ABSTRACT
Mycorrhizal fungi symbiotically associate with 80% of land plants, extend extraradical hyphae in the soil, and promote phosphorus (P) uptake in host plants. It is necessary to utilize the function of mycorrhizal symbiosis to cope with future depletion of P resources. Elucidation of the mycorrhizal colonization mechanism for utilizing mycorrhizal fungi, inoculation methods in agriculture and forestry, and new function of extraradical hyphae are described. Root exudates from P-deficient plants promoted extraradical hyphal growth and a hydrophobic fraction of the exudates promoted the formation of appressorium and subsequent colonization of the roots. Inoculation with a mycorrhizal fungus at the nursery stage reduced phosphate fertilizer application in Welsh onion cultivation and thus production cost. Inoculation with ectomycorrhizal and arbuscular mycorrhizal fungi increased the growth of tropical tree species under nursery and field conditions. Acid phosphatase was secreted from the extraradical hyphae of Rhizophagus clarus and its activity was increased under low P conditions. We found that utilization of AM fungi is useful as a strategy for sustainable P resource use.
1. Introduction
Mycorrhizas are a symbiosis between fungi in the soil and plant roots. Mycorrhizas can be categorized into arbuscular mycorrhiza, ectomycorrhiza, ectendomycorrhiza, arbutoid mycorrhiza, monotropoid mycorrhizas, ericoid mycorrhiza, and orchid mycorrhiza according to their morphology and the combination of host plant and fungus (Smith and Read Citation2008). The fungi that form mycorrhizas are called mycorrhizal fungi. It is estimated that 80% of land plants have mycorrhizal roots. Here we describe arbuscular mycorrhizas, which most plants form. Arbuscular mycorrhizal (AM) fungi extend extraradical hyphae in the soil and promote phosphorus (P) uptake in the host plants. The function of these fungi has rarely been considered in crop production under conditions where phosphate fertilizers is inexpensive and sufficiently supplied. It is necessary to utilize the function of the mycorrhizal symbiotic system in order to cope with the future depletion of P resources (Tawaraya et al. Citation2012). In this review, a series of research results by the author’s group about elucidation of mycorrhizal colonization mechanism for utilizing mycorrhizal fungi, inoculation method in agriculture and forestry, and new function of extraradical hyphae are described.
2. Phosphate inhibition mechanism of arbuscular mycorrhizal colonization
Heavy application of P fertilizer inhibits AM colonization (Thomson, Robson, and Abbott Citation1986). Little has been known about the mechanisms of the inhibition. It was first necessary to clarify the mechanism of mycorrhizal colonization in roots in order to utilize mycorrhizal fungi. The first systematic field survey of arbuscular mycorrhizal (AM) colonization in major field crops in Japan was conducted in 1982–1983 and mycorrhizas were rarely formed in the roots of crops growing in the soils to which a large amount of phosphate fertilizer was applied. It was confirmed that mycorrhizal colonization was reduced by the application of P and that mycorrhizal colonization promoted P uptake and growth in the pot experiments (Tawaraya et al. Citation1996). Therefore, focusing on the regulation mechanism of mycorrhizal colonization by application of phosphate, the inhibition of mycorrhizal colonization by application of a large amount of P is regulated by the P concentration in the host plant and the number of appressorium is decreased by P application. Inhibition of mycorrhizal colonization by P was considered to occurs at the stage of hyphal entry into the root epidermis (Tawaraya et al. Citation1994). P application suppressed new colonization of the fungi but did not affect respiratory metabolic activity in the roots. P concentration in the medium had a little effect on spore germination and extraradical hyphal elongation (Tawaraya et al. Citation1996). These observations suggest that AM colonization is regulated through internal P status of the host plant.
