ABSTRACT
In Lotus japonicus, quantitative trait loci (QTLs) for nitrogen fixation activity (acetylene reduction activity, ARA) and seed weight are located in the same region on chromosome 4 as the gene responsible for the Fix− mutant sen1. The SEN1 gene of L. japonicus Gifu B129 has a deletion of three nucleotides in comparison with that of L. japonicus Miyakojima MG20, which results in a deletion of one amino acid. Here, we examined whether SEN1 is responsible for these QTLs. Because the MG20 allele of the QTL for ARA and seed weight has a positive effect, we generated a near-isogenic line (NIL) in which the SEN1 gene region of B129 was replaced with that of MG20. Growth parameters of this NIL (MG20-type) 21 days after inoculation with Mesorhizobium loti were significantly higher than those of B129-type, and ARA was also significantly higher. Nodules formed on hairy roots in which the sen1 mutant was complemented with the MG20 SEN1 gene had higher ARA than those of B129. The seeds of the MG20-type NIL were significantly heavier than those of B129-type. These results indicate that the SEN1 gene is a very likely candidate for the QTL for ARA and seed weight on chromosome 4.
1. Introduction
Many legumes form root nodules on the roots upon infection with soil bacteria called rhizobia. In the root nodules, rhizobia convert atmospheric nitrogen into ammonia using the nitrogenase system, and the fixed nitrogen is supplied to the host plants. Host legumes establish this symbiotic relationship by supplying assimilation products produced by photosynthesis to the rhizobia as a carbon source. Owing to these symbioses, legumes are generally thought to be able to grow on poor soils. In soybean (Glycine max), about 60–75% of grain nitrogen is reportedly derived from nitrogen fixed by rhizobia (Ohyama et al. Citation1992). Therefore, symbiotic nitrogen fixation has a great effect not only on plant growth but also on crop production.
To identify loci involved in symbiotic nitrogen fixation, Tominaga et al. (Citation2012) analyzed QTLs for acetylene reduction activity (ARA) using recombinant inbred lines (RILs) generated from the Gifu B129 and Miyakojima MG20 accessions of Lotus japonicus, a model leguminous plant. QTLs with a positive effect derived from MG20 were detected on chromosomes 2–5. Among them, a QTL on chromosome 4 was located near marker TM0832. By QTL analysis using MG20 and B129 RILs, Klein and Grusak (Citation2009) detected QTLs related to seed mass, one of which is present near TM0832 (). The MG20 allele of this QTL has a positive effect (Klein and Grusak Citation2009).
Figure 1. Molecular linkage map of L. japonicus and QTLs (a) Partial molecular linkage map of L. japonicus chromosome 4. Circles containing arrowheads indicate the positions of previously reported QTLs with significant LOD scores: the size of each circle indicates the LOD score, and the direction of each arrow indicates the direction of effect of the B129 allele. SM1 (at 10.6 cM), SM2 (at 9.6 cM), ARA/P (at 9.6 cM) and ARA/NN (7.6 cM) indicate seed mass 1, seed mass 2, acetylene reduction activity per plant, and ARA per nodule number, respectively. (b) Molecular linkage map of L. japonicus and genotype of RI-088 and NIL. Genotype of the RI-088 is shown on the left of the linkage group and the genotype of NIL generated by the cross with RI-088 and B129 is shown on the right. Open circles, filled circles, and filled triangles indicate the genotype of the SSR marker at that position, respectively. Details of the SSR marker can be found on the HP of NBRP Lotus and Glycine
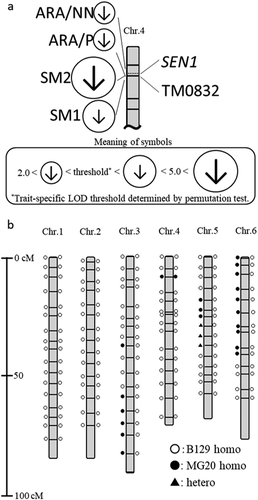
Lotus japonicus Fix− mutants such as sst1, fen1, ign1, and Ljsym105 form morphologically normal nodules with rhizobia but show lower nitrogen fixation activity than the wild type (Krusell et al. Citation2005; Hakoyama et al. Citation2009; Kumagai et al. Citation2007; Hossain, Umehara, and Kouchi Citation2006). The sen1 mutant sen1-1 is able to form nodules but has no nitrogen fixation activity and therefore grows remarkably poorly in N-free condition (Kawaguchi et al. Citation2002; Suganuma et al. Citation2003; Hakoyama et al. Citation2012). Interestingly, the gene responsible for the sen1 mutant is also located near TM0832 (). These facts suggest a hypothesis that the gene corresponding to the QTLs for ARA and seed mass is SEN1. In this study, we tested this hypothesis.
