ABSTRACT
The selection of effective rhizobia for higher efficiency nitrogen fixation is one of the most important steps for inoculant production. Therefore, this experiment was conducted to select the most effective type A and type B strains for specific Rj-gene harboring soybean varieties and to test the symbiotic effectiveness of selected strains on different Rj-gene harboring soybean varieties. Screening experiments using the specific soybean varieties were done with a completely randomized design and three replications in this study. Evaluation of the effective Myanmar Bradyrhizobium strains for plant growth, nodulation and N2 fixation were studied in pot experiments using sterilized vermiculite in the Phytotron (controlled-environmental condition). Then, a pot experiment was conducted using Futsukaichi soil in the screen house (natural environmental condition). The N2 fixation ability of soybean was evaluated by acetylene reduction activity (ARA) and the relative ureide index method. In the first screening experiment, type A and type B strains with higher nitrogen fixation and proper nodulation on their respective soybean cultivars were selected for the next screening. In the second screening, Bradyrhizobium elkanii AHY3-1 (type A), Bradyrhizobium japonicum SAY3-7 (type A), B. elkanii BLY3-8 (type B) and B. japonicum SAY3-10 (type B) isolates, which showed higher nitrogen fixation and nodulation in Yezin-3 (Rj4) and Yezin-6 (non-Rj), were selected for the next experiment. In the third screening experiment, SAY3-7 and BLY3-8, which had higher nitrogen fixing potential and proper nodulation, were selected as effective isolates. These two isolates were compatible with non-Rj and Rj4 soybean varieties for nodulation and nitrogen fixation. Based on the results of the screening experiment, these two strains were tested for their symbiotic efficacy in Futsukaichi soil. This study shows that inoculation treatment of SAY3-7 and BLY3-8 significantly increased plant growth, nodulation, and N2 fixation at the V6, R3.5 and R8 stages in Yezin-3 (Rj4) and/or Yezin-6 (non-Rj), and the seed yield at R8 stage, in Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean varieties compared with the control treatment. It can be concluded that SAY3-7 and BLY3-8 are suitable for inoculant production because of their higher nitrogen fixation ability, proper nodulation and better productivity of Myanmar soybean cultivars.
1. Introduction
Soybean seeds are a major source of protein, oil, carbohydrate, and minerals for human and animal nutrition. Proteins, oil, carbohydrates, and minerals constitute 36%, 19%, 35%, and 5%, respectively, of the total soybean dry weight (Liu Citation1997). In addition to providing nutritious food, the soybean increases soil fertility (FAO Citation1984). The yield of soybeans (1.51 tons ha−1) in Myanmar is still low compared to the world average yield (2.52 tons ha−1; MOAI Citation2015). This reduction of yield is related to inadequate nitrogen application and lack of inoculation of an alternative source of nitrogen fertilizer.
Inoculation is the treatment of the seed or soil with inoculants of specific bacterial strains (NifTAL Citation1990). The aim of inoculation is to provide sufficient numbers of viable, effective rhizobia to enhance rapid colonization in the rhizosphere to form nodules as soon as possible after germination and thereby promote optimal crop yields (Catroux Citation1991). In many soils, the strain, number, quality, or virulence of indigenous nodulating bacteria is not adequate for nitrogen fixation (FAO Citation1984). Therefore, inoculation against seeds or soils with effective strains is necessary for successful nodulation because biological nitrogen fixation (BNF) depends on effective nodules.
Soybeans, in symbiosis with Bradyrhizobium, have the ability to fix nitrogen at a rate of up to 300 kg N ha−1 under favorable conditions (Smith and Hume Citation1987). However, the symbiosis is dependent on host specificity. This specificity might also be related to the nodulation regulatory genes of soybean cultivars and nodulation types of rhizobia, especially in the soybean. Ishizuka et al. (Citation1991a); (Citation1991b) tested the compatibility and preference of Rj-genotype soybean cultivars with specific Bradyrhizobium strains. The Bradyrhizobium strains are classified into nodulation types A, B, and C, based on their compatibility with Rj cultivars. Type A strains are preferred by the non-Rj genotype soybean cultivars and nodulate with all the Rj-genotype soybean cultivars. Type B strains are preferred by Rj4 genotype soybean cultivars and inhibit nodulation with the Rj2Rj3 genotype soybean cultivars. Type C strains are preferred by the Rj2Rj3 cultivars and restrict effective nodule formation with the Rj4 genotype soybean cultivars.
The responses of soybean cultivars vary with the Rhizobium strains. While some cultivars are fully compatible with other Rhizobium, others cannot form nodules, although the Rhizobium belongs to same serogroups (Van et al. Citation2007). This might be due to nodulation regulatory genes called Rj genes found in the soybean cultivars. The Rj-genotypes (non-Rj, rj1, Rj2, Rj3, and Rj4) have been found to exist in nature (Devine and Kuykendall Citation1996). In Myanmar, non-Rj, Rj2Rj3, and Rj4 genotypes soybean cultivars were reported in a previous study (Htwe et al. Citation2015b). Among them, Rj4 genotype soybean cultivars are widely grown in Myanmar and account for 60% of all the cultivars. Devine and Kuykendall (Citation1996) reported that more than 60% of the soybean cultivars in Southeast Asia have the Rj4 gene. In Myanmar, Soe et al. (Citation2013) reported that six cultivars, such as Hinthada, Southern Shan local, Northern Shan local, Yezin-3, Yezin-11, and Daewoncong, were Rj4 genotype soybean cultivars, but the remaining eight cultivars, such as Shan Sein, Shan Wha, Yezin-6, Yezin-8, Yezin-14, Daepung, Cheongga-3, and Zhongpin-661, were non-Rj genotype soybean cultivars among the 14 soybean cultivars. From their results, non-Rj and Rj4 genotype soybean cultivars are widely grown in Myanmar. Therefore, selection of the type A strain, which prefers non-Rj genotype soybean cultivar, and type B strains, which prefer to Rj4 genotype soybean cultivars, are important steps for increasing nodulation and nitrogen fixation. Moreover, selected type A and type B strains can be useful as effective inoculants for soybean cultivation in Myanmar, where non-Rj and Rj4-gene harboring soybean cultivars are widely grown.
