 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An unusual phenomenon occurred in new display cases at the Rijksmuseum four months after their installation in April 2013. White deposits were visible on glass windows, silicone door gaskets, black structural adhesive seals, and on works of art. The works of art most affected by these deposits were bronze sculptures, wooden and waxed objects, tempera, and oil paintings. In this study the deposits were chemically characterised using (pyrolysis-)gas chromatography-mass spectrometry ((Py-)GC-MS), µ-Raman spectroscopy (µ-Raman), ion chromatography (IC), and scanning electron microscopy combined with energy dispersive X-ray spectroscopy (SEM-EDX). Five chemically different crystalline deposits were found. They were identified as organic salts of the base 2,2,6,6-tetramethyl-4-piperidinol (TMP-ol), a secondary amine, and five different carboxylic acids. It was found that TMP-ol, which is part of the UV-light stabiliser Tinuvin-770, emitted from the structural adhesive Terostat-9220. Terostat-9220 was used in large quantities in the display cases to adhere glass windows to metal parts. The carboxylic acids derived from both construction materials used to build the cases and from conservation materials present on the exhibited works of art. The carboxylic acids involved were 2,4-dichlorobenzoic acid, formic acid, methacrylic acid, palmitic acid, and an unknown carboxylic acid, respectively emitted from peroxide-cured silicone gaskets, panels of medium-density fibreboard (MDF), UV-adhesive, beeswax containing products, and from an unknown acidic conservation product or binding medium. The identification of the crystalline deposits was supported by their syntheses in the laboratory. Since 2013, similar deposits have been observed in a number of museum collections worldwide. A treatment for preventing further growth of the deposits was developed and applied in the Rijksmuseum showcases.
Introduction
Several hundred display cases were designed, built, and installed by different companies for the newly renovated Rijksmuseum in April 2013. Four months after the installation, white needle-shaped deposits (hereafter also referred to as ‘crystals’) were observed in all 180 display cases built by Goppion S.p.A., on the glass windows, along the silicone door gaskets, and on the black structural adhesive seals (). Moreover, 473 objects of the 1438 works of art exhibited in the affected cases were covered with a thin whitish layer of these deposits. Objects affected were bronze sculptures, wooden frames and furniture, veneered panels, polychromes, lacquered chests, seals, objects containing turtle skin, waxed metal objects and leather, a terracotta sculpture, gilded objects, panel paintings with tempera, and two oil paintings on copper (). Glazed ceramics and objects made of gold and silver remained unaffected. Concerns were raised whether the crystalline deposits were harmful to the works of art and to what extent their appearance would impact display. It appeared that the soft and fragile deposits adhered only loosely to the objects and that most of the whitish haze could be easily removed by using a dry brush, cotton swab, or vacuum cleaner, or a wet brush with a mixture of ethanol and water. No optical evidence was found for surface damage to any type of object by using (digital) microscopy at high magnification. The deposits were neither acidic nor alkaline, and were found not to affect Cu, Ag, and Pb coupons after incubating the deposits with the coupons for 28 days at 60°C. The Rijksmuseum started research in September 2013 in collaboration with the Cultural Heritage Agency of the Netherlands, to find the cause of the deposit formation and a way to eliminate it.
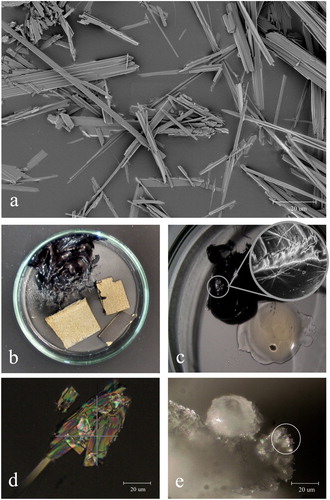
Figure 1. (a) Deposit of crystals on the window glass of a freestanding display case with hinged door and transparent silicone door gasket. (b) Close-up of crystals on silicone door gasket. (c) Long white crystals in the bottom corner of a wall-mounted display case with medium-density fibreboard (MDF-WJ) back panel, deposited on the black seals of structural adhesive Terostat-9220. (d) Close-up of crystals on the black adhesive seal. Crystals in a and b were identified as type K1, in c and d as type K2.

Figure 2. (a) Bronze Walking man, BK-16083, Anonymous, c.1580–1600, with a whitish deposit of crystals. (b) Close-up of arm covered with crystals. (c) Microscopic image of sparkling crystals on right leg. Crystals in a, b, and c were identified as type K4.

Figure 3. (a) Tempera on panel (pendant) of Two evangelists, SK-A-3980, Gherardo Starnina, c.1407 before exposure in the showcase. (b) Detail of the same object in raking light after exposure in the showcase, showing the crystalline deposit on the gold leaf in the upper right corner. Crystals in b were identified as type K5.

Figure 4. (a) Oil painting on copper Still life with flower in a glass, SK-A-2102, Jan Brueghel (II), c.1625–1630 before exposure in the showcase. (b) The same object after exposure in the showcase, showing tiny sparkling crystals, easily visible on dark paint using raking light. (c) Micrograph showing rosette-shaped crystals. Crystals in b and c were identified as type K5.