P nutritional status of the host plant affected metabolites in the root and root exudates and the P deficiency of the host plant affected the concentration of amino acids and sugars in the root and rhizosphere (Tawaraya and Saito Citation1994; Tawaraya et al. Citation1994). The P deficiency promoted the formation of appressorium by extraradical hyphae (Tawaraya et al. Citation1994; Tawaraya et al. Citation2007). P-deficient onion root exudates promoted extraradical hyphal growth but P-sufficient onion root exudates did not affect (Tawaraya et al. Citation1996). Appressoirum formation-promoting substance secreted from the P-deficient onion roots was crudely purified and its hydrophobic fraction promoted the formation of appressorium and subsequent colonization of the roots (Tawaraya, Hashimoto, and Wagatsuma Citation1998). Mycorrhizal colonization was not observed in the root of 36 species in the genus Lupinus in the Fabaceae in which most species form AM but was observed in seven genera related to Lupinus (Oba, Tawaraya, and Wagatsuma Citation2001). Root exudates of Lupinus spp. inhibited the elongation of extraradical hyphae (Oba, Tawaraya, and Wagatsuma Citation2002). These results suggest that plants control AM colonization through root exudates according to their P status.
3. Reduction of phosphate fertilization by inoculation of arbuscular mycorrhizal fungi
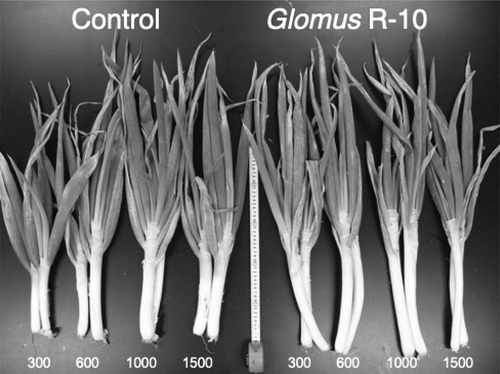
Differences in the response to AM colonization have been observed among plant species (Plenchette, Fortin, and Furlan Citation1983; Jasper and Davy Citation1993; Schweiger, Robson, and Barrow Citation1995) and cultivars (Bakarr and Janos Citation1996; Baon et al., Citation1993; Bryla and Koide Citation1990). Although many studies showed that the inoculation of AM fungi improved growth of Allium species in pot experiments (e.g., Mosse and Hayman Citation1971; Stribley, Tinker, and Rayner et al. Citation1980; Smith et al. Citation1986), little had been known about crop species difference in responses to AM colonization under field conditions. The relationships between mycorrhizal fungi and the host plants are mutualism, parasitism and commensalism, depending on the chemical characteristics of the soil and on the host plant-fungus combination. The conditions for mutualistic relationship between crops and inoculant fungi have hardly been clarified. Therefore, various environmental and plant factors for the onset of inoculation effect were examined. Mycorrhizal dependency that is an index used for the assesment is defined as follows (Plenchette, Fortin, and Furlan Citation1983); Mycorrhizal dependency (%) = [(dry weight of mycorrhizal plant – dry weight of non-mycorrhizal plant)/dry weight of mycorrhizal plant] x 100. Mycorrhizal dependency was 79% in trees, 70% in wild grasses, 56% in forage crops, and 44% in field crops and also different among plant species and cultivars (Tawaraya Citation2003). Mycorrhizal dependency was negatively correlated with the ability of roots to absorb phosphate without mycorrhizas (Tawaraya, Imai, and Wagatsuma Citation1999; Tawaraya, Tokairin, and Wagatsuma Citation2001). Growth response to inoculation was higher in Allium spp. such as Welsh onion, onions, and garlic because these plants have a coarse root system compared with other crops. We showed that the inoculation with a commercial mycorrhizal fungus at the nursery stage could reduce the cost of Welsh onion production significantly by saving P fertilizer application, indicating the importance of adequate soil phosphate concentration in combination with responsive cultivars (Tawaraya et al. Citation2012) (). The effect of AM fungal inoculation under field conditions is different from that in pot experiments because of the presence of indigenous AM fungi in the field, indicating the importance of considering interactions between the introduced and indigenous fungi (Sato, Cheng, and Tawaraya Citation2018). Belowground AM fungi also interact with aboveground fauna (Tawaraya et al. Citation2012). In summary, responses of crops to AM fungal inoculation are significantly affected by soil P concentration and root morphology and further, the inoculation of AM fungi can reduce P fertilizer in the production of the crops with a coarse root system.