In Myanmar, the use of Rhizobium for soybean production is still limited. To promote soybean productivity, it is necessary to select strains compatible with cultivars. Therefore, the goal of the present study was to select the most effective type A and type B strains for non-Rj and Rj4-gene harboring soybean cultivars and to evaluate the symbiotic effectiveness of selected Bradyrhizobium type A and type B isolates on non-Rj and Rj4-gene soybean cultivars.
2. Materials and methods
2.1. Inoculant preparation
Bradyrhizobium diazoefficiens USDA110 was obtained from the Laboratory of Plant Nutrition, Kyushu University. Indigenous Bradyrhizobium strains were obtained from a previous experiment (Htwe et al. Citation2015a). The origins of Myanmar indigenous Bradyrhizobium strains are presented in supplementary Table 1. Bradyrhizobium strains were cultured in A1E liquid media (Kuykendall Citation1987) on a rotary shaker (100 rpm) at 30°C for 7 days.
2.2. Soybean varieties
Myanmar soybean varieties [Yezin-3 (Rj4), Yezin-6 (non-Rj), Yezin-11 (Rj4), and Yezin-14 (non-Rj)] were collected from the Food Legumes Section, Department of Agricultural Research, Yezin, Myanmar. Their Rj genes were identified by (Soe et al. Citation2013; Htwe et al. Citation2015b). Rj4 and non-Rj in the parenthesis refer to the nodulation regulatory genes (Devine and Kuykendall Citation1996).
2.3. Cultivation, crop management, plant sampling, and analysis of screening experiments under controlled environmental conditions
The 1 L pots were filled with vermiculite and 0.6 L of half-strength modified Hoagland nutrient (MHN) solution (Nakano et al. Citation1997). The pots were autoclaved at 120°C for 20 min. Surface sterilization of the seeds was done by soaking them in 2.5% sodium hypochlorite solution for 5 min, rinsing five times with 10 mL of 99.5% ethanol, and washing five times with sterilized MHN solution. Five surface sterilized seeds were sown in the pots. The liquid bacterial cultures were diluted with sterilized MHN solution to 107 cells mL−1. Each seed was inoculated with 5 mL of bacterial suspension. The plants were cultivated in an environmentally controlled room (25°C and 75% relative humidity) for 30 days. In Myanmar, soybean is cultivated from November to February with residual moisture after rice cultivation. From November to February, the average monthly temperature is between 20°C and 25°C. Therefore, we set the temperature at 25°C for screening experiments to obtain better soybean growth. Completely randomized design was used with three replications. Watering was done as necessary using autoclaved deionized water. At the harvest time, N2 fixation was analyzed using an acetylene reduction assay (ARA) as described by Haider et al. (Citation1991). After the assay, nodules were counted. Shoots, roots and nodules were collected separately and oven dried at 70°C for 24 h to record dry weights. This experiment was conducted from January 2017 to June 2017.
2.4. Cultivation, crop management, plant sampling and analysis of pot experiment under natural environmental conditions
Futsukaichi soil was collected from Kamigoka, Chikushino city, Fukuoka prefecture, Japan. Before cultivation, the indigenous rhizobial population of the Futsukaichi soil was evaluated by the most probable number (MPN) method (Vincent Citation1970) using Yezin-3 (Rj4) as the host plant. The estimated rhizobial population was 2.9 × 103 rhizobia per 1 g dried soil. For pot preparation, the a/5000 Wagner pot was filled with 3.7 kg (oven dry basis) of Futsukaichi soil. Futsukaichi soil was collected from Kamigoka, Chikushino city, Fukuoka prefecture, Japan. The physiochemical properties of Futsukaichi soil were analyzed by Myint et al. (Citation2011). This soil had a sandy loam with pH 6.11 (soil: water, 1:2.5). The soil had a total nitrogen (N) content of 0.68 g Kg−1; and total phosphorus (P) content of 0.37 g Kg−1. The cation exchange capacity (CEC) of the soil was 9.75 cmolc kg−1. Exchangeable potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), and Iron (Fe) contained 0.32, 10.76, 0.89, 0.14, and 0.10 cmolc kg−1, respectively. We adjusted the soil from pH 6.1 to pH 6.5 by using CaMg(CO3)2 powder. Then, compound fertilizer (Kumiai Mame-kasei 300, Ryoto Fertilizer Co., Ltd., Ooita, Japan) containing 3% N, 10% P2O5, and 10% K2O, was applied at a rate of 1.6 g pot−1 at the time of pot preparation. The water content was maintained at 60% of the water holding capacity at the time of sowing. In this experiment, the seed inoculation method was used prior to seed sowing. A total of 100 soybean seeds were mixed thoroughly with 10 g peat soil, 7 mL 20% liquid solution of gum arabic, and 0.1 mL 1 × 108 cell mL−1 Bradyrhizobium to obtain the required inoculation density (1 × 105 cells seed−1). Four inoculated seeds were planted in one pot and covered with soil just after seed sowing. At 20 days after sowing, thinning was done to maintain one plant per pot. Pesticides were sprayed as necessary. Plant samples were collected from three growing stages: V6 (six unfolded trifoliate leaves), R3.5 (early pod-fill stage), and R8 (maturity stage). The growth stages referred to Fehr et al. (Citation1971).
