated for their positive host-PGPR interaction by improving nutrients status, P-solublization, antifungal and antibacterial activity, as well as their salt tolerance potential (Aziz et al. Citation2012; Yadav et al. Citation2013).
The persistence of PGPR in the rhizosphere soil and the limited shelf life of the biofertilizers are the major constraints for the farmers in the commercialization of biofertilizers. Selection of root powder from halophytes was aimed to evaluate the role of root powder as carrier for the PGPR and further to procure better tolerance to temperature, moisture, and salt stresses.
The effectiveness of PGPR endophyte from the roots of halophytes has never been explored previously for the induction of salinity tolerance in plants. Keeping in view, the present attempt was aimed to evaluate the role of C. ciliaris root powder on soil fertility, wheat growth, and physiology. The potential of root powder as an alternative carrier was determined by the application on wheat under induced salt stress (NaCl) and in natural saline-sodic of field.
2. Material and methods
2.1. Plant material and growing condition
Six halophytes viz. Conyzanthus saqumatus, Solanum surattense Burm. f., Cenchrus ciliaris L., Aerva javanica (Burman f.) Peganum harmala L., and Chrysopogon aucheri L. were collected from Khewra salt range (EC = 4.7 dS m−1 and SAR = 25.7). Buffel grass (C. ciliaris) was selected because it contained higher sugar, protein, and organic matter (Table S1). The Buffel grass (Cenchrus ciliaris L.), a naturally grown halophyte, was uprooted when the plant was 13–15 cm high. The roots of the plants were washed with sterilized water, shade dried for 5–7 d, and ground into powder form, using grinder (Anex KC106, Rawalpindi, Pakistan). Pseudomonad moraviensis (accession No. LN714047), B. cereus (accession No. LN714048), and S. maltophilia (accession No. LN714049) were isolated from the root powder. Seeds of wheat (Triticum aestivum L.) were obtained from Soil Salinity Research Institute, Pindibhattian, District Hafizabad, Pakistan and grown in saline-sodic soil (EC = 4.8 dS m−1, SAR = 25.7) (FAO-UNESCO system (Citation1974). Experiments were conducted for two consecutive years 2013–2014 and 2014–2015. For field experiment, plot measuring 10 × 10 m2 was made at Soil Salinity Research Institute, Pindibhattian, District Hafizabad, Pakistan. Distance between two rows of wheat was 36 cm. For each treatment, five replicates were made. Along with field experiment, pot experiment was conducted in green house of Quaid-i-Azam University, Islamabad. For each treatment, six replicates were used. For pot experiment, earthen pots measuring 17 × 20 cm2 were filled with autoclaved soil and mixed with sand 3:1 ratio. Salinity was induced by adding 150 mM NaCl (EC = 3.6 dS m−1) to pots after sowing. The treatments consisted of application of halophyte root powder in sterilized form (T0), application of halophyte root powder in unsterilized form (T), and untreated control. The root powder was mixed in the saline field at the rate of 100 g/10 m2 by hand drill at the time of sowing. In pot experiment, root powder (20 g/pot) was mixed in 8 kg autoclaved garden soil. No chemical or organic fertilizer was added. Seeds (10–15) were sown in each pot and thinned to five plants/pots (20 d after germination). These plants were allowed to grow till maturity. Plants were sampled at early vegetative phase 57 d after sowing (DAS) for physiological parameters and at 122 DAS for yield parameters.
2.2. Isolation of endophytic microbes and determination of colony-forming unit
For isolation of endophytic bacteria, root powder (1 g) of Buffel grass was suspended in 9 ml autoclaved distilled water and an aliquot (100 µl) from decimal dilution was used to inoculate Lauria Bertani culture media. Lauria Bertani media is considered as the appropriate medium for bacterial growth with minimum risk of contamination (Ramalashmi et al. Citation2018). The culture plates were incubated for 24–72 h at 27°C. The number of viable cell counts at 107 dilution was calculated following the formula adapted by James (Citation1978):
Viable cell count (cfu g−1) = (number of colonies/volume of inocula) × dilution factor.
2.3. Salt tolerance potential of PGPR
Salt tolerance potential of PGPR isolates were determined in vitro using the filtrate of saline-sodic soil of the field. Soil sample was collected 15 cm from upper surface of the rhizospheric soil in the farmer field at Soil Salinity Research Institute, Pindibhatian. A composite soil sample (50 g) was suspended with 250 ml autoclaved water, stirred for 2 h on magnetic stirrer, and filtered using Whatman No.42 filter paper. From stock solution of soil filtrate, four dilutions were made and EC of the filtrate was determined as 1, 1.4, 3.5, and 4 dS m−1, respectively. The soil filtrate (250 ml) was added to 1 l of culture media. Media containing autoclaved water was treated as control. An aliquot (20 µl) of inoculum from decimal dilution of each PGPR was added to the culture. The plates were incubated at 30°C for 24 h. The tolerance of PGPR against added filtrate from saline soil of the field was measured by counting their colony-forming unit.