4. Remediation of degraded tropical forest by inoculation of mycorrhizal fungi
Tropical forests are disappearing due to illegal logging, burning and clearing for agricultural land. Recent timber production in tropical developing countrires has resulted in the transformation of natural forest into over-logged, poorly managed and degraded forests. AM associaton is also prevalent in tropical forest (Bakarr and Janos Citation1996; Moyersoen, Becker, and Alexander Citation2001). It may be possible that AM fungal inoculation improves growth of tree species in degraded forest. The inoculation effect of AM fungi is generally larger in soil with lower available phosphate. It was predicted, therefore, that the effect of AM fungal inoculation would be great in tropical forests in which phosphate concentration is extremely low and thus the mycorrhizal dependency of the trees is high. Tree species in Guttiferae, Tetrameristaceae, Sapotaceae, Melastomataceae, Thymelaeaceae, Euphorbiaceae, Anacardiaceae, and Dipterocarpaceae dominate in tropical forest in Indonesia. Many of the tree species in those families formed arbuscular mycorrhizas (Tawaraya et al. Citation2003). Tree species of Proteaceae is nonmycorrhizal plant (Lambers and Teste Citation2013). Inoculation with AM fungi isolated from the soil of tropical forest increased phosphate uptake and the growth of a variety of the species (Turjaman et al. Citation2006; Turjaman et al. Citation2008). Inoculation with ectomycorrhizal fungi also increased nitrogen (N) and P uptake, growth and survival rate of seedlings of Shorea pinanga and Shorea seminis, which are important species in tropical forests (Turjaman et al. Citation2005; Turjaman et al. Citation2006). Inoculation with ectomycorrhizal fungi also promoted the growth of Shorea balangeran 40 months after transplanting in disturbed peatlands and increased survival rate (Turjaman et al. Citation2011). In addition, the inoculation of AM fungi to Albizia saman and Paraserianthes falcataria in the nursery promoted the P uptake and growth six months after transplanting, and also increased the growth and survival rates seven months after transplanting in a post opencast coal mining site (Wulandari, Saridi, and Tawaraya Citation2016). It was considered that not only the increase in N and P uptake, but also the enhancement of water uptake, and disease tolerance contributed to the increases in the survival rates. We also showed that endophytic fungi contributed to the growth of Paraserianthes falcataria in tropical forests (Maulana et al. Citation2018). In summary, the inoculation of native AM fungi improves nutrient uptake, growth and survival rates of tropical tree species in Indonesia and thus can be applicable to the remediation of degraded tropical forest.
