ABSTRACT
To examine the exchangeability of 137Cs and K in soils of agricultural fields after decontamination, 173 soil samples were collected in 2016 from the plowed layer (0–15 cm depth) of decontaminated agricultural fields in Tomioka town, Fukushima prefecture, Japan. We investigated total 137Cs, exchangeable 137Cs, exchangeable K, and nonexchangeable K content for these soils. The total 137Cs content in soils was, on average, 1,200 Bq kg−1 (value range: 20–4,400 Bq kg−1), which was an 80% decrease from the values determined before the decontamination. This result indicated that the decontamination process considerably reduced the total 137Cs content in the plowed layer. The exchangeable K content in soils was on average 172 mg kg−1 (value range: 27.9 – 743 mg kg−1). More than 80% of soils showed the exchangeable K content lower than the recommended threshold value for paddy soils needed in order to reduce the 137Cs transfer from soil to plant (i.e. 200 mg kg−1). The nonexchangeable K content showed a wide range of values (value range: 98.8–1,280 mg kg−1) and no significant correlation with the exchangeable K content. Because nonexchangeable K is released more slowly but is comparable to exchangeable K as a reservoir of phytoavailable K, more frequent K application is recommended for soils with lower contents of both exchangeable and nonexchangeable K. The total 137Cs content in soils showed a significant positive correlation with the exchangeable K content in soils (p < 0.01), which means that decontamination efforts intended to reduce the total 137Cs content in soils might also lead to a reduction in the exchangeable K content. In conclusion, the application of K fertilizer is strongly recommended in the decontaminated agricultural fields because the decontamination causes a considerable decrease in exchangeable K content along with a reduction in total 137Cs content.
1. Introduction
The Great East Japan Earthquake and the subsequent tsunami on 11 March 2011 caused TEPCO’s (Tokyo Electric Power Company Holdings, Inc.) Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. Radioactive fission products, including 131I, 134Cs, and 137Cs, were released into the environment (Evangeliou et al. Citation2013), and soils over a wide area of Fukushima and the neighboring prefectures were contaminated by these radionuclides (Christoudias and Lelieveld Citation2013). Of all these radionuclides, 137Cs has been a major concern in terms of contamination even though seven years have passed since the 2011 accident, because the amount released was very large (1.3 × 1016 Bq) (Chino et al. Citation2011) and it has a longer half-life (30.2 y) than 131I (8.04 d) or 134Cs (2.06 y). Because the maximum standard limit of radiocesium in food was set to 100 Bq kg−1 (Ministry of Health, Labor and Welfare Citation2012), it was necessary to reduce radiocesium in the food produced from these contaminated agricultural fields. Hence, by 2018, as much as 2.4 × 104 km2 in Fukushima and the neighboring prefectures had been decontaminated to reduce the total radiocesium in agricultural soils derived from the accident, although the decontaminated area did not include the ‘difficult-to-return’ zones (Ministry of the Environment Citation2018).
After the FDNPP accident, Tomioka town, located approximately 10 km south of FDNPP, was designated as a residence restriction area. The evacuation order for this town was lifted on 1 April 2017, except for the difficult-to-return zone in the northeastern part of town. The decontamination of agricultural fields in this town involved the following steps: (1) removing the contaminated surface soil layer (0–5 cm depth); (2) dressing typically with uncontaminated granitic sand (0–5 cm thickness) in place of the removed soil; and (3) mixing the fresh soil with the subsurface soil (5–15 cm depth). After the decontamination, the total radiocesium (137Cs + 134Cs) content in the plowed layer (0–15 cm depth) had been reduced from 9,100 Bq kg−1 to 1,260 Bq kg−1 in a representative sample of the fields (n = 17) (Tomioka town Citation2016). The lower 137Cs content in soils directly contributes to a smaller external radiation exposure during agricultural activity in the fields (Satoh et al. Citation2014), whereas it does not necessarily reduce the 137Cs transfer from soil to plant (Saito et al. Citation2012) if the soils have high exchangeability of 137Cs or low exchangeability of K. The 137Cs transfer becomes smaller if the soils have a lower exchangeable 137Cs content (Kondo et al. Citation2015); however, the 137Cs transfer is also reduced under conditions of higher phytoavailable K content (Eguchi et al. Citation2015; Kato et al. Citation2015; Zhu and Smolders Citation2000). Phytoavailable K can be separated into exchangeable K (Ex-K) and nonexchangeable K (Nex-K) (Kitagawa et al. Citation2018). Ex-K, electrostatically adsorbed on the surface of minerals and organic matter, is readily available for uptake by plants. Although Nex-K is released more slowly and retained more strongly by minerals such as 2:1 phyllosilicates, it is also an important reservoir of phytoavailable K (Moritsuka et al. Citation2004). The uptake of radiocesium by plants was controlled by these phytoavailable K fractions, especially by the Ex-K content in soils. In spite of their importance, no previous study has elucidated this exchangeability of 137Cs and K, or the levels of their exchangeable fractions, over a wide area of decontaminated agricultural fields.