2.4. Biochemical characterization
The C/N source utilization pattern for bacterial strains was determined using QTS-24 miniaturized identification system (DESTO Laboratories Karachi, Pakistan). The bacterial colonies were suspended in 5 ml of 0.85% NaCl solution (dissolve 0.85 g NaCl in 100 ml of water), and inoculated into the tubes containing QTS kit. The colonies were incubated at 30°C for 18 h and results were compared with standard species documented in Bergey’s Manual of determinative bacteriology (Bergey et al. Citation1974).
Bacterial isolates were characterized on the basis of utilization of 24 carbon/nitrogen sources available in QTS 24 miniaturized identification kit. The results were compared with standard species as per Bergey’s Manual of Determinative Bacteriology (Bergey et al. Citation1974). The bacterial isolates utilized the C/N sources included ortho nitro phenyl-β-d-galactopyranoside, sodium citrate, sodium melonate, lysine decarboxylase, arginine dihydrolase, urea hydrolysis, tryptophan deaminase, indole, acid from glucose, acid from maltose, acid from sucrose, acid from mannose, and acid from melibiose.
2.5. Physicochemical and nutrient analysis of rhizosphere soil
The rhizosphere soil samples of wheat were collected at early vegetative phase (57 DAS), 7–10 cm from surface both for control and treated plants. The samples were homogenized, sieved through 2 mm sieve, and processed for the isolation of rhizobacteria and determination of physicochemical properties. The pH of rhizosphere soil was measured by preparing 1:1 (soil:water) suspension, while organic matter of soil and root powder was determined by the method of Walkley and Black (Citation1934). Soil nitrate (NO3-N), phosphorus (P), and bicarbonate ions were extracted from rhizosphere soil following the method of Reitemeier (Citation1943).
2.6. Elemental analysis of soil and wheat
Macro and micronutrients of soil were determined by the method of Soltanpour and Schwab (Citation1977), while accumulation of nutrients in treated leaves was determined by the method of Piper (Citation1947). Leaf P was determined by the method of Jackson (Citation1967).
2.7. Analysis of physiological parameters
The SPAD (Minolta SPAD-502, Osaka, Japan) was used for the measurement of leaf chlorophyll content. Fully expanded young grown leaf was selected for the measurements. Proline accumulation in wheat leaves was measured by the method of Bates et al. (Citation1973). Soluble protein content of wheat leaves and root powder were determined by the method of Lowry et al. (Citation1951). For the measurement of soluble sugar contents of leaves and root powder, method of Johnson et al. (Citation1966) was used. The peroxidase (POD) activity was determined following the method of Vetter et al. (Citation1958) while superoxide dismutase (SOD) activity was determined by the method of Beauchamp and Fridovich (Citation1971). The extraction and purification for indole acetic acid (IAA), gibberellic acid (GA), and abscisic acid (ABA) were made following the method of Kettner and Doerffling (Citation1995).
2.8. DNA extraction and sequencing
The genomic DNA of bacterial strain was extracted by the Gen Elute Bacterial Genomic DNA Kit. The purified PCR products were sequenced on an Applied Bio Systems model 3730XL automated DNA sequencing system (Applied Bio Systems, USA) at the Macrogen, Inc., Seoul, Korea.
2.9. Statistical design
Statistical analyses of the data were conducted using analysis of variance in Statistix program, version 8.1. The experiment was laid by RCBD design in field and CRD in pot experiment. Five replicates were used for field and pot experiment and most healthy and heighted plant was selected from each replicate. Mean values were compared according to Steel and Torrie (Citation1980) by least significant difference (LSD) at p = 0.05.
3. Results
Among six halophytes used for screening; the root powder of C. ciliaris was rich in soluble proteins, sugar, and organic matter contents (Table S1). Cenchrus ciliaris root powder also contained higher K, Ca, and Mg contents as compared to other halophytes (Table S2). Three PGPR, identified from 16s rRNA gene sequence, revealed that for isolate 1 the comparison of the nucleotide sequence with data nucleotide bank showed 99% sequence similarity for 1477/1482 nucleotide bases with that of B. cereus strain MSU AS 16s ribosomal RNA gene, partial sequence (ACC. No.: LN714048). For isolate 2, the comparison of the nucleotide sequence with data nucleotide bank showed 99% sequence similarity for 1477/1485 nucleotide bases with that of P. moraviensis isolate PSB34 16s ribosomal RNA gene, partial sequence (ACC. No.: LN714047).For isolate 3, the comparison of the nucleotide sequence with data nucleotide bank showed 99% sequence similarity for 1466/1472 nucleotide bases. For S. maltophilia strain 2681/2685 nucleotide bases with 16s ribosomal RNA gene, partial sequence (ACC. No.: LN714049).