2. Materials and methods
2.1. Growth conditions
Seeds were surface sterilized by immersion in sodium hypochlorite [2% (v/v) containing 0.1% (v/v) Tween 20] for 20 min and rinsed several times with sterile distilled water. After imbibing water, the swollen seeds were sown on 1.2% (w/v) agar medium and the plates were incubated at 24°C in the dark for 3 days. Germinated seeds were transplanted to vermiculite-filled pots and watered with N-free B&D medium (Broughton and Dilworth Citation1971) containing 1.0 × 107 cells/mL of Mesorhizobium loti MAFF303099 (Keele, Hamilton, and Elkan Citation1969; Kaneko et al. Citation2000; Saeki and Kouchi Citation2000), which had been grown in yeast–mannitol liquid medium (Keele, Hamilton, and Elkan Citation1969). The plants were grown at 24°C under 16 h light/8 h dark at a light intensity of 150 µmol m−2 s−1.
2.2. Acetylene reduction assay for nitrogenase activity
Five plants inoculated with M. loti as described in the previous subsection were placed in a 34-mL test tube, which was then covered with a rubber serum cap and degassed for 30 s. Acetylene diluted five times was injected into the tube, which was then incubated in a growth chamber at 25°C for 1 h. The amount of ethylene formed was determined by gas chromatography (Suzuki et al. Citation2008; Tominaga et al. Citation2009).
2.3. Induction of hairy roots and nodulation experiment
Since there is no intron in SEN1 gene of L. japonicus, the coding region of SEN1 gene for B129 or MG20 were amplified by PCR with primers, 5ʹ-CACCATGGCTGCTGGTGCACCCAACCAT-3ʹ and 5ʹ-TTATATTTCCAACCCACCAGCACTT-3ʹusing genomic DNA as a template. Each amplified fragment was cloned into the pENTR/D-TOPO entry vector (Invitrogen, Carlsbad, CA, USA). The inserts of the entry clones were recloned into the pUBGWS-GFP vector (Maekawa et al. Citation2008), which was obtained from the National BioResource Project (NBRP) Lotus and Glycine (Japan), by LR reaction using the Gateway cloning system (Invitrogen). The resultant plasmid (EV, negative control) was introduced into Agrobacterium rhizogenes LBA1334 by electroporation. Seeds of the sen1 mutant were germinated for 2 days in the dark. Seedling hypocotyls were cut off, the cut surface was inoculated with A. rhizogenes, and shoots without roots were cocultured for 5 days in the dark. To induce hairy root formation, cocultured shoots were grown for 14 days (16 h light/8 h dark) on 1.5% B5 agar medium (Gamborg, Miller, and Ojima Citation1968). Plants with green fluorescent protein-positive hairy roots were selected under a fluorescence microscope (SZX9, Olympus, Tokyo, Japan). Selected transformants were inoculated with rhizobia (1.0 × 107 cells), grown on vermiculite, and watered with N-free B&D medium for 28 days (16 h light/8 h dark).
2.4. Preparation of a near-isogenic line by crossing RI-088 and B129
We selected RI-088 from the RILs published on the NBRP website (https://www.legumebase.brc.miyazaki-u.ac.jp/). Its genotype of TM0832 was MG20, but among the genotypes in other chromosome regions, RI-088 had the highest proportion of B129-type markers (). RI-088 and B129 were crossed, and BC3F1 was obtained by crossing B129 with its progeny three times. Through these crossings, MG20 homo regions on chromosomes 3, 5, and 6 were replaced by B129 homo. Eventually, BC3F2 plant possessing MG20-type or B129-type SEN1 gene was selected, then these plants were used as NILs. In this study, we used BC3F4, which was harvested in terms of around 6 months after seeding, for measuring growth parameters and seed weight.