The objective of this study was, therefore, to investigate the exchangeability of 137Cs and K and to discuss the relationship between these exchangeabilities and other soil properties for soils over a wide area of the decontaminated agricultural fields in Fukushima.
2. Materials and methods
2.1. Soil samples
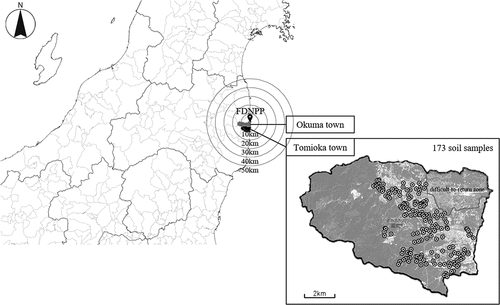
In total, 173 soil samples were collected on November 8–10, 2016 from the plowed layer (0–15 cm depth) of 173 agricultural fields distributed widely in Tomioka town, Fukushima prefecture, Japan (N37°20′, E141°00′) (). No fertilizer had been applied after decontamination when we collected the soil samples, i.e. the soil samples were in the post-decontamination state just after decontamination. The soils investigated were classified into five soil groups based on the classification of original soils described in the Soil-Inventory (National Agricultural and Food Research Organization): Muck soils, Andosols (Wet Andosols and Andosols), Lowland soils (Gray Lowland soils and Brown Lowland soils), Yellow soils, and Brown Forest soils (Cultivated soil classification committee Citation1995). The number of samples for Muck soils, Andosols, Lowland soils, Yellow soils, and Brown Forest soils was 22, 57, 64, 11, and 7, respectively, with an additional 12 unclassified samples. In each field, subsamples of soil were collected from five points (at the center and the four vertices of a square with a side length of 15 m) and mixed together. The hourly air dose rate (µSv h−1) at a height of 1 m was also determined at the center of each field using a gamma-ray radiation detector (Dose e, Fuji Electric Co., Ltd., Japan). Three extraordinarily high air dose rates were detected in those fields with solar panel installations; these values of air dose rates were not used in this study owing to possibly unreliable measurements of the air dose rates due to electromagnetic malfunction. Soil samples were dried at 50°C for more than two days and then sieved to less than 2 mm before analysis.
Figure 1. Locations of the sampling sites in Tomioka town, Fukushima prefecture, Japan. The pin indicates the location of the Fukushima Daiichi Nuclear Power Plant (FDNPP) in Okuma town. This map is based on the blank map and aerial photograph published by Geospatial Information Authority of Japan (https://maps.gsi.go.jp/development/ichiran.html)
2.2. Determination of total and exchangeable 137Cs content
A 20 cm3 volume of soil sample corresponding to approximately 20–40 g was filled in a polyethylene vial (20 cm3). The vial was set in an auto gamma counter with a NaI (Tl) detector (2480WIZARD2, PerkinElmer, Waltham, Massachusetts, USA, Radioisotope Research Center of Kyoto Prefectural University) to determine the gamma activity (between 589 and 735 keV) in soils. The counting time was set to 1,800–7,200 s to decrease the statistical counting error to less than 1.7%. One of these 173 soil samples showed a total 137Cs content that was less than the detection limit. As the resolution of the NaI detector is not high enough to differentiate the gamma ray of 134Cs from that of 137Cs, the results were calibrated with a germanium (Ge) detector. More specifically, approximately 60–80 g portions of four representative soil samples were filled into styrene U-8 vessels (100 mL), and then their respective gamma activities from 137Cs and 134Cs were analyzed using a Ge semiconductor detector connected to a multichannel analyzer system (GC-4018, Canberra, Japan, Radioisotope Research Center of Fukushima University). The counting time was set to 3,845–5,004 s to decrease the statistical counting error to less than 0.3%. To elucidate the mobility of 137Cs with relatively high levels of contamination, the exchangeable 137Cs content in soils with a total 137Cs content of higher than 2,000 Bq kg−1 was determined for 32 out of the 173 soil samples (). Although sample extraction in this way may cause some statistical bias in the following results, soils with