At the V6 stage, the plants were uprooted and washed to collect data. ARA measurements and collected data were the same as described above. Shoots, roots, and nodules were oven-dried at 70°C for 72 h to record their dry weights.
At the R3.5 stage, the plants were cut just under the cotyledonary nodes, inserted into a silicon tube, and the xylem sap was collected within 1 h after cutting. The sap samples were stored at −30°C before analysis. Then, the amino acid (Moore and Stein Citation1954), nitrate (Cataldo et al. Citation1975), and ureide (Young and Conway Citation1942) concentrations were analyzed from the root bleeding saps. The relative ureide index (RUI) of the root bleeding sap was calculated using the following formula (Peoples et al. Citation1989);
The percentage of N derived from N fixation (%Ndfa), was calculated using the following formula (Herridge and Peoples Citation1990):
where y is RUI (%) and x is %Ndfa, respectively.
At R8, the plants were cut at the cotyledon nodes to determine yield components such as the number of pods per plant, number of seeds per pod, 100-seed weight, and seed yield. This experiment was conducted in the screen house under open environmental conditions from July 2017 to November 2017.
3. Results
3.1. Screening of effective Bradyrhizobium type A strains
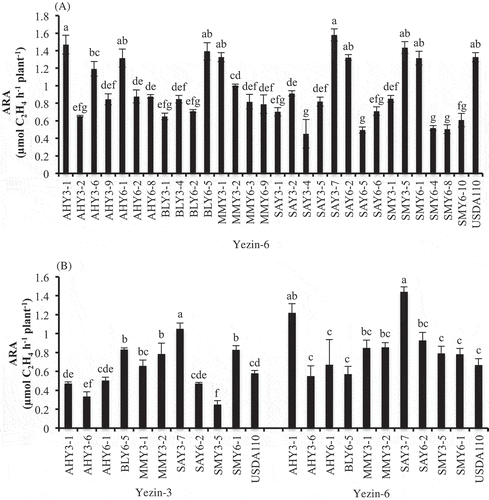
In the first screening, Yezin-6 (non-Rj) was used as a host plant for the determination of the nitrogen fixing potential and nodulation ability of 30 bradyrhizobial type A strains. USDA110 was used as the positive control. There were no significant differences in the shoot and root dry weights (supplementary Table 2). AHY3-1, AHY6-1, BLY6-5, MMY3-1, MMY3-2, SAY3-7, SAY6-2, SMY3-5, SMY6-1 and USDA110 significantly enhanced nitrogen fixation compared to the other tested strain. Therefore, these strains with higher nitrogen fixation and proper nodulation were selected for the next screening.
In the second screening experiment, the strains were compared for their ability to fix nitrogen, in terms of the ARA per plant using Yezin-3 (Rj4), and Yezin-6 (non-Rj). The results of the plant growth and nodulation are shown in . The results of nitrogen fixation are shown in . In both soybean varieties, shoot biomass and root biomass were not significantly different among inoculated strains (). Nodule numbers of Yezin-3 were significantly different among inoculated bacteria, but the nodule dry weights were not significantly different (). B. elkanii AHY3-1 and SAY6-2 produced significantly higher nodule numbers than other tested isolates except BLY6-5 in Yezin-3. In Yezin-6 soybean cultivars, nodule number and nodule mass were significantly different among inoculated bacteria (). AHY3-1, SAY6-2, and SMY3-5 produced significantly higher nodule numbers, but they were not different from USDA110. Moreover, AHY3-1, SAY6-2, and SMY3-5 produced higher nodule dry weights than other tested strains, except SAY3-7. Nitrogen fixation of B. japonicum SAY3-7 was significantly higher than that of other tested isolates and USDA110 () in Yezin-3 (Rj4). In Yezin-6 (non-Rj), the nitrogen fixation of B. japonicum strains SAY3-7 and AHY3-1 was significantly higher than that of other tested isolates and USDA110 (). It was interesting that SAY3-7 consistently showed higher nitrogen fixation in both soybean varieties, whereas AHY3-1 fixed higher nitrogen in Yezin-6. Therefore, AHY3-1 and SAY3-7 was selected for the next screening.
Table 1. Effect of inoculation of selected Bradyrhizobium type A strains on NN, NDW, RDW and SDW of Yezin-3 and Yezin-6 soybean varieties at 30 days after sowing.
Figure 1. Effect of inoculation of Bradyrhizobium type A strains on acetylene reduction activity (ARA) of Yezin-6 in the first screening (A), and Yezin-3 and Yezin-6 in the second screening (B) at 30 days after sowing. The histograms with the same letter at each variety are not significantly different at P < 0.05 (Tukey’s test). The bar on each histogram indicates standard deviation (SD).