Seven tests – H2S production, Voges–Proskauer, gelatin hydrolysis, acid production from sorbitol, acid production from inositol, acid production from adonitol, and acid production from raffinose – were negative in all bacteria. Acid production from glucose, indole, and tryptophan deaminase test were found positive in all bacterial isolates.
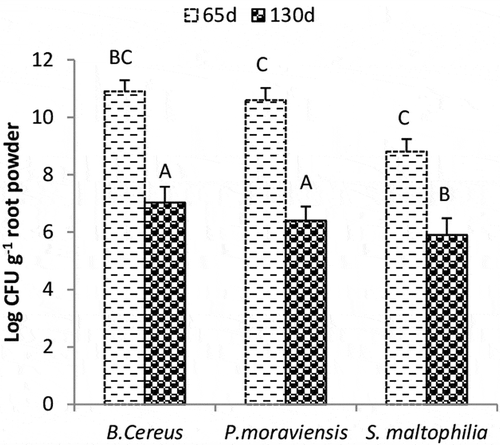
The survival of efficiency of PGPR in root powder stored at room temperature, measured at 65 d showed 24% and 20% better survival for B. cereus and P. moraviensis, respectively over S. maltophilia (). The cfu of B. cereus, P. moraviensis, and S. maltophilia was decreased by 36%, 40%, and 33%, respectively at 130 d as compared to cfu calculated at 65 d.
Figure 1. Colony-forming units of endophytic bacteria isolated from Cenchrus ciliaris root powder. Measurements were made from root powder stored at room temperature after 65 and 130 d. Values given are mean of four replicates. Values followed by different letters are significantly different (p = 0.05).
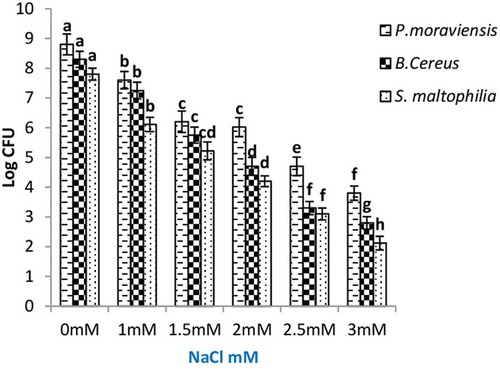
A severe decline in cfu was recorded for all PGPR, with increasing NaCl concentration in culture media, except for P. moraviensis at 1.5 and 2 mM concentration. At 3 mM NaCl, the declines in cfu of B. cereus and P. moraviensis were 66% and 57%, respectively over control (0 mM NaCl) (). Decrease in cfu of S. maltophilia was 73% at 3 mM of NaCl as compared to its cfu at 0 mM.
Figure 2. Colony-forming unit of endophytic PGPR measured at different concentration of NaCl in culture media. Values given are mean of four replicates ± SE. Values followed by different letters heading the bars are significantly different (p = 0.05).
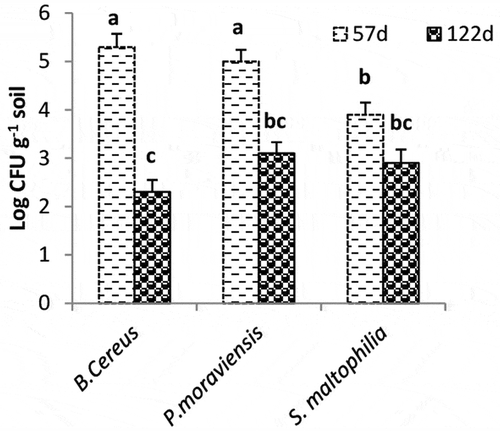
The cfu of B. cereus was higher than that of P. moraviensis and S. maltophilia at early vegetative phase (57 DAS) in the rhizosphere soil of wheat of potted plants (EC = 3.7 dS m−1) (). After 122 d of sowing (booting phase), the cfu of B. cereus, P. moraviensis, and S. maltophilia was declined by 57%, 58%, and 27%, respectively as compared to cfu measured at 57 d.
Figure 3. Colony-forming units of rhizosphere soil (cfu g−1 soil) of wheat treated with Cenchrus ciliaris root powder in pot experiment after 57 d of sowing (2–3 leaf stage) and 122 d of sowing (booting stage). Values given are mean of four replicates.
During 2013–2014, organic matter of soil was 24% and 17% higher in wheat rhizosphere soil of pots and field, respectively receiving sterilized root powder treatment (). Root powder in unsterilized form with PGPB increased the organic matter by 43% and 41% in pots and field, respectively, over control. In pot experiment, sterilized root powder treatment (T0) decreased SAR of soil by 19%, whereas unsterilized root powder resulted in 24% decrease over control. Unsterilized root powder treatment decreased SAR by 16% as compared to control in field-grown plants without affecting pH of the rhizosphere soil. The percent decrease in EC and SAR and increase in organic matter were higher during 2014–2015.