5. Utilization of unavailable phosphate by arbuscular mycorrhizal fungi
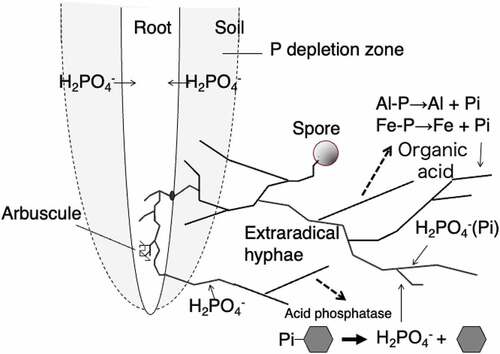
Soil P consists of plant-available and plant-unavailable forms. The available form is mono – and divalent orthophosphates whereas the unavailable forms are organic phosphate such as inositol phosphate, nucleic acid, phospholipids and sugar phosphate and, and sparingly soluble phosphate bound to iron-, aluminum-, and calcium. It is necessary (1) to reduce P fertilizer application to soil in the short term (until around 2030), (2) to improve the efficiency of phosphate acquisition and utilization in crops in the medium term (until around 2050), and finally (3) to promote the utilization of the unavailable phosphates accumulated in the soil, and establish the recirculation of phosphorus resources in Japan in order to cope with the depletion of P resources in the long term (until around 2100). Utilization of unavailable soil P by AM colonization had been suggested (Tarafdar and Marschner Citation1994; Koide and Kabir Citation2000; Feng et al. Citation2003). We hypothesized that AM fungi access plant-unavailable phosphate as the concentration of plant-unavailable phosphate decreased after cultivation of AM plants. (). The hyphal exudates from the extraradical hyphae of AM fungi were collected by combining two-compartment pot cultivation that can divide the roots and extraradical hyphae in the soil and it was shown that extraradical hyphae of Gigaspora margarita secreted organic acids (Tawaraya, Naito, and Wagatsuma Citation2006). Furthermore, it was shown that acid phosphatase was secreted from the extraradical hyphae of Rhizophagus clarus isolated from peat soil. Root extracts and root exudates were analyzed by SDS-PAGE and 187 kDa acid phosphatase activity was obtained by active staining of the gel in the soil solution of the inoculum and the extraradical hyphae obtained by sand and sterile culture (Sato et al. Citation2015). In addition, secretion of acid phosphatase from extraradical hyphae of R. clarus was promoted under low P conditions (Sato et al. Citation2019). These results suggest that AM fungi access the plant-unavailable forms of phosphate and are useful for sustainable use of P resource.
6. Elucidation of low phosphorus tolerant mechanism by metabolome analysis
Organic compounds secreted from roots play an important role in the acquisition of phosphate in plants. Among various kinds of metabolites in root exudates, organic acids and phosphatase are considered to be involved in the P acquisition (Gardner, Barber, and Parbery Citation1983; Tadano and Sakai Citation1991). but the roles of other metabolites in low-P tolerance of plants have not yet been clarified. Metabolite profiling of root exudates could be useful tools to clarify mechanism of P acquisition of plant. Root exudates under hydroponic and soil-cultivated conditions were recovered, and the metabolites contained therein were comprehensively analyzed by metabolome analysis. By this method, it was found that rice and soybean actively secreted metabolites into the rhizosphere under low P conditions (Tawaraya et al. Citation2013, Citation2014). Lipids contained in the leaves were measured by LC-MS in two rice cultivar, low-P tolerant Akamai (Yamagata) and low-P susceptible Koshihikari. The degree of remodeling of phospholipids to non-phospholipids (glycolipids and sulfolipids) under low P conditions was found to be higher in Akamai than in Koshihikari (Tawaraya et al. Citation2018). These results suggest that metabolome profiling of root exudates detect various kinds of compound and clarify their possible roles in P acquisition of plant.
7. Conclusion
We found that plants control AM colonization through root exudates according to their P status. We also found that the responses of plants to AM colonization are affected by soil P availability and dependent on host species and cultivars. We successfully demonstrated that the reduction of P fertilizer is feasible in crop production by the inoculation of AM fungi. We found that the inoculation of AM fungi improves growth and survival rates of tropical tree species and can be used for the remediation of disturbed tropical forest in Indonesia. We first clarified that AM fungi access plant-unavailable organic phosphates and are useful for sustainable use of P resources.
Acknowledgments
I thank all the students at laboratory of Plant Nutrition and Soil Science, Faculty of Agriculture, Yamagata University and all the co-authors of research for their kind help, deep discussion, and encouragement.
Disclosure statement
Not potential conflict of interest was reported by the author.
Additional information
Funding
References
- Bakarr, M. I., and D. P. Janos. 1996. “Mycorrhizal Associations of Tropical Legume Trees in Sierra Leone, West Africa.” Forest Ecology and Management 89 (1–3): 89–92. doi:10.1016/S0378-1127(96)03866-2.