3.2. Screening of effective Bradyrhizobium type B strains
In the first screening, Yezin-3 (Rj4) was inoculated with 26 bradyrhizobial type B strains. USDA110 was used as a positive control. In the first screening experiment, shoot biomass and root biomass were not significantly different among the inoculated bacteria (supplementary Table 3). However, the nodule number, nodule mass and nitrogen activities of the inoculated bacteria were significantly different (supplementary Table 3 and ). The isolates showed the proper nodulation and higher nitrogen fixing potential were selected for the next screening experiment. They were B. elkanii strains BLY3-8 and BLY6-1, B. japonicum strains SAY3-3 and SAY3-10, and Bradyrhizobium spp. strains SHY3-1, SHY3-3, SHY6-1, SHY6-5, SHY6-6, and SHY6-10.
Figure 2. Effect of inoculation of Bradyrhizobium type B strains on ARA of Yezin-3 in the first screening (A), and Yezin-3 and Yezin-6 in the second screening (B) at 30 days after sowing. The histograms with the same letter at each variety are not significantly different at P < 0.05 (Tukey’s test). The bar on each histogram indicates SD.
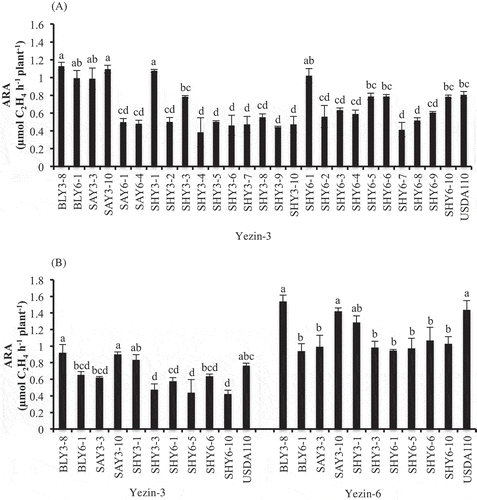
In the second screening experiment, the strains were compared for their nitrogen fixation ability in terms of the ARA per plant using Yezin-3 (Rj4) and Yezin-6 (non-Rj). The results of the plant growth and nodulation are shown in . The nitrogen fixation results are shown in . In both soybean varieties, the shoot biomass and root biomass were not significantly different among the inoculated strains (). However, the nodule number, nodule mass, and nitrogen fixing potential were significantly different among the inoculated bacteria ( and ). Nodule number, nodule mass and nitrogen fixation of B. elkanii BLY3-8, and B. japonicum strains SAY3-10 were significantly higher than those of the other tested isolates, but these strains were not different from Bradyrhizobium spp. SHY3-1, and USDA110 () in both varieties. Therefore, BLY3-8 and SAY3-10 were selected for the next screening.
Table 2. Effect of inoculation of selected Bradyrhizobium type B strains on NN, NDW, RDW, and SDW of Yezin-3 and Yezin-6 soybean varieties at 30 days after sowing.
3.3. Effects selected type A and type B strains on symbiotic effectiveness of various soybean varieties
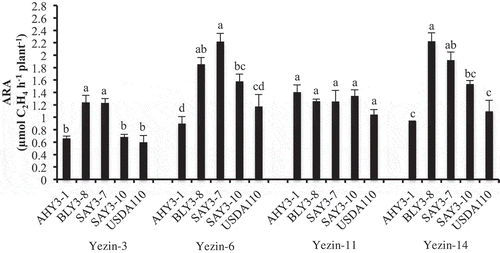
n this experiment, the selected Bradyrhizobium type A and type B strains were compared for their nitrogen fixation potential using Yezin-3 (Rj4), Yezin-6 (non-Rj), Yezin-11 (Rj4) and Yezin-14 (non-Rj). The plant growth and nodulation results are shown in . The nitrogen fixation results are shown in . Shoot and root dry weights were not significant in all tested soybean varieties. Nodule numbers were significantly different among inoculated bacteria, especially in Yezin-3 varieties, but not in other tested varieties (). BLY3-8 produced significantly higher nodule numbers compared with SAY3-10 and USDA110, but it was not different from AHY3-1 and SAY3-7. Nodule dry weights were significantly different among inoculated bacteria, especially in Yezin-3, Yezin-6, and Yezin-14 varieties, but not in Yezin-11 variety. SAY3-7 produced significantly higher nodule dry weights in Yezin-3, Yezin-6, and Yezin-14 varieties, but it was not different from BLY3-8 in Yezin-3 and Yezin-14 varieties. Nitrogen fixation rates differed significantly in the Yezin-3, Yezin-6 and Yezin-14 varieties, but not in the Yezin-11 variety (). AHY3-1 and USDA110 showed significantly lower nitrogen fixation than the other tested isolates in Yezin-3, Yezin-6, and Yezin-14 varieties. However, BLY3-8 and SAY3-7 showed higher nitrogen fixing potential than other tested isolates in Yezin-3, Yezin-6, and Yezin-14 varieties, but nitrogen fixation of BLY3-8 and SAY3-7 was not significantly different compared to SAY3-10, Yezin-6, and Yezin-14 varieties, respectively. Therefore, BLY3-8 and SAY3-7 were more compatible in all the tested varieties.
Table 3. Effect of inoculation of selected B. elkanii AHY3-1 (type A), B. elkanii BLY3-8 (type B), B. japnicum SAY3-7 (type A) and B. japnicum SAY3-10 (type B) strains on NN, NDW, RDW, and SDW of Yezin-3, Yezin-6, Yezin-11 and Yezin-14 soybean varieties at 30 days after sowing.
Figure 3. Effect of inoculation of selected B. elkanii AHY3-1 (type A), B. elkanii BLY3-8 (type B), B. japnicum SAY3-7 (type A) and B. japnicum SAY3-10 (type B) strains on ARA of Yezin-3, Yezin-6, Yezin-11 and Yezin-14 soybean varieties at 30 days after sowing. The histograms with the same letter at each variety are not significantly different at P < 0.05 (Tukey’s test). The bar on each histogram indicates SD.