a total 137Cs content of lower than 2,000 Bq kg−1 was removed in this experiment to ensure data reliability. Duplicates of 50 g of soil sample were shaken with 250 mL of 1 mol L−1 ammonium acetate (CH3COONH4) for 24 h. The suspension was centrifuged, and the supernatant was collected by filtration. The duplicate supernatant solutions were combined into a 500 mL beaker after filtration through a 0.45 µm Millipore filter and heated gently until the volume of the supernatant decreased to approximately 20 mL. To determine the exchangeable radiocesium (137Cs + 134Cs) content by counting the gamma activity between 589 and 735 keV, approximately 20 mL of the concentrated supernatant in a polyethylene vial (20 mL) was analyzed using the auto gamma counter. The counting time was set to 1,800 s to decrease the statistical counting error to less than 2%. The activity ratio of 137Cs/134Cs emitted from FDNPP in March 2011 was reported to be approximately 1:1 (Komori et al. Citation2013). Considering the difference in their half-lives, this ratio was expected to be approximately 8:1 when we measured the exchangeable 137Cs content in November 2017. The 137Cs desorption ratio (%), i.e. the percentage of exchangeable 137Cs content in the total 137Cs content, was calculated by the following equation: [137Cs desorption ratio (%)] = [exchangeable 137Cs content in soils (Bq kg−1)]/[total 137Cs content in soils (Bq kg−1)] × 100. Although Nex-137Cs is as phytoextractable as Nex-K, we considered that it is much less phytoavailable due to high competition with K+ at the ionic channel on plant root.
Table 1. Selected physicochemical properties of the soils
2.3. Determination of phytoavailable K content
The Ex-K content in soils was determined using the 1 mol L−1 CH3COONH4 extraction method (Thomas Citation1982). More specifically, 5.0 g of the soil sample was shaken with 25 mL of 1 mol L−1 CH3COONH4 for 30 min. The suspension was centrifuged, and the supernatant was collected by filtration. These processes were repeated three times. The approximately 75 mL of supernatant was brought up to a total volume of 100 mL with 1 mol L−1 CH3COONH4, and the K content of this solution was determined by atomic absorption spectroscopy (AAS) (AA-6200, Shimadzu, Kyoto, Japan).
The Nex-K content in soils was determined using the 1 mol L−1 hot nitric acid (HNO3) extraction method (Helmke and Sparks Citation1996). More specifically, 2.5 g of the soil sample was heated gently with 25 mL of 1 mol L−1 HNO3 for 15 min after boiling began. After cooling for 5 min, the sample was filtered, and the extract volume was brought up to 100 mL with 0.1 mol L−1 HNO3. The K content of the extract solution was determined by AAS. As the K in the extract includes both Ex-K and Nex-K, the amount of Nex-K was calculated by subtracting the amount of Ex-K from the hot HNO3 extractable K.
2.4. Determination of selected physicochemical properties
Selected physicochemical properties of soils with a total 137Cs content of higher than 2,000 Bq kg−1 were determined for 32 out of 173 soil samples () to elucidate the mobility of 137Cs with relatively high levels of contamination. Oxalate acid extractable aluminum (Alo) and iron (Feo) (McKeague and Day Citation1966) were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) (iCAP 4400 Duo Full MFC, Thermo Fisher Scientific, Waltham, USA) after extraction with 0.2 mol L−1 acid ammonium oxalate solution (pH 3) for 4 h in darkness followed by filtration through a 0.45 µm Millipore filter. The total carbon (C) content in soils, as an indicator of total organic matter content, was determined by the dry combustion method with an NC analyzer (NC-95A, Smika Chem. Anal. Service, Tokyo, Japan) and gas chromatography (GC-8A, Shimadzu, Kyoto, Japan) after fine grinding. The sand (2 mm to 20 µm), silt (20–2 µm), and clay (<2 µm) contents were determined by sieving and pipetting methods after organic matter removal by oxidation with hydrogen peroxide and ultrasonic dispersion.