- Baon, J. B., S. E. Smith, and A. M. Alston. 1993. “Mycorrhizal Responses of Barley Cultivars Differing in P Efficiency.” Plant and Soil 157 (1): 97–105. doi:10.1007/BF02390231.
- Bryla, D. R., and R. T. Koide. 1990. “Role of Mycorrhizal Infection in the Growth and Reproduction of Wild Vs. Cultivated Plants II. Eight Wild Accessions and Two Cultivars of Lycopersicon Esculentum Mill.” Oecologia 84 (1): 82–92. doi:10.1007/BF00665599.
- Feng, G., Y. C. Song, X. L. Li, and P. Christie. 2003. “Contribution of Arbuscular Mycorrhizal Fungi to Utilization of Organic Sources of Phosphorus by Red Clover in a Calcareous Soil.” Applied Soil Ecology 22 (2): 139–148. doi:10.1016/S0929-1393(02)00133-6.
- Gardner, W. K., D. A. Barber, and D. G. Parbery. 1983. “The Acquisition of Phosphorus by Lupinus Albus L. III. The Probable Mechanisms by Which Phosphorus Movement in the Soil/root Interface Is Enhanced.” Plant and Soil 70 (1): 107–124. doi:10.1007/BF02374754.
- Jasper, D. A., and J. A. Davy. 1993. “Root Characteristics of Native Plant Species in Relation to the Benefit of Mycorrhizal Colonization for Phosphorus Uptake.” Plant and Soil 155-156 (1): 281–284. doi:10.1007/BF00025037.
- Koide, R. T., and Z. Kabir. 2000. “Extraradical Hyphae of the Mycorrhizal Fungus Glomus Intraradices Can Hydrolyse Organic Phosphate.” New Phytologist 148 (3): 511–517. doi:10.1046/j.1469-8137.2000.00776.x.
- Lambers, H., and F. P. Teste. 2013. “Interactions between Arbuscular Mycorrhizal and Non-mycorrhizal Plants: Do Non-mycorrhizal Species at Both Extremes of Nutrient Availability Play the Same Game?” Plant, Cell & Environment 36 (11): 1911–1915. doi:10.1111/pce.12117.
- Maulana, A. F., M. Turjaman, T. Sato, Y. Hashimoto, W. Cheng, and K. Tawaraya. 2018. “Isolation of Endophytic Fungi from Tropical Forest in Indonesia.” Symbiosis 76 (2): 151–162. doi:10.1007/s13199-018-0542-7.
- Mosse, B., and D. S. Hayman. 1971. “Plant Growth Responses to Vesicular-arbuscular Mycorrhiza. Ii. In Unsterilized Field Soils.” New Phytologist 70 (1): 29–34. doi:10.1111/j.1469-8137.1971.tb02505.x.
- Moyersoen, B., P. Becker, and I. J. Alexander. 2001. “Are Ectomycorrhizas More Abundant than Arbuscular Mycorrhizas in Tropical Heath Forests?” New Phytologist 150 (3): 591–599. doi:10.1046/j.1469-8137.2001.00125.x.
- Oba, H., K. Tawaraya, and T. Wagatsuma. 2001. “Arbuscular Mycorrhizal Colonization in Lupinus and Related Genera.” Soil Science and Plant Nutrition 47 (4): 685–694. doi:10.1080/00380768.2001.10408433.
- Oba, H., K. Tawaraya, and T. Wagatsuma. 2002. “Inhibition of Pre-symbiotic Hyphal Growth of Arbuscular Mycorrhizal Fungus Gigaspora Margarita by Root Exudates of Lupinus Spp.” Soil Science and Plant Nutrition 48 (1): 117–120. doi:10.1080/00380768.2002.10409180.
- Plenchette, C., J. A. Fortin, and V. Furlan. 1983. “Growth Responses of Several Plant Species to Mycorrhizae in a Soil of Moderate P-fertility I. Mycorrhizal Dependency under Field Conditions.” Plant and Soil 70 (2): 199–209. doi:10.1007/BF02374780.