3.4. Effects of selected type A and type B strains on symbiotic effectiveness of Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean varieties at different growth stages
The plant growth and nodulation results of Yezin-3 and Yezin-6 at different growth stages are shown in . The N2 fixation results for both varieties are shown in . At the V6 growth stage, except nitrogen fixation, other parameters such as nodule numbers, nodule dry weight, root dry weight, and shoot dry weight were not significantly different among the treatments in Yezin-3 variety (), whereas the nodule dry weight, root dry weight, shoot dry weight, and nitrogen fixation were significantly different by inoculation in Yezin-6 (). These results show that the inoculation of SAY3-7 induced more symbiotic effectiveness at the early growth stage (V6) in the Yezin-6 variety. Moreover, inoculation of SAY-7 and BLY3-8 significantly enhanced N2 fixation in terms of the C2H4 production compared with the control treatment () in both varieties.
Table 4. Effect of inoculation of B. japonicum SAY3-7 (type A) and B. elkanii BLY3-8 (type B) on NN, NDW, RDW, and SDW of Yezin-3 and Yezin-6 soybean varieties at different growth stages.
Figure 4. Effect of inoculation of B. japonicum SAY3-7 (type A) and B. elkanii BLY3-8 (type B) on (A) ARA at V6 stage, (B) relative ureide index (%) at R3.5 stage, (C) N derived from N fixation (%Ndfa) at R3.5 of Yezin-3 and Yezin-6 soybean varieties. The histograms with the same letter at each variety are not significantly different at P < 0.05 (Tukey’s test). The bar on each histogram indicates SD.
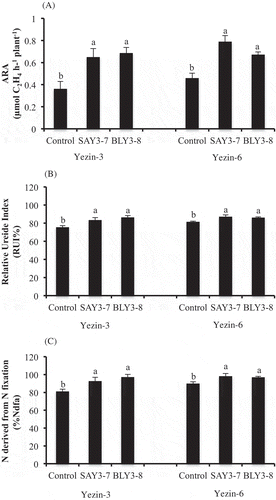
At the R3.5 growth stage, the nodule number, nodule dry weight, and shoot dry weight were significantly different among the treatments in both varieties, but the root dry weight was not significant in Yezin-3. In the Yezin-3 variety, inoculation of BLY3-8 produced significantly higher nodule number, nodule dry weight, shoot dry weight and nitrogen fixation in terms of RUI (%) and %Ndfa compared with the control, but not SAY3-7 ( and ). In the Yezin-6 variety, inoculation of SAY3-7 and BLY3-8 produced a higher nodule number, nodule dry weight, root dry weight, and shoot dry weight compared with the control. At R3.5 stage, nodule number of inoculated plants with BLY3-8 (86.0 plant−1) increased 35.7% compared with non-inoculated plant (55.3 plant−1) in Yezin-3. Nodule number of inoculated plants with SAY3-7 (93.7 plant−1) and BLY3-8 (92.0 plant−1) and increased 23.2% and 21.7%, respectively, compared with non-inoculated plant (72.0 plant−1) in Yezin-6. These results suggested that competitiveness of SAY3-7 and BLY3-8 strains for nodulation with indigenous rhizobia existing in the soil.
At the R8 growth stage, shoot biomass was significantly different in both the soybean varieties (). Inoculation of SAY3-7 and BLY3-8 produced at higher shoot biomass than the control treatment.
The yield component results are shown in . Yield components, such as the number of pods per plant, number of seeds per pod, and 100-seed weight, were not significantly different among the treatments in both varieties. However, the seed yields were significantly different. Inoculation of SAY3-7 and BLY3-8 resulted in significantly higher seed yields than the control treatment in both varieties. This result suggests that SAY3-7 and BLY3-8 are effective bacteria, which are compatible with Yezin-6 (non-Rj) and Yezin-3 (Rj4) because of the higher production of the seed yield.
Table 5. Effect of inoculation of B. japonicum SAY3-7 (type A) and B. elkanii BLY3-8 (type B) on yield components and seed yield of Yezin-3 and Yezin-6 soybean varieties at maturity stage.
4. Discussion
Rhizobia have been used extensively in agricultural systems to enhance the ability of legumes to fix atmospheric N2 (Elkan Citation1992). Therefore, the isolation and selection of indigenous bacteria for their respective soybean cultivars is necessary. Recently, Soe et al. (Citation2013) and Htwe et al. (Citation2015b) identified Myanmar soybean cultivars with non-Rj and Rj4 genotypes. Soe et al. (Citation2013) and Htwe et al. (Citation2015c) noted that the cultivars Yezin-6, harboring the non-Rj gene, and Yezin-3, harboring the Rj4 gene, have enhanced nodulation and nitrogen fixation compared to indigenous strains. However, their initial screening was based on using Yezin-6 (non-Rj) soybean cultivars. As a consequence, type A strains, which prefer non-Rj soybean cultivars, have more potential for selection. In this study, the type A and type B groups were separately screened using the respective cultivars, Yezin-6 (non-Rj) and Yezin-3 (Rj4), using the USDA110, which is used in many countries as an inoculant. In the first screening, the type A and type B strains that showed proper nodulation and higher nitrogen fixing potential were selected for the next screening experiment (supplementary Table 2 and for type A) and (supplementary Table 3 and for type B). According to the results of this study, the isolates belonging to the same species showed different responses for nitrogen fixation. This was in line with the previous findings of our group (Soe and Yamakawa Citation2013; Htwe et al. Citation2015c).