3. Results and discussion
3.1. Changes in total 137Cs content and air dose rate after decontamination
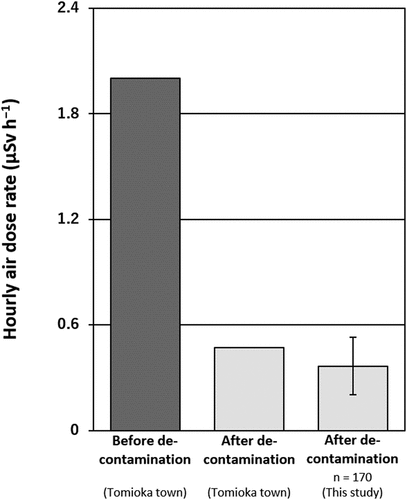
According to Tomioka town (Tomioka town 2016), the total radiocesium (137Cs + 134Cs) content for local agricultural soils before soil decontamination was reported to be, on average, 9,100 Bq kg−1 (collected in May 2014). Rate of 137Cs content in this value can be calculated to be 6,700 Bq kg−1 since activity ratio of 137Cs/134Cs was reported to be approximately 1:1 just after the FDNPP accident (Komori et al. Citation2013). Furthermore, the activity concentration of 137Cs during our sampling period was calculated to be 6,000 Bq kg−1, based on the effective half-life of 137Cs in plowed layer (16.9 y) (Komamura et al. Citation1999). In addition, the hourly air dose rate in the agricultural fields in Tomioka town before decontamination was, on average, 2.00 µSv h−1 (Tomioka town 2016).
After decontamination, our results showed that the total 137Cs content in the soils ranged from 20 to 4,400 Bq kg−1, with median and arithmetic mean (±standard deviation) values of 940 and 1,200 ± 1,000 Bq kg−1, respectively. Total 137Cs content in the decontaminated soils may vary depending on both the difference in the amount of removed and dressed soils among fields and the original contamination. We considered the former is more important because of the significant positive correlation between the Ex-K content and the total 137Cs content in soils (). The hourly air dose rate at a height of 1 m ranged from 0.10 to 0.94 µSv h−1, with median and arithmetic mean (±standard deviation) value of 0.34 and 0.36 ± 0.16 µSv h−1, respectively. Both an average value of the total 137Cs content in soils () and the hourly air dose rate () were about 80% smaller than those surveyed before the decontamination (Tomioka town 2016), whereas very similar to those surveyed after decontamination (Tomioka town 2016). The reductions were much larger than those caused only by the natural decay of radiocesium (total 137Cs content: 10% decrease). These results strongly support the effectiveness of decontamination for the decrease of the total 137Cs content in soils and the hourly air dose rate in Fukushima.
Figure 2. Boxplots of the distribution of the total 137Cs content in soils before and after decontamination. The quartiles and median value are shown as the boxes and the maximum and minimum values are shown as the whiskers. The total 137Cs content (collected in May 2014 and February 2016) before and after decontamination are from Tomioka town (2016) and corrected for decay from the sampling date
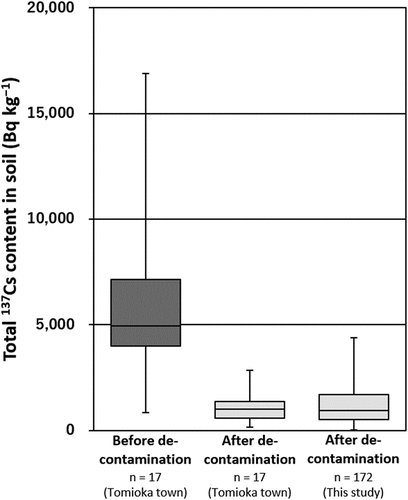
Figure 3. Comparison of the hourly air dose rate at a height of 1 m before and after decontamination (bars indicate standard deviation). The dose rate (measured from 30 July 2013 to 9 May 2016) before and after decontamination are from Tomioka town (2016)
The total 137Cs content in soils showed a significant positive correlation with the hourly air dose rate (p < 0.01) (). Fields with higher air dose rates than expected from the regression line were those located close to the border of forests or difficult-to-return zone where the decontamination (including removal of the surface soil layer) had been less thorough. Thus, the gamma radiation from these areas may have increased the air dose rate in the fields. On the contrary, a lower air dose rate than expected from the regression line was detected in a few of the fields, which is probably because of insufficient plowing after the fields had been covered with dressed soils. Except for these specific situations, the air dose rate in the agricultural fields was found to be mainly controlled by the total 137Cs content in the surface soil layer, in addition to a certain background level. Kohyama et al. (Citation2013) also showed a similar positive relationship between the total 137Cs content in soils and the hourly air dose rate in fields plowed after the FDNPP accident even across different conditions of sampling date and decontamination.