- Sato, T., S. Hachiya, N. Inamura, T. Ezawa, W. G. Cheng, and K. Tawaraya. 2019. “Secretion of Acid Phosphatase from Extraradical Hyphae of the Arbuscular Mycorrhizal Fungus Rhizophagus Clarus Is Regulated in Response to Phosphate Availability.” Mycorrhiza 29 (6): 599–605. doi:10.1007/s00572-019-00923-0.
- Sato, T., T. Ezawa, W. G. Cheng, and K. Tawaraya. 2015. “Release of Acid Phosphatase from Extraradical Hyphae of Arbuscular Mycorrhizal Fungus Rhizophagus Clarus.” Soil Science and Plant Nutrition 61 (2): 269–274. doi:10.1080/00380768.2014.993298.
- Sato, T., W. G. Cheng, and K. Tawaraya. 2018. “Effects of Indigenous and Introduced Arbuscular Mycorrhizal Fungi on the Growth of Allium Fistulosum under Field Conditions.” Soil Science and Plant Nutrition 64 (6): 705–709. doi:10.1080/00380768.2018.1543534.
- Schweiger, P. F., A. D. Robson, and N. J. Barrow. 1995. “Root Hair Length Determines Beneficial Effect of a Glomus Species on Shoot Growth of Some Pasture Species.” New Phytologist 131 (2): 247–254. doi:10.1111/j.1469-8137.1995.tb05726.x.
- Smith, S. E., B. J. S. John, F. A. Smith, and J. L. Bromley. 1986. “Effect of Mycorrhizal Infection on Plant Growth, Nitrogen and Phosphorus Nutrition in Glasshouse-grown Allium Cepa L.” New Phytologist 103 (2): 359–373. doi:10.1111/j.1469-8137.1986.tb00622.x.
- Smith, S. E., and D. J. Read. 2008. Mycorrhizal Symbiosis Third Edition. London, UK: Academic Press.
- Stribley, D. P., P. B. Tinker, and J. H. Rayner . 1980. “Relation of Internal Phosphorus Concentration and Plant Weight in Plants Infected by Vesicular-arbuscular Mycorrhizas.” New Phytologist 86 (3): 261–266. DOI:10.1111/j.1469-8137.1980.tb00786.x.
- Tadano, T., and H. Sakai. 1991. “Secretion of Acid Phosphatase by the Roots of Several Crop Species under Phosphorus-deficient Conditions.” Soil Science and Plant Nutrition 37 (1): 129–140. doi:10.1080/00380768.1991.10415018.
- Tarafdar, J. C., and H. Marschner. 1994. “Phosphatase Activity in the Rhizosphere and Hyphosphere of VA Mycorrhizal Wheat Supplied with Inorganic and Organic Phosphorus.” Soil Biology & Biochemistry 26 (3): 387–395. doi:10.1016/0038-0717(94)90288-7.
- Tawaraya, K., K. Hashimoto, and T. Wagatsuma. 1998. “Effect of Root Exudate Fractions from P-deficient and P-sufficient Onion Plants on Root Colonisation by the Arbuscular Mycorrhizal Fungus Gigaspora Margarita.” Mycorrhiza 8 (2): 67–70. doi:10.1007/s005720050214.
- Tawaraya, K., K. Sasai, and T. Wagatsuma. 1994. “Effect of Phosphorus Application on the Contents of Amino Acids and Reducing Sugars in the Rhizosphere and VA Mycorrhizal Infection of White Clover.” Soil Science and Plant Nutrition 40 (3): 539–543. doi:10.1080/00380768.1994.10413332.
- Tawaraya, K., K. Tokairin, and T. Wagatsuma. 2001. “Dependence of Allium Fistulosum Cultivars on the Arbuscular Mycorrhizal Fungus, Glomus Fasciculatum.” Applied Soil Ecology 17 (2): 119–124. doi:10.1016/S0929-1393(01)00126-3.