Isolation and selection were aimed at producing rhizobial inoculants. The first step in manufacturing rhizobial inoculants is to obtain the most effective strain, since rhizobial strains within a species vary in their effectiveness (Boonkerd et al. Citation1978). Therefore, the second screening was done to determine the symbiotic effectiveness of the selected isolates on Yezin-3 (Rj4) and Yezin-6 (non-Rj) because the selected isolates were isolated from Yezin-3 (Rj4), and Yezin-6 (non-Rj). Among the selected type A strains, SAY3-7 consistently showed higher nodulation and nitrogen fixation in both soybean varieties compared with the other type A strains ( and ). However, AHY3-1 fixed higher nitrogen and produced proper nodulation in the Yezin-6 soybean cultivar, but not in Yezin-3 ( and ). Among the selected type B strains, the nodulation and nitrogen fixation of BLY3-8 and SAY3-10 were shown to be significantly higher than those of the other tested isolates, in both the Yezin-3 and Yezin-6 soybean cultivars ( and ). To confirm the results of the second screening and to select the most effective strains, a third screening was done. In the third screening, BLY3-8 and SAY3-7 showed higher nitrogen fixing potential than the other tested isolates in the Yezin-3, Yezin-6 and Yezin-14 varieties, but nitrogen fixation of BLY3-8 and SAY3-7 was not significant from SAY3-10 in both the Yezin-6 and Yezin-14 varieties (). Therefore, BLY3-8 and SAY3-7 seemed to be more compatible for proper nodulation and nitrogen fixation in all the tested varieties (). This result supports the finding of others in which BNF in soybean production can be increased by the selection of effective Bradyrhizobium japonicum strains and efficient soybean cultivars as cultivar-bacterial strain pairs (Israel et al. Citation1986; Soe and Yamakawa Citation2013; Htwe et al. Citation2015c). However, plant growth was not significant among the inoculated treatments. This was in agreement with (Sarr et al. Citation2016) in which N2 fixation was increased significantly by Bradyrhizobium strains, but it was not accompanied by a significant increase in plant growth.
It has been reported that soybean growth parameters and yield were significantly increased due to the inoculation of bradyrhizobial isolates (Purcino et al. Citation2000; Pant and Prasad Citation2004). In this study, inoculation of SAY3-7 and BLY3-8 significantly increased N2 fixation at the V6 and R3.5 stages; plant growth, nodulation, N2 fixation and N uptake at the V6, R3.5 and R8 stages ( and ), and the seed yield at the R8 stage (), in Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean varieties were compared with the control treatment. These results support the findings of our group in which inoculation of bradyrhizobial strains increased the plant growth, nodulation, N2 fixation, and seed yield of soybeans cultivated in the soil condition (Soe et al. Citation2012; Yamakawa and Fukushima Citation2014).
A successful legume-Rhizobium symbiosis mainly depends on the presence of a compatible strain in the soil for a particular legume. Therefore, inoculation with the compatible strain is important for successful legume-Rhizobium symbiosis. However, one of the major problems in the inoculation technology with soybeans is the establishment of an inoculated strain from populations of indigenous bradyrhizobia existing in the soil (Tang Citation1979). Therefore, the occupancy of inoculated strains in nodules is relatively low compared with indigenous rhizobia because of their competition to occupy root nodules (Weaver and Frederick Citation1974; Kvien et al. Citation1981). However, in this study, the nodule number and nodule dry weight in inoculation treatment was significantly higher than those of the control at R3.5 stage. The abundance of nodulation in the plants inoculated with SAY3-7 and BLY3-8 shows that the inoculated strain might have a competitive nodulation ability with indigenous rhizobia. These results were observed because of the use of proper variety, compatible strain and suitable inoculation methods with proper inoculation density as described in (Yamakawa et al. Citation2003) and (Yamakawa and Fukushima Citation2014). Nodule occupancy of inoculated strain can be increased by the use of reciprocal relationship between Rj-genotype soybean cultivars and rhizobia (Yamakawa et al. Citation2003). Better competitiveness for nodulation with indigenous rhizobia can be promoted by using suitable inoculation methods such as inoculation of seeds with 1000-fold higher density than that in the soil (Yamakawa and Fukushima Citation2014).
Soybean-Bradyrhizobium symbiosis provides the required nitrogen to soybean plants through the symbiotic nitrogen fixation of Bradyrhizobium. To optimize this symbiosis, selection of compatible strains for currently used soybean cultivars is considered to be one of the major factors in improving the nodulation and symbiotic effectiveness. Moreover, Rj genes of soybean varieties and the nodulation types of inoculated strains need to be considered because many scientists reported that the indigenous Bradyrhizobium strains in the soil exhibit preferences for nodulation on compatible Rj-genotypes (Saeki et al. Citation2008; Minami et al. Citation2009). The Rj-genotypes play a role in controlling the plant’s compatibility with specific rhizobial strains, and the preference for indigenous soybean-nodulating rhizobia (Ishizuka et al. Citation1991a, Citation1991b). In addition, non-Rj genotype soybean cultivars are compatible with all types of bradyrhizobial strains, but Rj4 has unique features that restrict nodulation with specific strains of Bradyrhizobium (Vest and Caldwell Citation1972). In the present study, the symbiotic effectiveness on plant growth, nodulation, nitrogen fixation and productivity were observed in SAY3-7 (type A strain) and BLY3-8 (type B strain) compared with the control, in both Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean cultivars. The improvement of the symbiotic efficacy in inoculation treatments is due to the preferable use of Rj-genotype soybean cultivars by inoculated Bradyrhizobium strains.