Figure 4. Relationship between the total 137Cs content in soils and the hourly air dose rate at a height of 1 m (n = 170) (** indicates P ≤ 0.01). The darker plots indicate fields located close to the border of forests or difficult-to-return zone
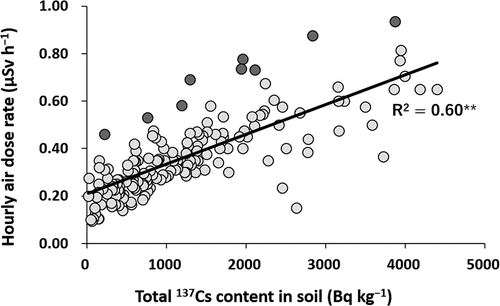
Assuming that one spends 8 h outside and 16 h at home (Ministry of the Environment Citation2011), the average hourly air dose rate in Tomioka town, i.e. 0.36 µSv h−1, corresponds to an annual additional exposure dose of 1.9 mSv y−1. In Japan, 10 mSv y−1 is the criterion for lifting the evacuation order, and 1 mSv y−1 is the criterion for designating zones for which a decontamination plan is to be formulated. To achieve the latter criterion, therefore, agricultural fields with a high air dose rate that were often located close to the border of forests or difficult-to-return zones should be carefully managed.
3.2. Evaluation of phytoavailable K
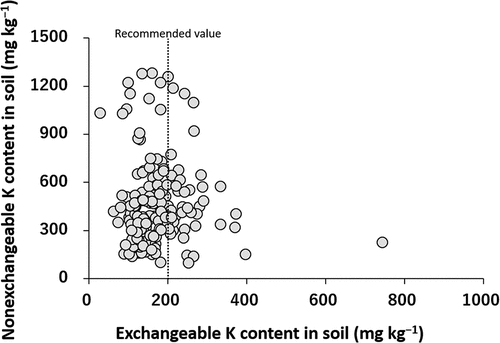
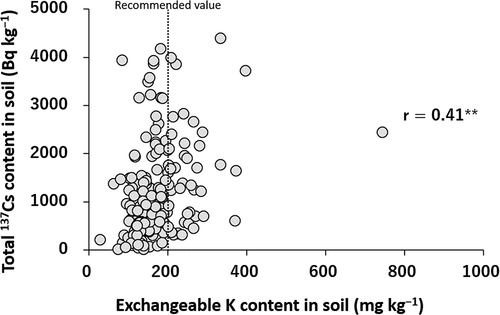
The Ex-K content in soils ranged from 27.9 to 743 mg kg−1, with median and arithmetic mean (±standard deviation) values of 159 and 172 ± 74.2 mg kg−1, respectively. More than 80% of the soil samples showed an Ex-K content of less than 200 mg kg−1, i.e. less than the recommended threshold value needed to reduce 137Cs transfer from soil to plant (Kato et al. Citation2015). The Ex-K content was positively correlated with the total 137Cs content in soils (p < 0.01) (). This correlation was not expected because their origins and adsorption sites were supposed to be largely different. Ex-K in agricultural soils mainly originated from K input as fertilizer before the FDNPP accident, which would probably be weakly adsorbed on the surface of minerals and organic matter in an exchangeable form (Helmke and Sparks Citation1996). 137Cs in agricultural soils in Fukushima was mainly derived from the FDNPP accident and was strongly bound partly in the interlayer of micaceous minerals. This correlation suggests that the mixing of dressed soils in the decontamination processes reduced not only the total 137Cs content in soils but also the Ex-K content, possibly because of the lower contents of 137Cs and Ex-K in the dressed soils than in the surface soil layer. Therefore, fertilization with K after decontamination to improve the level of Ex-K is strongly recommended.
Figure 5. Relationship between the exchangeable K content in soils and the total 137Cs content in soils (n = 172) (** indicates P ≤ 0.01)
The Nex-K content in soils ranged from 98.8 to 1,280 mg kg−1, with median and arithmetic mean (±standard deviation) values of 391 and 460 ± 266 mg kg−1, respectively. The Nex-K content showed no significant correlation with the Ex-K content (). Because the Nex-K content showed a wide range of values, the potential to reserve Ex-K varied widely among the soils. This means that the determination of not only Ex-K but also Nex-K is important in efforts to design appropriate countermeasures to reduce the 137Cs transfer. Further studies will be required to determine the recommended threshold value of Nex-K content in soils needed in order to reduce the transfer of 137Cs from soil to plant. Kitagawa et al. (Citation2018) showed that Andosols and Wet Andosols in agricultural fields in Japan tended to have lower Nex-K contents (110 and 132 mg kg−1, respectively) than the other soil types. However, the Nex-K content of Andosols in this study was, on average, 536 mg kg−1, which was much higher than that of the previous study. Dressed soils may be rich in K-bearing minerals such as micaceous minerals, which would account for the increased Nex-K content. Mixing with dressed soil may have changed other properties of the surface soils as well. As shown in , none of the soils that have been classified as Andosols satisfy the diagnostic criterion of andic properties (i.e. Alo+1/2Feo contents > 20 g kg−1) (Soil Survey Staff Citation2014). This discrepancy is also caused by the dilution effect of dressed soils on surface soils.