3. Results
3.1. Generation of a near-isogenic line and its genotype
Because the QTLs for ARA per plant, ARA per nodule, and seed mass all have positive effects (Tominaga et al. Citation2012; Klein and Grusak Citation2009), the replacement of these alleles in B129 with those from MG20 should increase the values of these traits. Thus, we generated a near-isogenic line (NIL) by crossing RI-088 and B129, in which TM0832 was MG20 homozygous and all other SSR marker genotypes were B129-type ().
3.2. Growth and nitrogen fixation activity in NIL
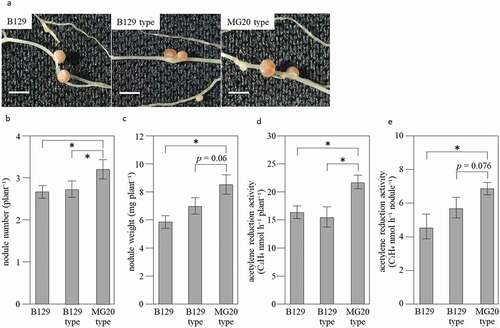
Typical B129, B129-type, and MG20-type plants 21 days after inoculation with M. loti are shown in . Shoots of MG20-type were significantly longer than those of B129 and B129-type (). Shoots of MG20-type were significantly heavier than those of B129 (). The length and weight of roots were significantly higher in MG20-type than in B129 and B129-type (). Typical nodules formed on the roots of B129, B129-type, and MG20-type plants are shown in . MG20-type formed more nodules than B129 and B129-type (). Nodules of MG20-type tended to be heavier than those of B129-type and were significantly heavier than those of B129 (). ARA per plant was significantly higher in MG20-type than in B129 and B129-type (). ARA per nodule in MG20 type tended to be higher than that in B129-type (p = 0.076) and was significantly higher than that in B129 (). Taken together, these results indicate that the SEN1 region of MG20 has a positive effect on nitrogen fixation activity.
Figure 2. Growth of NILs at 21 days after inoculation with M. loti. (a) Wild-type B129, NILs of B129 type and MG20-type plants. Bar = 1 cm. Arrow heads indicate nodules. (b-e) Shoot length (b), shoot weight (c), root length (d) and root weight (e). B129; n = 75, B129 type, MG20 type; n = 50. Error bars represent standard error (SE). *P < 0.05 by Student’s t-test
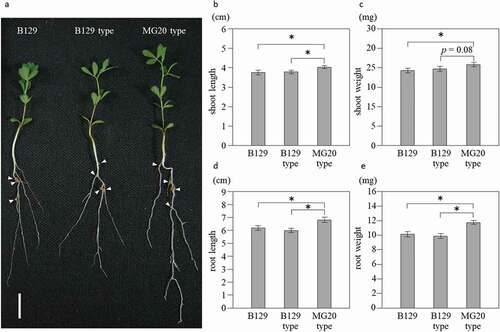
Figure 3. Nodules and ARA of NILs at 21 days after inoculation with M. loti. (a) Nodules formed on root of Wild-type B129, NILs of B129 type and MG20 type. Bar = 2 mm. (b-e) Nodule number per plant (b), Nodule weight per plant (c), ARA per plant (d) and ARA per nodule (e). B129; n = 15, B129 type, MG20 type; n = 10. Error bars represent standard error (SE). *P < 0.05 by Student’s t-test
3.3. Complementation of sen1-1 mutant by SEN1 gene
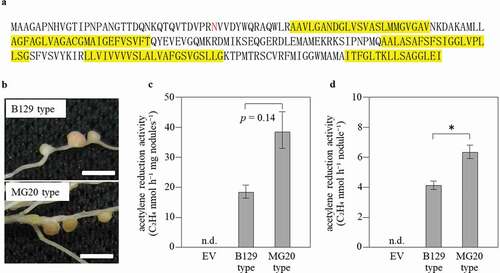
We reasoned that if the gene responsible for the QTL for nitrogen fixation activity is SEN1, there should be a difference between the SEN1 nucleotide sequences of B129 and MG20. So, the nucleotide sequence of MG20 SEN1 gene was determined and compared with that of B129 deposited in DNA Data Bank of Japan (AB573230). Comparison of these sequences confirmed that bases 100–102 of MG20 (AAC; encoding asparagine 34) were deleted in B129 (). To confirm that this genotypic difference directly affects nitrogen fixation activity, we introduced the B129-type and MG20-type SEN1 genes into the sen1-1 mutant by hairy root transformation, using the EV as a control. Mutant roots complemented with the B129 or MG20 SEN1 gene formed functional pink nodules (). Nitrogen fixation activity per nodule () was significantly higher and that per nodule weight tended to be higher () after complementation with MG20-type SEN1 than after complementation with B129-type SEN1. These results support the hypothesis that MG20-type SEN1 increases nitrogen fixation activity in L. japonicus.