Table 1. Effects of halophyte root powder in sterilized or unsterilized form on electrical conductivity, pH, organic matter, and sodium absorption ratio (SAR) in rhizosphere soil of wheat, in pot or field experiments during 2013–2014 and 2014–2015. Measurements were made at 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
Increase in NO3-N of soil was 25% in pot-grown plants as compared to control following the application of root powder in sterilized form during 2013–2014 (). In unsterilized form of root powder, increase in NO3-N was 30% in pot experiment over control. In field-grown plants, sterilized root powder augmented NO3-N by 21% over control. This increase was 51% when root powder was applied in sterilized form. Root powder in sterilized form increased P contents by 46% and 42% in pots and field-grown plants, respectively over control. Unsterilized root powder increased P by 68% and 32% over control in pots and field-grown plants, respectively. Similar trend was observed during 2014–2015.
Table 2. Effects of halophyte root powder in sterilized or unsterilized form on nutrients contents (mg kg−1) of wheat rhizosphere soil in pot or field experiments during 2013–2014 and 2014–2015. Measurements were made after 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
The Na content was significantly decreased (20%) over control in the rhizosphere soil of field-grown wheat plants when unsterilized root powder was applied during 2013–2014. The Mg, K, and Ca () were increased both in the rhizosphere of field and pot treated with unsterilized root powder. The K contents were increased by 30% and 31% as compared to control in field and pot-grown plants, respectively. In pots and field-grown plants, accumulations of Mg and Ca were 35% and 45% higher over control. Similar trends were observed during 2014–2015 for all the treatments (). However, the percent increase in NO3-N and Mg, K, and Ca was higher as compared to 2013–2014.
Root powder treatment in unsterilized form accumulated 32% and 44% higher NO3-N over control in wheat leaves of pots and field-grown plants, respectively (). Unsterilized root powder increased P content of leaves by 50–57% as compare to control both in pots and field-grown plants. The accumulations of K and Mg in the leaves of treated plants were higher during 2014–2015. Root powder treatment in unsterilized form decreased Na contents by 44% and increased Ca by 39% in leaves over control plant grown in pots.
Table 3. Effects of halophyte root powder in sterilized or unsterilized form on nutrients accumulation in wheat leaves, in pot or field experiments during 2013–2014 and 2014–2015. Measurements were made after 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
During 2013–2014, Na/K ratio (data not presented) of wheat rhizosphere soil was reduced by 21% and 33% as compared to control in the rhizosphere soil of field and pot-grown plants, treated with unsterilized root powder. The magnitude of decrease in Na+/Ca+ was significantly higher (83%) in leaves of potted plants as compared to that of field-grown plants (19%). Similar trend was observed during 2014–2015.
Sterilized root powder increased plant height by 27% in potted plants and 16% in field-grown plants over control (). The same treatment increased the protein contents of leaves significantly (24%) both in pots and field-grown plants as compared to control.
Table 4. Effects of halophyte root powder in sterilized or unsterilized form on growth and physiological parameters of wheat, in pot or field experiments during 2013–2014 and 2014–2015. Measurements were made after 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
The plants treated with halophyte root powder in unsterilized form showed 52% and 47% greater plant height and fresh weight over control in pot-grown plants (). Field-grown plants treated with unsterilized root powder exhibited 41% greater plant height and 39% greater fresh weight over control. Increases in protein contents of unsterilized root powder-treated plants were 29% and 50% over control in pots and field-grown plants. Sugar contents of leaves were 28% higher in potted and 48% in field-grown plants. Proline contents of leaves were 30% and 24% higher in pots and field-grown plants over control. The SOD activity of leaves was higher by 20% and 41% in pots and field-grown plants, whereas POD was 30% higher in both pots and field-grown plants.
The root powder in unsterilized form -induced similar increases in growth and physiology of wheat in both years, but the effects of unsterilized root powder were more pronounced (5–10% higher over control) as compared to similar treatment during 2014–2015.
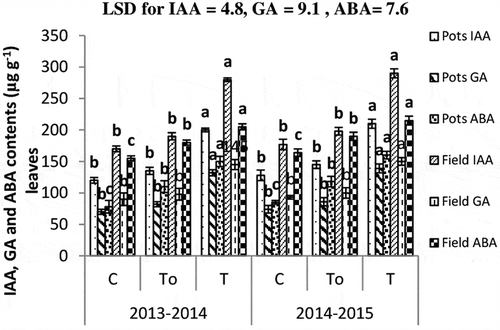
Phytohormone ABA content was significantly improved (40% in pots and 18% in field over control) following the application of sterilized root powder (). Unsterilized root powder treatment increased IAA contents of leaves by 65% as compared to control both in pots and field-grown plants. Increase in GA contents was 88% in pots and 61% in field-grown plants over control. Unsterilized root powder was more effective in improving ABA contents of leaves and there were 92% and 35% greater ABA contents in pots and field-grown plants, respectively over control. Accumulation pattern of phytohormones was similar during 2014–2015.