3.3. Factors influencing the 137Cs desorption ratio
The exchangeable 137Cs content in soils ranged from 40 to 520 Bq kg−1, with median and arithmetic mean (±standard deviation) values of 200 and 230 ± 140 Bq kg−1, respectively. The 137Cs desorption ratio ranged from 1.0% to 22%, with median and arithmetic mean (±standard deviation) values of 6.0% and 8.1% ± 5.3%, respectively. The low 137Cs desorption ratio indicated that most of the 137Cs was strongly adsorbed in the soils and was not readily available for uptake by plants. These values are similar to but lower than those reported for soils investigated soon after the accident in Fukushima (Saito et al. Citation2014). This difference in the desorption ratio corresponded reasonably well to the research that showed the desorption ratio of the added 137Cs to decrease with time (Takeda et al. Citation2013).
A Spearman correlation analysis indicated that the 137Cs desorption ratio was positively correlated with the exchangeable 137Cs content (p < 0.01) and negatively correlated with the Nex-K content (p < 0.05), whereas it was not significantly correlated with the total C content or the Alo + 1/2Feo content (). The positive correlation with the exchangeable 137Cs content indicates that the variability in the 137Cs desorption ratio was mainly controlled by the variability in the exchangeable 137Cs content rather than the total 137Cs content. Therefore, the exchangeable 137Cs content, as the phytoavailable fraction, may be more useful in the estimation of 137Cs transfer from soil to plant. The negative correlation with the Nex-K content (p < 0.05) may be an indirect relationship; both a lower desorption ratio of 137Cs and a higher amount of K release from the nonexchangeable fraction may be directly related to the abundance of weathered micaceous minerals, because the interlayer of these minerals can be both a fixation site for 137Cs (Saito et al. Citation2014; Nakao et al. Citation2014) and a reservoir of Nex-K (Fanning et al. Citation1989). Therefore, nonexchangeable K content can be a rough index to estimate the immobility or non-exchangeability of 137Cs. However, the lack of any significant correlations of the 137Cs desorption ratio with the total C content or the Alo + 1/2Feo content was unexpected. Yamaguchi et al. (Citation2017) and Nakao et al. (Citation2015a) reported that the radiocesium interception potential (RIP), as an indicator of the capacity and selectivity for the sorption of radiocesium by soil (Cremers et al. Citation1988), showed a significant negative correlation with the total C content and andic soil properties (e.g. phosphate absorption capacity). These correlations would occur because the organic matter coating on micaceous minerals may inhibit 137Cs sorption (Tashiro et al. Citation2018), and Andosols tend to have lesser amounts of micaceous minerals in comparison to the large content of non-crystalline minerals (Nakao et al. Citation2015b). The factors controlling 137Cs desorption may be different from those controlling its selective adsorption (Vandebroek et al. Citation2012). Another possible reason is that the inclusion of dressed soils on surface soils largely modified soil characteristics. Further investigation into the relationship between RIP and 137Cs desorption ratio will provide some important information for designing appropriate countermeasures to reduce the 137Cs transfer from soil to plant.
Table 2. Correlation coefficients between the 137Cs desorption ratio and selected physicochemical properties of the soils
4. Conclusions
We investigated exchangeability of 137Cs and K, which will affect 137Cs transfer from soil to plant, i.e. total 137Cs, exchangeable 137Cs, exchangeable K, and nonexchangeable K content, for the soils collected from 173 decontaminated fields in Tomioka town in the eastern coastal area of Fukushima. We found that the decontamination largely reduced the exchangeable K content in soils as well as the total 137Cs content in soils with low 137Cs desorption ratios. In fact, more than 80% of the soil samples showed an exchangeable K content less than the recommended threshold value needed to reduce 137Cs transfer from soil to plant (200 mg kg−1). We also found that nonexchangeable K content can be a rough index to estimate the immobility of 137Cs. In conclusion, K fertilization of decontaminated surface soils is strongly recommended, taking account of both exchangeable K and nonexchangeable K contents, because the decontamination caused a considerable decrease in the exchangeable K content.