- Tawaraya, K., M. Naito, and T. Wagatsuma. 2006. “Solubilization of Insoluble Inorganic Phosphate by Hyphal Exudates of Arbuscular Mycorrhizal Fungi.” Journal of Plant Nutrition 29 (4): 657–665. doi:10.1080/01904160600564428.
- Tawaraya, K., and M. Saito. 1994. “Effect of Vesicular-arbuscular Mycorrhizal Infection on Amino Acids Composition in Roots of Onion and White Clover.” Soil Science and Plant Nutrition 40 (2): 339–343. doi:10.1080/00380768.1994.10413308.
- Tawaraya, K., M. Saito, M. Morioka, and T. Wagatsuma. 1994. “Effect of Phosphate Application to Arbuscular Mycorrhizal Onion on the Development and Succinate Dehydrogenase Activity of Internal Hyphae.” Soil Science and Plant Nutrition 40 (4): 667–673. doi:10.1080/00380768.1994.10414306.
- Tawaraya, K., M. Saito, M. Morioka, and T. Wagatsuma. 1996a. “Effect of Concentration of Phosphate on Spore Germination and Hyphal Growth of Arbuscular Mycorrhizal Fungus, Gigaspora Margarita Becker & Hall.” Soil Science and Plant Nutrition 42 (3): 667–671. doi:10.1080/00380768.1996.10416336.
- Tawaraya, K., R. Hirose, and T. Wagatsuma. 2012. “Inoculation of Arbuscular Mycorrhizal Fungi Can Substantially Reduce Phosphate Fertilizer Application to Allium Fistulosum L. And Achieve Marketable Yield under Field Condition.” Biology and Fertility of Soils 48 (7): 839–843. doi:10.1007/S00374-012-0669-2.
- Tawaraya, K., R. Horie, A. Saito, T. Shinano, T. Wagatsuma, K. Saito, and A. Oikawa. 2013. “Metabolite Profiling of Shoot Extracts, Root Extracts, and Root Exudates of Rice Plant under Phosphorus Deficiency.” Journal of Plant Nutrition 36 (7): 1138–1159. doi:10.1080/01904167.2013.780613.
- Tawaraya, K., R. Horie, T. Shinano, T. Wagatsuma, K. Saito, and A. Oikawa. 2014. “Metabolite Profiling of Soybean Root Exudates under Phosphorus Deficiency.” Soil Science and Plant Nutrition 60 (5): 679–694. doi:10.1080/00380768.2014.945390.
- Tawaraya, K., S. Honda, W. Cheng, M. Chuba, Y. Okazaki, K. Saito, A. Oikawa, H. Maruyama, J. Wasaki, and T. Wagatsuma. 2018a. “Ancient Rice Cultivar Extensively Replaces Phospholipids with Non-phosphorus Glycolipid under Phosphorus Deficiency.” Physiologia Plantarum 163 (3): 297–305. doi:10.1111/ppl.12699.
- Tawaraya, K., S. Shiozawa, K. Ueda, H. Murayama, T. Nishizawa, T. Toyomasu, T. Murayama, S. Sato, T. Wagatsuma, and H. Yasuda. 2012. “Leaf Herbivory by Spodoptera Litura Increases Arbuscular Mycorrhizal Colonization in Roots of Soybean.” Soil Science and Plant Nutrition 58 (4): 445–449. doi:10.1080/00380768.2012.704520.
- Tawaraya, K., S. Watanabe, E. Yoshida, and T. Wagatsuma. 1996b. “Effect of Onion (Allium Cepa) Root Exudates on the Hyphal Growth of Gigaspora Margarita.” Mycorrhiza 6 (1): 57–59. doi:10.1007/s005720050106.