According to the results of this study, it can be concluded that B. japonicum SAY3-7 (type A strain) and B. elkanii BLY3-8 (type B strain) were the most effective isolates with proper nodulation and higher nitrogen fixation in all the Rj4 and non-Rj soybean cultivars cultivated in rhizobial free vermiculite conditions. Moreover, these two effective isolates showed significantly higher plant growth, nodulation, N2 fixation, and seed yield of soybeans cultivated in Futsukaichi soil, where rhizobia existed at a density of 2.9 × 103 cells g−1 compared with the control treatment. In this study, the symbiotic effectiveness of inoculated isolates was observed in soil containing low level of indigenous rhizobial population (2.9 × 103 cells g−1). As further experiment, these two isolates should be tested in soil containing not only low density of rhizobia but also high density of native rhizobia to know their competitiveness to occupancy in nodules by inoculum and to evaluate their symbiotic effectiveness on soybean.
Supplemental Material
Download MS Word (42.7 KB)Acknowledgments
We are grateful to Ministry of Education, Culture, Sports, Sciences and Technology (MEXT) of Japan for financial support of this study.
Supplementary materials
Supplementary data for this article are accessed here
Additional information
Funding
References
- Boonkerd N, Weber DF, Bezdicek DF 1978: Influence of Rhizobium japonicum strains and inoculation methods on soybeans grown in rhizobia-populated soil. Agron. J., 70, 547–549.
- Cataldo DA, Haroon M, Schrader LE, Youngs VL 1975: Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal., 6, 71–80. doi:10.1080/00103627509366547
- Catroux G 1991: Inoculant quality standards and controls in France. In Expert Consultation on Legume Inoculant Production and Quality Control, Thompson JA. Ed., pp 113–120. FAO, Rome.
- Devine TE, Kuykendall LD 1996: Host genetic control of symbiosis in soybean (Glycine max L.). Plant Soil., 186, 173–187. doi:10.1007/BF00035072
- Elkan GH 1992: Taxonomy of the rhizobia. Can. J. Microbiol., 38, 46–450. doi:10.1139/m92-075
- FAO 1984: Legume Inoculants and Their Use, NifTAL Project, U.S.A.
- Fehr WR, Caviness CE, Burmood DT, Pennington JS 1971: Stage of development descriptions for soybeans, Glycine max (L) Merrill. Crop Sci., 11, 929–931. doi:10.2135/cropsci1971.0011183X001100060051x
- Haider J, Hussam AKMA, Ikeda M, Yamakawa T, Ishizuka J 1991: Effects of nitrate application on growth, nodulation and nitrogen fixation ofnitrate-tolerant mutant soybean. Soil Sci. Plant Nutr., 37, 521–529. doi:10.1080/00380768.1991.10415065
- Herridge DF, Peoples MB 1990: Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol., 93, 495–503. doi:10.1104/pp.93.2.495
- Htwe AZ, Saeki Y, Moe K, Yamakawa T 2015b: Determining nodulation regulatory (Rj) genes of Myanmar soybean cultivars and their symbiotic effectiveness with Bradyrhizobium japonicum USDA110. Am. J. Plant Sci., 6, 2799–2810. doi:10.4236/ajps.2015.618276
- Htwe AZ, Yamakawa T, Moe K, Dien DC 2015c: Symbiotic effectiveness of different indigenous Bradyrhizobium strains on selected Rj-genes harboring Myanmar soybean cultivars. African J. Microbiol. Res., 9(49), 2345–2353.
- Htwe AZ, Yamakawa T, Sarr PS, Sakata T 2015a: Diversity and distribution of soybean-nodulation bradyrhizobia isolated from major soybean-growing regions in Myanmar. African J. Microbiol. Res., 9(43), 2183–2196. doi:10.5897/AJMR2015.7702
- Ishizuka J, Suemasu Y, Mizogami K 1991a: Preference of Rj-soybean cultivars for Bradyrhizobium japonicum for nodulation. Soil Sci Plant Nutr., 37, 15–21. doi:10.1080/00380768.1991.10415005
- Ishizuka J, Yokoyama A, Suemasu Y 1991b: Relationship between serotypes of Bradyrhizobium japonicum and their compatibility with Rj-cultivars for nodulation. Soil Sci Plant Nutr., 37, 23–30. doi:10.1080/00380768.1991.10415006
- Israel DW, Mathis JN, Barbour WM, Elkan GH 1986: Symbiotic effectiveness and host-strain interaction of Rhizobium fredii USDA 191 on different soybean cultivars. Appl. Environ. Microbiol., 51, 898–903.
- Kuykendall LD 1987: Isolation and identification of genetically marked strains of nitrogen-fixing microsymbionts of soybeans. In Practical Symbiotic Nitrogen Fixation Methodology, Elkan GH. Ed., pp 205–220. Marcel Dekker, New York.
- Kvien CS, Ham GE, Lambert JW 1981: Recovery of introduced Rhizobium japonicum strains by soybean genotypes. Agronomy J., 73, 900–905. doi:10.2134/agronj1981.00021962007300050034x
- Liu K 1997: Chemistry and nutritional value of soybean components. In Soybean: chemistry, Technology, and Utilization, Liu K. Ed., pp 25–113. Chapman and Hall, New York.