Acknowledgments
The experiment on measurement of 137Cs content was carried out in the Laboratory of Radioisotopes of Kyoto Prefectural University and Fukushima University. This work was financially supported by the JSPS KAKENHI (grant number 16H06188) and research grant from Tomioka town. We would like to thank Mr. Sho Ogasawara, Ms. Shiori Uno, Ms. Yuka Kitagawa, Mr. Ryo Ito, Ms. Sumika Kataoka, Ms. Mai Terashima, Ms. Mayu Tomita, Ms. Yuki Matsuyama, Ms. Honoka Ota, Mr. Rikuo Kitayama, Mr. Yuki Tanaka, Ms. Mina Hirose, Ms. Natsumi Noguchi, Ms. Sae Ito, Ms. Yurie Nishii, Ms. Fukiko Masai, and Ms. Nanami Murashita of Laboratory of Soil Chemistry, Kyoto Prefectural University for collecting soil samples and sample pretreatments.
Additional information
Funding
References
- Chino M, Nakayama H, Nagai H, Terada H, Katata G, Yamazawa H 2011: Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi nuclear power plant into the atmosphere. J. Nucl. Sci. Technol., 48, 1129–1134. doi:10.1080/18811248.2011.9711799
- Christoudias T, Lelieveld J 2013: Modelling the global atmospheric transport and deposition of radionuclides from the Fukushima Dai-Ichi nuclear accident. Atmos. Chem. Phys., 13, 1425–1438. doi:10.5194/acp-13-1425-2013
- Cremers A, Elsen A, Depreter P, Maes A 1988: Quantitative analysis of radiocaesium retention in soils. Nature, 335, 247–249. doi:10.1038/335247a0
- Cultivated soil classification committee 1995: Classification of Cultivated Soils in Japan (3rd Approximation). Misc. Publ. Natl. Inst. Agro-Environ. Sci., 17, 1–75.
- Eguchi T, Ohta T, Ishikawa T, Matsunami H, Takahashi Y, Kubo K, Yamaguchi N, Kihou N, Shinano T 2015: Influence of the nonexchangeable potassium of mica on radiocesium uptake by paddy rice. J. Environ. Radioact., 147, 33–42. doi:10.1016/j.jenvrad.2015.05.002
- Evangeliou N, Balkanski Y, Cozic MAP 2013: Global transport and deposition of 137Cs following the Fukushima nuclear power plant accident in Japan: emphasis on Europe and Asia using high–resolution model versions and radiological impact assessment of the human population and the environment using interactive tools. Environ. Sci. Technol., 47, 5803–5812. doi:10.1021/es400372u
- Fanning D, Keramidas V, El-Desoky M 1989: Micas. In Minerals in Soil Environments, Ed. Dixon JB, Weed SB, pp. 551–634. Soil Science Society of America, Madison.
- Helmke PA, Sparks DL 1996: Potassium, Rubidium, and Cesium. Methods of soil analysis part 3. Chemical methods, pp. 559–562. SSSA Book Series. no. 5, Madison, WI.
- Kato N, Kihou N, Fujimura S, Ikeba M, Miyazaki N, Saito Y, Eguchi T, Itoh S 2015: Potassium fertilizer and other materials as countermeasures to reduce radiocesium levels in rice: results of urgent experiments in 2011 responding to the Fukushima Daiichi nuclear power plant accident. Soil Sci. Plant Nutr., 61, 179–190. doi:10.1080/00380768.2014.995584
- Kitagawa Y, Yanai J, Nakao A 2018: Evaluation of nonexchangeable potassium content of agricultural soils in Japan by the boiling HNO3 extraction method in comparison with exchangeable potassium. Soil Sci. Plant Nutr., 64, 116–122. doi:10.1080/00380768.2017.1411168
- Kohyama K, Takata Y, Obara H, Taniyama I, Saito T 2013: A distribution map of radio cesium concentration in farmland soils. Inventry, 11, 2–9.
- Komamura M, Tsumura A, Kodaira K 1999: Residence half-time of 137Cs in Japanese paddy top-soils. Radioisotopes, 48, 635–644. doi:10.3769/radioisotopes.48.635
- Komori M, Shozugawa K, Nogawa N, Matsuo M 2013: Evaluation of radioactive contamination caused by each plant of Fukushima Daiichi nuclear power station using 134Cs/137Cs activity ratio as an index. Bunseki Kagaku, 62, 475–483. doi:10.2116/bunsekikagaku.62.475
- Kondo M, Maeda H, Goto A et al. 2015: Exchangeable Cs/K ratio in soil is an index to estimate accumulation of radioactive and stable Cs in rice plant. Soil Sci. Plant Nutr., 61, 133–143. doi:10.1080/00380768.2014.973347
- McKeague JA, Day JH 1966: Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci., 46, 13–22. doi:10.4141/cjss66-003
- Ministry of Health, Labor and Welfare 2012: New maximum standard limit of radiocesium in food. https://www.mhlw.go.jp/shinsai_jouhou/dl/leaflet_120329.pdf (December, 2018, in Japanese).