Figure 4. Polymorphism of the amino acid sequence of SEN1 and its effect on the phenotypes. (a) The amino acid sequence of MG20 SEN1 ORF. The 34th amino acid (N, asparagine) is deleted in B129, and transmembrane domains are enclosed in yellow boxes. The transmembrane helix of the membrane protein was predicted by TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). (b) Nodules phenotype with either B129 SEN1 or MG20 SEN1 introduced into sen1-1 mutant at 28 days after inoculation. (c) Acetylene reduction activity per nodule weight. (d) Acetylene reduction activity per nodule. B129 type, MG20 type; n = 22, 24. Error bars represent standard error (SE). *P < 0.05 by Student’s t-test
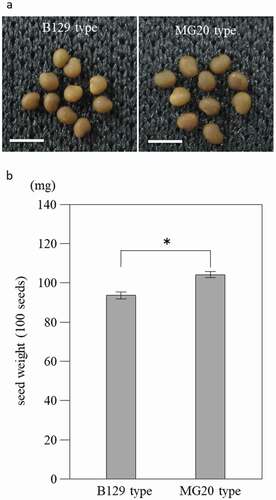
3.4. Effect of the genotypes of SEN1 region on seed production
BC3F4 generation seeds of MG20-type looked larger than those of B129-type (), and the seed weight of MG20-type was significantly higher (by 11%) than that of B129-type (). These results confirm that a QTL for seed weight is present near marker TM0832 and that its MG20 allele has a positive effect.
4. Discussion
Although some studies have analyzed QTLs for nitrogen fixation in legumes, few have identified these QTLs by investigating the nitrogen fixation activity of root nodules. Using a population of RILs obtained by crossing cultivated soybean (C08) with wild-type Glycine soja (W05) and the relative ureide abundance method, Muñoz et al. (Citation2016) detected a QTL with a high LOD score on chromosome 17. Hwang et al. (Citation2013) reported several QTLs for ureide and nitrogen concentrations using an RIL population obtained by crossing the soybean cultivars ‘KS4895ʹ and ‘Jackson.’ Ramaekers et al. (Citation2013) detected a QTL by the 15N natural abundance method in common bean (Phaseolus vulgaris). No genes responsible for QTLs for symbiotic nitrogen fixation have been reported in any of these studies, and no genes with high sequence identity to SEN1 were identified in the regions where these QTLs were detected. Therefore, SEN1 is a novel gene underlying a QTL for symbiotic nitrogen fixation.
We analyzed the effects of SEN1 genotypes on nitrogen fixation activity and seed weight. In the hairy roots of the sen1-1 mutant complemented with MG20 SEN1, ARA per nodule was significantly higher and ARA per nodule weight tended to be higher than in those complemented with B129 SEN1. These results indicate that nitrogen fixation activity is greatly influenced by SEN1. In phenotypic analyses of NILs 21 days after inoculation, plant growth was promoted in MG20-type in comparison with B129-type. Furthermore, seed weight was significantly higher in MG20-type than in B129-type. There are reports of the effect of nitrogen fixation activity on plant biomass production in soybean. EN1282, a non-nodulating mutant, had poorer growth than wild-type ‘Enrei’ (Francisco and Akao Citation1993; Shimada et al. Citation2012). Overexpression of GmINS1, encoding a cell-wall-expansion , increased the number, biomass, infection cell abundance, and nitrogenase activity of large nodules and subsequently increased the nitrogen content and biomass of soybean plants (Li et al. Citation2018). In this study, although not only the SEN1 gene was replaced in the NILs, the MG20-type SEN gene likely contributed to plant growth and nitrogen fixation activity.