Figure 4. Indole acetic acid (IAA) and gibberellic acid (GA3) contents of leaves. Values are mean of four replicates with ±SE bar of mean. Values heading over the bars with different letters are significantly different (p = 0.05). To: sterilized root powder; T: unsterilized root powder; C: untreated control.
In pot-grown plants, increases in seeds/spike, spike length, and seed weight were 28%, 30%, and 18% higher over control in plants treated with unsterilized root powder (). Under field condition, increases in plant/m2 and spike length were significantly higher (20–23%), following the application of sterilized root powder (). Unsterilized root powder addition improved number of plant/m2, spike length, and seeds/spike by 45%, 48%, and 44%, respectively over control, while increase in seed’s weight was 7% higher in field-grown plants (). Increase in grain yield and biological yield (ton ha−1) were 42% and 21% higher over control in unsterilized root powder-treated plants, while the harvest index was 20% higher.
Table 5. Effects of halophyte root pieces in powder form on yield parameters of wheat in pot experiment during 2013–2014 and 2014–2015. Measurements were made after 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
Table 6. Effects of halophyte root pieces in powder form on yield parameters of wheat in field experiment during 2013–2014 and 2014–2015. Measurements were made after 57 d of sowing (2–3 leaf stage). Values are mean of five replicates.
4. Discussion
The increase in cfu of bacterial strains in root powder may be attributed to the availability of C/N sources from root powder, required for the proliferation of the bacteria (). The greater cfu of B. cereus is perhaps due to endospore forming ability and survial of Bacillus sp under adverse environmental conditions. Soni et al. (Citation2016) demonstrated the thermal resistance of B. cereus spore to grow under adverse environmental conditions.
Survival efficiency of B. cereus, P. moraviensis, and S. maltophilia (present in the root powder) measured at 3 mM NaCl demonstrates the ability of bacteria to tolerate salt stress (). The observed tolerance of B. cereus, P. moraviensis, and S. maltophilia () may be explained on the basis of the colonization of halotolerant bacteria to halophytic plants. Bibi et al. (Citation2011) isolated halotolerant bacteria Haloferula luteola sp. nov from halophyte Rosa rugosa.
The significant effects of root powder on EC, SAR, and pH of rhizosperic soil under field condition may be attributed to the synergistic effect of organic matter, protein, and sugar contents of the root powder which may serve as C/N sources for the plethora of microorganisms (). The PGPR associated with root powder may sequester the salts in their cells consequently decreasing EC and SAR of rhizosphere soil (Zhu Citation2002). Previously, the use of maize straw, sugarcane husk, and rice husk also increased organic matter of soil and assisted PGPR survival (Ogbo and Odo Citation2011; Hassan and Bano Citation2015). Increase in organic matter and soil nutrients and decrease in EC and SAR are also correlated with better rainfall in 2014–2015 (Figure S1). Possibly, the change in moisture content of soil augmented microbial activities (Eilers et al. Citation2012).
In the present study, higher organic matter was determined at 57 DAS. Added halophyte root powder served as additional source of organic matter (). The contribution of plant roots in stability of soil aggregates through the inherent abundance of organic matter production stimulates microbial activity as well as exopolysacchride production (Schmidt et al. Citation2011).
Better availability of nutrients in rhizosphere soil and their accumulation in leaves (), following the application of root powder reveals the role of associated PGPR which assisted nutrients availability in soil and their translocation to the leaves (Cakmakci et al. Citation2007). Root powder of C. ciliaris contained higher content of K, Ca, and Mg and may act as an additional source of these nutrients in the soil (Table S3).
The increase in fresh weight of aerial parts of field-grown wheat plants (), after treatment with root powder residing PGPR, might be attributed to increased nutrient content of the soil. Our results are in agreement with Abbasi et al. (Citation2011) who demonstrated that application of PGPR associated with wheat rhizosphere enhanced fresh weight and plant height of wheat. Similarly, co-inoculation of Bacillus amyloliquefaciens strain LL2012 and Bradyrhizobium japonicum has previously been demonstrated to induce significant effects on the growth of soybean (Masciarelli et al. Citation2014).