- Ministry of the Environment 2011: The concept of annual additional exposure dose. https://www.env.go.jp/press/files/jp/18437.pdf (December, 2018, in Japanese).
- Ministry of the Environment 2018: Environmental Remediation in Japan. http://josen.env.go.jp/en/pdf/progressseet_progress_on_cleanup_efforts.pdf (December, 2018)
- Moritsuka N, Yanai J, Kosaki T 2004: Possible processes releasing nonexchangeable potassium from the rhizosphere of maize. Plant Soil, 258, 261–268. doi:10.1023/B:PLSO.0000016556.79278.7f
- Nakao A, Ogasawara S, Sano O, Ito T, Yanai J 2014: Radiocesium sorption in relation to clay mineralogy of paddy soils in Fukushima, Japan. Sci. Total Environ., 468–469, 523–529. doi:10.1016/j.scitotenv.2013.08.062
- Nakao A, Takeda A, Ogasawara S, Yanai J, Sano O, Ito T 2015a: Relationships between paddy soil radiocesium interception potentials and physicochemical properties in Fukushima, Japan. J. Environ. Qual., 44, 780–788. doi:10.2134/jeq2014.10.0423
- Nakao A, Nakao A, Tanaka R, Ogasawara S, Yanai J 2015b: Aeolian-dust-derived micaceous minerals control radiocesium retention in andosols in Japan. Soil Sci. Soc. Am. J., 79, 1590–1600. doi:10.2136/sssaj2015.05.0173
- National Agricultural and Food Research Organization: Soil-Inventory. https://soil-inventory.dc.affrc.go.jp/index.html (December, 2018, in Japanese).
- Saito T, Makino H, Tanaka S 2014: Geochemical and grain-size distribution of radioactive and stable cesium in Fukushima soils: implications for their ong-term behavior. J. Environ. Radioact., 138, 11–18. doi:10.1016/j.jenvrad.2014.07.025
- Saito T, Ohkoshi S, Fujimura S, Iwabuchi K, Saito M, Nemoto T, Sato M, Sato M, Yoshioka K, Tsukada H 2012: Effect of potassium application on root uptake of radiocesium in rice. KUR Res. Program Sci. Basis Nucl. Saf., 165–169. doi:10.1007/s10967-014-3609-9
- Satoh D, Furuta T, Takahashi F, Endo A, Lee C, Bolch WE 2014: Calculation of dose conversion coefficients for external exposure to radioactive cesium distributed in soil. JAEA-Research, 2014–017. doi:10.11484/jaea-research-2014-017
- Soil Survey Staff 2014: Keys to Soil Taxonomy (12th Ed.), Washington, DC: United Departmant of Agriculture & Natural Resorces Conservation Service. pp. 1–360.
- Takeda A, Tsukada H, Nakao A, Takaku Y, Hisamatsu S 2013: Time-dependent changes of phytoavailability of Cs added to allophanic Andosols in laboratory cultivations and extraction tests. J. Environ. Radioact., 122, 29–36. doi:10.1016/j.jenvrad.2013.02.005
- Tashiro Y, Nakao A, Wagai R, Yanai J, Kosaki T 2018: Inhibition of radiocesium adsorption on 2:1 clay minerals under acidic soil environment: effect of organic matter vs. hydroxy aluminum polymer. Geoderma, 319, 52–60. doi:10.1016/j.geoderma.2017.12.039
- Thomas GW 1982: Exchangeable cations. Methods of soil analysis part 2. Chemical and microbiological properties, pp. 159–165. SSSA Book series. no. 9, Madison, WI.
- Tomioka town 2016: Decontamination report in Tomioka town. http://www.tomioka-town.jp/living/Files/2016/10/07/report.pdf (December, 2017, in Japanese).
- Vandebroek L, Van HM, Delvaux B, Spaargaren O, Thiry Y 2012: Relevance of radiocaesium interception potential (RIP) on a worldwide scale to wssess soil vulnerability to 137Cs contamination. J. Environ. Radioact., 104, 87–93. doi:10.1016/j.jenvrad.2011.09.002
- Yamaguchi N, Tsukada H, Kohyama K, Takata Y, Takeda A, Isono S, Taniyama I 2017: Radiocesium interception potential of agricultural soils in northeast Japan. Soil Sci. Plant Nutr., 63, 119–126. doi:10.1080/00380768.2017.1294467
- Zhu YG, Smolders E 2000: Plant uptake of radiocaesium: a review of mechanisms, regulation and application. J. Exp. Bot., 51, 1635–1645. doi:10.1093/jexbot/51.351.1635