SEN1 encodes an integral membrane protein with five transmembrane helices (predicted by TMHMM Server v. 2.0) homologous to Glycine max nodulin-21, and also to CCC1, a vacuolar iron/manganese transporter of Saccharomyces cerevisiae, and VIT1, a vacuolar iron transporter of Arabidopsis thaliana. The expression of SEN1 is detectable exclusively in infected nodule cells and increases during nodule development (Hakoyama et al. Citation2012). G. max nodulin-21 is reported as GmVTL1a, which facilitate iron transport into nodule symbiosomes (Bear et al. Citation2020; Liu et al. Citation2020). In addition, it is reported that the homolog of SEN1, MtVTL8 is also an iron transporter localized in the symbiosome membrane in Medicago truncatula (Jennifer et al. Citation2020). The amino acid inserted in MG20 is located in the N-terminal region before the first transmembrane region (Hakoyama et al. Citation2012). It is possible that this insertion changed the iron transport efficiency and consequently affected nitrogen fixation activity.
It is reported that seed weight was increased by nitrogen fertilizer in G. max non-nodulating lines (Manalo et al. Citation1998). Furthermore, the seed weight of G. max increased by the supply of nitrogen fertilizer or inoculation of rhizobia without the addition of fertilizer (Semu and Hume Citation1979). These results suggest that seed weight is affected by the nitrogen supply, such as fertilizer or symbiotic nitrogen fixation. Therefore, we thought that an increase in nitrogen fixation activity at least partially could lead to an increase in seed weight in L. japonicus. If the same strategy can be applied to crops, it can be expected to greatly contribute to their seed production.
Acknowledgments
The seeds of Lotus japonicus Miyakojima MG20, Gifu B129, and RI-088 were kindly provided by The National BioResource Project Lotus japonicus and Glycine max. Critical comments of the editor and anonymous reviewers helped the authors to improve the manuscript. This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research Grant Number (B) 25292014.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bear, E. M., F. Bedon, A. Garrin, I. S. Kryvoruchko, I. Torres-Jenez, M. K. Udvardi, D. A. Day, and P. M. C. Smith. 2020. “GmVTL1 is an Iron Transporter on the Symbiosome Membrane of Soybean with an Important Role in Nitrogen Fixation.” New Physiologist. doi:https://doi.org/10.1111/nph.16734.
- Bourion, V., S. M. H. Rizvi, S. Foutnier, S. F. H. Larambergue, F. Galmiche, P. Marget, G. Duc, and J. Burstin. 2010. “Genetic Dissection of Nitrogen Nutrition in Pea through a QTL Approach of Root, Nodule, and Shoot Variability.” Theoretical and Applied Genetics 121: 71–86. doi:https://doi.org/10.1007/s00122-010-1292-y.
- Broughton, W. J., and M. J. Dilworth. 1971. “Control of Leghaemoglobin Synthesis in Snake Beans.” Biochemical Journal 125: 1075–1080. doi:https://doi.org/10.1042/bj1251075.
- Francisco, P. B., and S. Akao. 1993. “Autoregulation and Nitrate Inhibition of Nodule Formation in Soybean Cv. Enrei and Its Nodulation Mutants.” Journal of Experimental Botany 44: 547–553. doi:https://doi.org/10.1093/jxb/44.3.547.
- Gamborg, O. L., R. A. Miller, and K. Ojima. 1968. “Nutrient Requirements of Suspension Cultures of Soybean Root Cells.” Experimental Cell Research 50: 151–158. doi:https://doi.org/10.1016/0014-4827(68)90403-5.
- Hakoyama, T., K. Niimi, H. Watanabe, R. Tabata, J. Matsubara, S. Sato, Y. Nakamura, et al. 2009. “Ost Plant Genome Overcomes the Lack of a Bacterial Gene for Symbiotic Nitrogen Fixation.” Nature 462:514–517. doi:https://doi.org/10.1038/nature08594.
- Hakoyama, T., K. Niimi, T. Yamamoto, S. Isobe, S. Sato, Y. Nakamura, S. Tabata, et al. 2012. “The Integral Membrane Protein SEN1 is Required for Symbiotic Nitrogen Fixation in Lotus Japonicus Nodules.” Plant Cell Physiology 53 :225–236. doi:https://doi.org/10.1093/pcp/pcr167.