In the present study, improved chlorophyll contents () in root powder-treated plant suggest the pivotal role of root-associated PGPR in water and mineral absorption (Pérez-Montaño et al. Citation2014). Increase in proline and sugar contributes to the osmotic adjustment and protects macromolecules degradation under environmental stresses (Ameur et al. Citation2011). Application of PGPR imparted stimulatory effects on proline accumulation and our results are in accordance with Vurukonda et al. (Citation2016) who found that higher proline content in maize leaves following the application of rhizobacteria. The effects of PGPR on growth and physiology of wheat during second year of experiment are associated with lower EC and SAR of soil. Zahir et al. (Citation2012) also observed better affectivity of PGPR in the soil having lower EC and SAR.
Increase in antioxidant activities of halophyte root powder-treated plants () insinuates the potential of antioxidant promoting system, which is induced by PGPR residing there in to ameliorate the deleterious effect of reactive oxygen species (Ghorbanpour et al. Citation2013). Significantly increased stress tolerance was observed in Acacia gerrardii following the co-inoculation of arbuscular mycorrhizal fungi and endophytic bacteria Bacillus subtilis, under salt stress (Hashem et al. Citation2016).
Root powder treatment modulated and enhanced IAA and GA production in plants (). Noteworthy, ABA was higher in the field-grown wheat plants receiving root powder treatments, which may be an additional benefit to the plant to combat stress by inducing the stress hormone ABA production. The ABA contribution in increasing water uptake under salt stress dictates its role in root-to-shoot stress signaling (Keskin et al. Citation2010). PGPR are directly involved in secretion of plant growth regulators (auxin and gibberellins) and these phytohrmones are associated with growth and development (Sokolova et al. Citation2011).
Root powder treatments in unsterilized form had significantly higher fresh weight, protein, glucose, antioxidants (SOD and POD), and phytohormones (IAA, GA, and ABA) as compared to plant treated with sterilized root powder. The presence of PGPR in unsterilized root powder may assist the better decomposition of root powder and availability of organic matter. Environmental factors, litter composition, soil moisture contents, and microbial community have strong influence on decomposition process.
Increase in yield attributes ( and ) ascribes the higher photosynthetic activities of plants and efficient uptake of water and nutrients (Baset-Mia et al. Citation2010). Pseudomonas and Bacillus associated with roots (endophytes) accelerate the nutrient availability and acquisition, thereby substantially increasing the yield of cereals (Abbaspour Citation2009; Tiwari et al. Citation2011).
Noteworthy, the effect of root powder was less pronounced in potted plants grown under induced salt stress when single salt (NaCl) was used. In field, plethora of microbes resides which may minimize the effect of salt stress (Martínez-Viveros et al. Citation2010). Variations in climate and edaphic factors in field also affect the severity of stress (Bandopadhyay Citation2015).
5. Conclusions
It is inferred that the halophytic root powder harbors the PGPR P. moraviensis, B. cereus, and S. maltophilia which can survive at higher temperature, even can tolerate salinity and mechanical stress of grinding. Added halophyte root pieces in powder form harboring PGPR alleviated osmotic, oxidative and dehydration stresses as depicted by better growth and yield of inoculated wheat. It is demonstrated that synergistic interaction of PGPR applied as bio-inoculants along with root powder used as carrier may augment the effects of PGPR and induced tolerance. The root powder of halophytes can be used as carrier for biofertilizer formulation to enrich the soil with organic matter and C/N sources; hence to assist the PGPR to cope with stresses. Selection of halophytes should be made taking into consideration of the available organic matter, sugar, and protein contents of root powder which can serve as C/N source for better proliferation of the PGPR therein.
Supplemental Material
Download MS Word (19.6 KB)Acknowledgments
We are thankful to Soil Salinity Research Institute (SSRI), Pindibhatian for providing field facility and assistance of Mr. Saqlain Shah.
Supplementary material
Supplementary material can be accessed here.
References
- Abbasi MK, Sharif S, Kazmi M, Sultan T, Aslam M 2011: Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst., 145, 159–168. doi: 10.1080/11263504.2010.542318
- Abbaspour H 2009: Investigation of the effect of vesicular arbuscular mycorrhiza on mineral nutrition and growth of Carthamus tinctorius under salt stress conditions. Russian J. Plant Physiol., 57, 526–531. doi: 10.1134/S1021443710040102
- Abd_Allah EF, Hashem A, Alqarawi AA, Bahkali AH, Alwhibi MS 2015: Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Bio. Sci., 22, 274–283. doi: 10.1016/j.sjbs.2015.03.004
- Ahmed M, Zahir ZA, Asghar N, Asghar M 2011: Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Canad J. Micro, 57(7), 578–589.doi: 10.1139/w11-044
- Ameur H, Ghoul M, Selvin J 2011: The osmoprotective effect of some organic solutes on Streptomyces sp. MADO2 and Nocardiopsis sp. MADO3 growth. Braz. J. Microbiol., 42, 543–553. doi: 10.1590/S1517-838220110002000019
- Aziz ZFA, Saud HM, Rahim KA, Ahmed OH 2012: Variable responses on early development of shallot (Allium ascalonicum) and mustard (Brassica juncea) plants to B. cereus inoculation. Malaysian J. Microbiol., 8(1), 47–50.