- Minami M, Yamakawa T, Yamamoto A, Akao S, Saeki Y 2009: Estimation of nodulation tendency among Rj-genotype soybeans using the bacterial community isolated from an Andosol. Soil Sci. Plant Nutr., 55, 65–72. doi:10.1111/j.1747-0765.2008.00333.x
- MOAI 2015: Myanmar agriculture in brief. In Department of Planning, Ministry of Agriculture and Irrigation, pp 16–23. Union of Myanmar, Naypyitaw.
- Moore S, Stein WH 1954: A Modified ninhydrin reagent for the photometric determination of amino acids and realted compounds. J. Biol. Chem., 211, 907–913.
- Myint AK, Yamakawa T, Zenmyo T, Thao HTB, Sarr PS 2011: Effects of organic-manure application on growth, grain yield, and nitrogen, phosphorus, and potassium recoveries of rice variety Manawthukha in paddy soils of differing fertility. Commun. Soil Sci. Plant Anal., 42, 457–474. doi:10.1080/00103624.2011.542223
- Nakano Y, Yamakawa T, Ikeda M, Ishizuka J 1997: Nodulation of Rj-soybean varieties with Rhizobium fredii USDA193 under limited supply of nutrients. Soil Sci. Plant Nutr., 43, 929–932. doi:10.1080/00380768.1997.10414659
- NifTAL 1990: Inoculating tree legume seeds and seedling with rhizobia. In Introducing the Micro-Production Unit (MPU) A Planning Guide, pp 8–14.
- Pant BD, Prasad BN 2004: Effectiveness of Bradyrhizobium isolates on seedling growth and nitrogen content in soybean [Glycine max (L.) Merr.]. Botanica Orientalis., 4, 1–3.
- Peoples MB, Faizah AW, Rerkasem B, Herridge DF 1989: Methods for evaluating nitrogen fixation by nodulated legumes in the field. ACIAR Monograph., 11(7), 39–40.
- Purcino HMA, Festin PM, Elkan GH 2000: Identification of effective strains of Bradyrhizobium for Arachis pintoi. Tropical Agriculture., 77, 226–231.
- Saeki Y, Minami M, Yamamoto A, Akao S 2008: Estimation of the bacterial community diversity of soybean-nodulating bradyrhizobia isolated from Rj-genotype soybeans. J. Soil Sci. Plant Nutri., 54, 718–724. doi:10.1111/j.1747-0765.2008.00300.x
- Sarr PS, Araki S, Begoude DA, Yemefack M, Manga GA, Yamakawa T, Htwe AZ 2016: Phylogeny and nitrogen fixation potential of Bradyrhizobium species isolated from the legume cover crop Pueraria phaseoloides (Roxb.) Benth. in Eastern Cameroon. Soil Sci. Plant Nutri., 62, 1 13–19. doi:10.1080/00380768.2015.1086279
- Smith DL, Hume DJ 1987: Comparison of assay methods for N2 fixation utilizing white bean and soybean. Canadian J. Plant Sci., 67, 11–19. doi:10.4141/cjps87-002
- Soe KM, Bhromsiri A, Karladee D, Yamakawa T 2012: Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant Nutr., 58, 319–325. doi:10.1080/00380768.2012.682044
- Soe KM, Yamakawa T 2013: Evaluation of effective Myanmar Bradyrhizobium strains isolated from Myanmar soybean and effects of coinoculation with Streptomyces griseoflavus P4 for nitrogen fixation. Soil Sci Plant Nutr., 59, 361–370. doi:10.1080/00380768.2013.794437
- Soe KM, Yamakawa T, Hashimoto S, Sarr P 2013: Phylogenetic diversity of indigenous soybean bradyrhizobia from different agro-climatic regions in Myanmar. ScienceAsia., 39, 574–583. doi:10.2306/scienceasia1513-1874.2013.39.574
- Tang S 1979: Study on the nodulation and nitrogen fixation of soybean in lessive soils. Acta Pediologica Sinica., 16, 9–16.
- Van K, Kim MY, Lee SH 2007: Genomic of root nodulation. Genomic-Assisted Crop Improvement., 2, 435–452.
- Vest G, Caldwell BE 1972: Rj4-A gene conditioning ineffective nodulation in soybean. Crop Sci., 12, 692–693. doi:10.2135/cropsci1972.0011183X001200050042x
- Vincent JM 1970: A Manual for the Practical Study of Root Nodule Bacteria, IBP Handbbok No.15 Blackwell Scientific Publications, Oxford, UK.
- Weaver RW, Frederick LR 1974: Effect of inoculum rate on competitive nodulation of Glycine max L. Merrill. II. field studies. Agronomy J., 66, 233–236. doi:10.2134/agronj1974.00021962006600020015x
- Yamakawa T, Fukushima Y 2014: Low inoculum densities of Bradyrhizobium japonicum USDA110 is effective on production of soybean (Glycine max L. Merr.) Cultivar Fukuyutaka. J. Fac. Agr. Kyushu Univ., 59, (1) 45–53.
- Yamakawa T, Hussain AKMA, Ishizuka J 2003: Soybean preference for Bradyrhizobium japonicum for nodulation: occupation of serogroup USDA110 in nodules of soybean plants harboring various Rj-genes grown in a field. Soil Sci. Plant Nutr., 49, 835–841. doi:10.1080/00380768.2003.10410345
- Young EG, Conway CF 1942: On the estimation of allantoin by the Rimini-Schryver reaction. J. Biol. Chem., 142, 839–853.