- Hossain, M. S., Y. Umehara, and H. Kouchi. 2006. “A Novel Fix– Symbiotic Mutant of Lotus Japonicus, Ljsym105, Shows Impaired Development and Premature Deterioration of Nodule Infected Cells and Symbiosomes.” Molecular Plant-Microbe Interactions 19: 780–788. doi:https://doi.org/10.1094/MPMI-19-0780.
- Hwang, S., C. A. King, M. K. Davies, J. D. Ray, P. B. Cregan, and L. C. Purcell. 2013. “QTL Analysis of Shoot Ureide and Nitrogen Concentrations in Soybean [Glycine Max (L.) Merr.].” Crop Science 53: 2421–2433. doi:https://doi.org/10.2135/cropsci2012.11.0641.
- Jennifer, H. W., G. Kontra-Kováts, R. T. Green, Á. Domonkos, B. Horváth, E. M. Bear, M. Franceschetti, P. Kaló, and J. Balk. 2020. “The Medicago Truncatula Vacuolar Iron Transporter-Like Proteins VTL4 and VTL8 Deliver Iron to Symbiotic Bacteria at Different Stages of the Infection Process.” New Physiologist. doi:https://doi.org/10.1111/nph.16735.
- Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, et al. 2000. “Complete Genome Structure of the Nitrogen-fixing Symbiotic Bacterium Mesorhizobium Loti.” DNA Research 7 :331–338. doi:https://doi.org/10.1093/dnares/7.6.331.
- Kawaguchi, M., H. Imaizumi-Anraku, H. Koiwa, S. Niwa, A. Ikuta, K. Syono, and S. Akao. 2002. “Root, Root Hair, and Symbiotic Mutants of the Model Legume Lotus Japonicus.” Molecular Plant-Microbe Interactions 15: 17–26. doi:https://doi.org/10.1094/MPMI.2002.15.1.17.
- Keele, B. B., P. B. Hamilton, and G. H. Elkan. 1969. “Glucose Catabolism in Rhizobium Japonicum.” Journal of Bacteriology 97: 1184–1191. doi:https://doi.org/10.1128/JB.97.3.1184-1191.1969.
- Klein, M. A., and M. A. Grusak. 2009. “Identification of Nutrient, Physical Seed Trait QTL in the Model Legume Lotus Japonicus.” Genome 52: 677–691. doi:https://doi.org/10.1128/JB.97.3.1184-1191.1969.
- Krusell, L., K. Krause, T. Ott, G. Desbrosses, U. Krämer, S. Sato, Y. Nakamura, et al. 2005. “The Sulfate Transporter SST1 Is Crucial for Symbiotic Nitrogen Fixation in Lotus Japonicus Root Nodules.” The Plant Cell 17 :1625–1636. doi:https://doi.org/10.1105/tpc.104.030106.
- Kumagai, H., T. Hakoyama, Y. Umehara, S. Sato, T. Kaneko, S. Tabata, and H. Kouchi. 2007. “A Novel Ankyrin-Repeat Membrane Protein, IGN1, Is Required for Persistence of Nitrogen-Fixing Symbiosis in Root Nodules of Lotus Japonicus.” Plant Physiology 143: 1293–1305. doi:https://doi.org/10.1104/pp.106.095356.
- Li, X., J. Zheng, Y. Yang, and H. Liao. 2018. “INCREASING NODULE SIZE1 Expression Is Required for Normal Rhizobial Symbiosis and NODULE Development.” Plant Physiology 178: 1233–1248. doi:https://doi.org/10.1104/pp.18.01018.
- Liu, S., L. L. Liao, M. M. Nie, W. T. Peng, M. S. Zhang, J. N. Lei, Y. J. Zhong, H. Liao, and Z. C. Chen. 2020. “A VIT-Like Transporter Facilitates Iron Transport into Nodule Symbiosomes for Nitrogen Fixation in Soybean.” New Physiologist 226: 1413–1428. doi:https://doi.org/10.1111/nph.16506.