- Bandopadhyay S 2015: Effect of dual inoculation of plant growth promoting rhizobacteria on different non-leguminous plants under pot condition. Indian J. Microbiol. Res., 2(1), 20–26.
- Baset-Mia MA, Shamsuddin ZH, Wahab Z, Marziah M 2010: Rhizobacteria as bioenhancer and biofertilizer for growth and yield of banana (Musa spp. cv. ‘Berangan’). Sci. Hort, 126(2), 80–87. doi: 10.1016/j.scienta.2010.06.005
- Bates LS, Waldren RP, Teare ID 1973: Rapid determination of free proline for water status studies. Plant Soil, 39, 205–207. doi: 10.1007/BF00018060
- Beauchamp C, Fridovich I 1971: Superoxide dismutase,improved assays and an assay applicable to acrylamidegels. Anal. Biochem. 44, 276–287.
- Bergey DH, Buchanan RE, Gibbons NE 1974: Bergey’s Manual of Determinative Bacteriology, Williams & Wilkins, Baltimore, MD.
- Bibi F, Chung EJ, Yoon HS, Song GC, Jeon CO, Chung YR 2011: Haloferula luteola sp. nov., an endophytic bacterium isolated from the root of a halophyte, Rosa rugosa, and emended description of the genus Haloferula. Int. J. Syst. Evol. Microbiol., 61, 1837–1841. doi: 10.1099/ijs.0.022772-0
- Cakmakci RF, Donmez MF, Erdoğan U 2007: The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J. Agri. Forest, 31, 189–199.
- Eilers KG, Debenport S, Anderson S, Fierer N 2012: Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol. Biochem., 50, 58–65. doi: 10.1016/j.soilbio.2012.03.011
- El-Awady MAM, Hassan MM, Al-Sodany YM 2015: Isolation and characterization of salt tolerant endophytic and rhizospheric plant growth-promoting bacteria (PGPB) associated with the halophyte plant (Sesuvium verrucosum) grown in KSA. Int. J. Appl. Sci. Biotechnol., 3(3), 552–560. doi: 10.3126/ijasbt.v3i3.13440
- FAO/UNESCO 1974: Soil Map of the World, pp.62. Legend, UNESCO, Paris.
- Flowers TJ, Colmer TD 2008: Salinity tolerance in halophytes. New Phytol., 179, 945–963. doi: 10.1111/j.1469-8137.2008.02531.x
- Ghorbanpour M, Hatami M, Khavazi K 2013: Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloid production of Hyoscyamus Niger under water deficit stress. Turk J. Biol., 37, 350–360.
- Hashem A, Abd_Allah EF, Alqarawi AA, Al-Huqail AA, Wirth S, Egamberdieva D 2016: The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Microbiol., 7, 1089. doi: 10.3389/fmicb.2016.01089
- Hassan TU, Bano A 2015: Role of carrier-based biofertilizer in reclamation of saline soil and wheat growth. Arch. Agron. Soil Sci., 61(12), 1719–1731. doi: 10.1080/03650340.2015.1036045
- Jackson ML 1967: Soil Chemical Analysis, Prentice Hall of India Ltd, New Delhi.
- James GC 1978: Native Sherman Rockland Community College, State University, The Benjamin/Coming Publishing Company. Inc., New York, NY.
- Johnson RP, Balwani TL, Johnson LJ, Meclure KE, Denority BA 1966: Corn plant maturity II effect on in vitro cellular digestibility and soluble carbohydrate content. J. Anim. Sci., 25, 617–623. doi: 10.2527/jas1966.253617x
- Keskin BC, Sarikaya AT, Yuksel B, Memon AR 2010: Abscisic acid regulated gene expression in bread wheat. Austr J. Crop Sci., 4, 617–625.
- Kettner J, Doerffling K 1995: Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta, 196, 627–634. doi: 10.1007/BF01106753
- King C, Thomas DSG 2014: Monitoring environmental change and degradation in the irrigated oases of the Northern Sahara. J. Arid Environ., 103, 36–45. doi: 10.1016/j.jaridenv.2013.12.009
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951: Protein measurement with the Folin-Phenol reagents. J. Biol. Chem., 193, 265–275.
- Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML 2010: Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr., 10(3), 293–319. doi: 10.4067/S0718-95162010000100006
- Masciarelli O, Llanes A, Luna V 2014: A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res., 169(7–8), 609–615. doi: 10.1016/j.micres.2013.10.001
- Nadeem SM, Ahmadm M, Zahir ZA, Javaid A, Ashraf M 2014: The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotech. Adv., 32, 429–448. doi: 10.1016/j.biotechadv.2013.12.005
- Ogbo FC, Odo MO 2011: Potential of rice husk and cassava peel as carriers for bio-fertilizer production. Nig. J. Biotech., 23, 1–4.