- Tawaraya, K., S. Watanabe, T. Wagatsuma, and T. Wagatsuma. 2007. “Formation of Appressoria by the Arbuscular Mycorrhizal Fungus Gigaspora Margarita on Roots of Allium Cepa Is Linked with Root Age.” Mycoscience 48 (5): 305–308. doi:10.1007/S10267-007-0367-3.
- Tawaraya, K., T. Imai, and T. Wagatsuma. 1999. “Importance of Root Length in Mycorrhizal Colonization of Welsh Onion.” Journal of Plant Nutrition 22 (3): 589–596. doi:10.1080/01904169909365654.
- Tawaraya, K., Y. Takaya, M. Turjaman, S. J. Tuah, S. H. Limin, Y. Tamai, J. Y. Cha, T. Wagatsuma, and M. Osaki. 2003. “Arbuscular Mycorrhizal Colonization of Tree Species Grown in Peat Swamp Forests of Central Kalimantan, Indonesia.” Forest Ecology and Management 182 (1–3): 381–386. doi:10.1016/S0378-1127(03)00086-0.
- Tawaraya, K. 2003. “Arbuscular Mycorrhizal Dependency of Different Plant Species and Cultivars.” Soil Science and Plant Nutrition 49 (5): 655–668. doi:10.1080/00380768.2003.10410323.
- Thomson, B. D., A. D. Robson, and L. K. Abbott. 1986. “Effects of Phosphorus on the Formation of Mycorrhizas by Gigaspora Calospora and Glomus Fasciculatum in Relation to Root Carbohydrates.” New Phytologist 103 (4): 751–765. doi:10.1111/j.1469-8137.1986.tb00850.x.
- Turjaman, M., E. Santoso, A. Susanto, S. Gaman, S. H. Limin, Y. Tamai, M. Osaki, and K. Tawaraya. 2011. “Ectomycorrhizal Fungi Promote Growth of Shorea Balangeran in Degraded Peat Swamp Forests.” Wetlands Ecology and Management 19 (4): 331–339. doi:10.1007/s11273-011-9219-1.
- Turjaman, M., Y. Tamai, E. Santoso, M. Osaki, and K. Tawaraya. 2006a. “Arbuscular Mycorrhizal Fungi Increased Early Growth of Two Nontimber Forest Product Species Dyera Polyphylla and Aquilaria Filaria under Greenhouse Conditions.” Mycorrhiza 16 (7): 459–464. doi:10.1007/s00572-006-0059-4.
- Turjaman, M., Y. Tamai, H. Segah, S. H. Limin, M. Osaki, and K. Tawaraya. 2006b. “Increase in Early Growth and Nutrient Uptake of Shorea Seminis Seedlings Inoculated with Two Ectomycorrhizal Fungi.” Journal of Tropical Forest Science 18 (4): 243–249.
- Turjaman, M., Y. Tamai, H. Segah, S. Limin, J. Y. Cha, M. Osaki, and K. Tawaraya. 2005. “Inoculation with Ectomycorrhizal Fungi Pisolithus Arhizus and Scleroderma Sp. Improves Early Growth of Shorea Pinanga Nursery Seedlings.” New Forests 30 (1): 67–73. doi:10.1007/s11056-004-1954-1.
- Turjaman, M., Y. Tamai, I. R. Sitepu, E. Santoso, M. Osaki, and K. Tawaraya. 2008. “Improvement of Early Growth of Two Tropical Peat-swamp Forest Tree Species Ploiarium Alternifolium and Calophyllum Hosei by Two Arbuscular Mycorrhizal Fungi under Greenhouse Conditions.” New Forests 36 (1): 1–12. doi:10.1007/s11056-008-9084-9.
- Wulandari, D., W. G. C. Saridi, and K. Tawaraya. 2016. “Arbuscular Mycorrhizal Fungal Inoculation Improves Albizia Saman and Paraserianthes Falcataria Growth in Post-opencast Coal Mine Field in East Kalimantan, Indonesia.” Forest Ecology and Management 376: 67–73. doi:10.1016/j.foreco.2016.06.008.