- Maekawa, T., M. Kusakabe, Y. Shimoda, S. Sato, Y. Murooka, and M. Hayashi. 2008. “Polyubiquitin Promoter-Based Binary Vectors for Overexpression and Gene Silencing in Lotus Japonicus.” Molecular Plant-Microbe Interactions 21: 375–382. doi:https://doi.org/10.1094/MPMI-21-4-0375.
- Manalo, D. D., S. Sawada, H. Miura, and K. Kato. 1998. “Seed Weight of Nodulating and Non-nodulating Soybeans at Different Nitrogen Levels and Years.” Plant Production Science 1: 264–268. doi:https://doi.org/10.1626/pps.1.264.
- Muñoz, N., X. Qi, M. W. Li, M. Xie, Y. Gao, M. Y. Cheung, F. L. Wong, and H. M. Lam. 2016. “Improvement in Nitrogen Fixation Capacity Could Be Part of the Domestication Process in Soybean.” Heredity 117: 84–93. doi:https://doi.org/10.1038/hdy.2016.27.
- Ohyama, T., Y. Takahashi, T. Chinushi, and T. Nakano. 1992. “Evaluation of N2 Fixation Activity and Nitrogen Absorption Rate in Field Grown Soybean Plants by Simple Relative Method.” Agriculture and Horticulture (Nogyo Oyobi Engei) 67: 1157–1164.
- Ramaekers, L., C. H. Galeano, N. Gazón, J. Vanderleyden, and M. W. Blair. 2013. “Identifying Quantitative Trait Loci for Symbiotic Nitrogen Fixation Capacity and Related Traits in Common Bean.” Molecular Breeding 31: 163–180. doi:https://doi.org/10.1007/s11032-012-9780-1.
- Saeki, K., and H. Kouchi. 2000. “The Lotus Symbiont, Mesorhizobium Loti: Molecular Genetic Techniques and Application.” Journal of Plant Research 113: 457–465. doi:https://doi.org/10.1007/PL00013956.
- Semu, E., and D. J. Hume. 1979. “Effects of Inoculation and Fertilizer in Levels on N2 Fixation and Yields of Soybeans in Ontario.” Canadian Journal of Plant Science 59: 1129–1137. doi:https://doi.org/10.4141/cjps79-175.
- Shimada, S., H. Hamaguchi, Y. Kim, K. Matsuura, M. Kato, T. Kokuryu, J. Tazawa, and S. Fujimori. 2012. “Effects of Water Table Control by Farm-Oriented Enhancing Aquatic System on Photosynthesis, Nodule Nitrogen Fixation, and Yield of Soybeans.” Plant Production Science 15: 132–143. doi:https://doi.org/10.1626/pps.15.132.
- Suganuma, N., Y. Nakamura, M. Yamamoto, T. Ohta, H. Koiwa, S. Akao, and M. Kawaguchi. 2003. “The Lotus Japonicus Sen1 Gene Controls Rhizobial Differentiation into Nitrogen-Fixing Bacteroids in Nodules.” Molecular Genetics and Genomics 269: 312–320. doi:https://doi.org/10.1007/s00438-003-0840-4.
- Suzuki, A., K. Yamashita, M. Ishihara, K. Nakahara, M. Abe, K. Kucho, T. Uchiumi, S. Higashi, and S. Arima. 2008. “Enhanced Symbiotic Nitrogen Fixation by Lotus Japonicus Containing an Antisense β-1,3-Glucanase Gene.” Plant Biotechnology 25: 357–360. doi:https://doi.org/10.5511/plantbiotechnology.25.357.
- Tominaga, A., T. Gondo, R. Akashi, S. Zheng, S. Arima, and A. Suzuki. 2012. “Quantitative Trait Locus Analysis of Symbiotic Nitrogen Fixation Activity in the Model Legume Lotus Japonicus.” Journal of Plant Research 125: 395–406. doi:https://doi.org/10.1007/s10265-011-0459-1.
- Tominaga, A., M. Nagata, K. Futsuki, H. Abe, T. Uchiumi, M. Abe, K. Kucho, et al. 2009. “Enhanced Nodulation and Nitrogen Fixation in the Abscisic Acid Low-Sensitive Mutant Enhanced Nitrogen Fixation1 of Lotus Japonicus.” Plant Physiology 151 :1965–1976. doi:https://doi.org/10.1104/pp.109.142638.