- Pérez-Montaño F, Alías-Villegas C, Bellogín RA, Cerro PD, Espuny MRE, Jiménez-Guerrero I, López-Baena FJ, Ollero FJ, Cubo T 2014: Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol. Res., 169, 325–336. doi: 10.1016/j.micres.2013.09.011
- Piper CS 1947: Soil and Plant Analysis, pp. 268. University of Adelaide press, Australia.
- Ramalashmi K, Prasanna Vengatesh K, Magesh K, Sanjana R, Siril Joe S, Ravibalan K 2018: A potential surface sterilization technique and culture media for the isolation of endophytic bacteria from Acalypha indica and its antibacterial activity. J. Med. Plants Studies, 6(1), 181–184.
- Reitemeier RF 1943: Semi microanalysis of saline soil solutions. Indus. And Engin. Chem. Analyt., 15, 393–402. doi: 10.1021/i560118a016
- Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C 2009: Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil, 321, 305–339.
- Ruiz-Lozano JM, Porcel R, Azcon R, Aroca R 2012: Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants. New challenges in physiological and molecular studies. J. Exp. Bot., 63, 4033–4044.doi: 10.1093/jxb/ers126
- Schmidt MW, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC 2011: Persistence of soil organic matter as an ecosystem property. Nature, 47, 49–56.doi: 10.1038/nature10386
- Shahid SA, Rahman K 2011: Soil salinity development, classification, assessment and management in irrigated agriculture. In Handbook of Plant and Crop, Ed. Pessarakli M, pp. 23–39. CRC Press, Boca Raton, FL.
- Singariya P, Kumari P, Mourya KK 2012: Evaluation of antibacterial activity and preliminary physicochemical studies of the stem of Cenchrus ciliaris and Cenchrus setigerus. Asian. J. Pharma Clini Res., 5(1), 163–167.
- Sokolova MG, Akimova GP, Vaĭshlia OB 2011: Effect of phytohormones synthesized by rhizosphere bacteria on plants. Prikl. Biokhim. Mikrobiol., 47(3), 302–307.
- Soltanpour PN, Schwab AP 1977: A new soil test for simultaneous extraction of macro- and micronutrients in alkaline soils. Commun Soil Sci. Plant Anal., 8, 195–207. doi: 10.1080/00103627709366714
- Soni A, Oey I, Silcock P, Bremer P 2016: Spores in the food industry: a review on resistance and response to novel inactivation technologies. Compr. Rev. Food Sci. Food Safety, 15, 1139–1148. doi: 10.1111/1541-4337.12231
- Steel RGD, Torrie JH 1980: Principles and Procedures of Statistics: A Biometric Approach, McGraw-Hill. Co, New York, NY.
- Tiwari S, Singh P, Tiwari R, Meena KK, Yandigeri M, Singh DP, Arora DK 2011: Salt-tolerant rhizobacteria mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol. Fertil. Soil, 47, 907–916. doi: 10.1007/s00374-011-0598-5
- Vetter JL, Steinberrg MP, Nelson AI 1958: Quantitive determination of peroxidase in sweet corn. J. Agri. Food Chem., 6, 39–41.doi: 10.1021/jf60083a006
- Vurukonda SSKP, Vardharajula S, Shrivastava M, Sk A 2016: Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res., 184, 13–24. doi: 10.1016/j.micres.2015.12.003
- Wachowska U, Okorski A, Głowacka K 2006: Population structure of microorganisms colonizing the soil environment of winter wheat. Plant. Soil Environ., 52, 39–44.
- Walkley A, Black IA 1934: An examination of the degtiareff method for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci., 37, 29–38. doi: 10.1097/00010694-193401000-00003
- Yadav S, Yadav S, Kaushik R, Saxena AK, Dk A 2013: Genetic and functional diversity of fluorescent Pseudomonas from rhizospheric soils of wheat crop. J. Basic Microbiol., 1, 1–13.
- Yang J, Kloepper JW, Ryu CM 2009: Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci., 14, 1–4. doi: 10.1016/j.tplants.2008.10.004
- Zahir ZA, Saqib AS, Maqshoof AS, Sm N 2012: Comparative effectiveness of Enterobacter aerogenes and Pseudomonas fluorescens for mitigating the depressing effect of brackish water on maize. Intr J. Agri Biol., 14, 337–344.
- Zhu F, Qu L, Hong X, Sun X 2011: Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid-Base Compl. Altr. Med., 1, 1–6.
- Zhu JK 2002: Salt and drought stress signal transduction in plants. Annal. Rev. Plant Biol., 53, 247–273. doi: 10.1146/annurev.arplant.53.091401.143329