The occurrence of deposits formed on works of art is an unintended but known phenomenon in micro environments (Grzywacz Citation2006; Schieweck and Salthammer Citation2009; Schieweck, Salthammer, and Watts Citation2009; Engemann-Wendt and Waurick Citation2010; Dzullkiflli et al. Citation2018). On different kinds of objects various deposits have been reported. For example, on paintings the deposition of fatty acids is described, on bronzes the deposition of sodium copper acetate carbonate, on fossils the efflorescence of calclacite, and on ceramics the efflorescence of calcium formate, calcium acetate, and calcium sulphate salts. Gaseous pollution due to outgassing of exhibition materials is often involved in the formation of deposits. Therefore, several methods have been developed to analyse common harmful gasses emitting from exhibition materials (Schieweck, Markewitz, and Salthammer Citation2007; Ashley-Smith, Burmester, and Eibl Citation2013; Grøntoft, Lankester, and Thickett Citation2015; Grøntoft et al. Citation2016; Hackney Citation2016; Leyva Pernia Citation2019). Even though the Rijksmuseum display materials had been tested for outgassing prior to application, deposits had formed soon after installation of the cases.
The deposits formed in the Rijksmuseum showcases in 2013 were believed to be unique, but soon after, similar deposits were observed in several other museum collections (Hatchfield et al. Citation2015; Newman et al. Citation2015; Diehl et al. Citation2016). This paper aims to provide information on the cause of the deposits in the Rijksmuseum showcases. It describes the characterisation of five different deposits, the construction and conservation materials involved in their formation, the reaction mechanism, and their impact. The deposits were investigated with light microscopy (LM), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX), (pyrolysis-)gas chromatography-mass spectrometry ((Py-)GC-MS), and µ-Raman spectroscopy (µ-Raman). Deposits were synthesised in the laboratory to support the identification. A brief description is given of the effort made to find a solution to stop the crystal growth and how it was applied in the showcases of the Rijksmuseum.
Materials
Design of affected display cases
The 180 display cases affected by deposits are designed to be slim, elegant, and airtight (). The glass windows are bright and transparent. The cases have no power supply, no internal lighting, and no active or passive ventilation. Different types vary in airtightness, size, and shape, from square and rectangular to oval and circular, freestanding, wall mounted, with sliding or hinged doors, with glass domes, and some with back panels, tables, or pedestals. The museum climate is stable: the temperature is generally 20 ± 2°C and the relative humidity (RH) 53 ± 5%. Air exchange rates vary in cases with hinged and sliding doors. Hinged door showcases are more airtight: leakage is approximately 0.1% of the volume of the showcase per day. In showcases with sliding doors leakage is 0.8%. In the galleries all light sources are LED without UV and air quality conforms to the EU9 standard (Gustavsson Citation1996).
Figure 5. Photograph showing elegant design of a freestanding showcase with hinged door, transparent silicone door gasket, powder coated bottom deck, and support table. The white arrows indicate the locations where the structural adhesive Terostat-9220 is applied; the adhesive connects the glass windows with metal parts in the top and bottom.

Materials used in affected display cases
The main case components are glass and metal. The metal base, top, and profiles are made of powder-coated steel with a DURPOL SM wrinkled black TS HMF (EuroPolveri S.p.A.) coating. The laminated Artglass Protect 66.2 windows are treated with a two-sided antireflection coating, using titanium oxide applied with magnetron sputtering techniques (SIA GroGlass). Materials other than glass and metal, except for MDF, were tested for outgassing of common harmful compounds by the external laboratory Labo Consult Srl. Oximes, isocyanates, VOC total, volatile sulphides, formaldehyde, formic acid, and acetic acid were determined using gas chromatography for headspace analysis (EPA 5021A-2003 + EPA 8015D-2003). Products used for the showcases were selected based on outgassing levels within the standards and, if not available, based on test results demonstrating lowest emission. The metal parts and glass windows are joined with the strong black structural adhesive Terostat-9220 (Henkel), also known as Teroson-9220, a silane-modified polymer. Vertical edges of the glass windows are adhered with the transparent UV-adhesive Delo®-Photobond®-4468 (DELO). Transparent V-shaped and grey tubular silicone door gaskets are used in cases which needed to be most airtight, and black brush hair door seals are used in other cases. The silicone gaskets are moulded silicone rubber SR-70 (Posa S.p.A. and Technical Rubber Srl). The brush hair Pile Seals are made of polypropylene fibres on a solvent-free adhesive (Schlegel®). Other products used include magnetic gaskets made of SBR rubber, Dow Corning® 895 structural glazing sealant, X-treme sealer (Promante), powder coated Doluflex aluminium sandwich side panels, and VHB™ acrylic foam tape 4910F by 3M™. Medium-density fibreboard (MDF-WF) is used for back panels and pedestals (Novolegno S.p.A.). The technical datasheet of the material showed that it met the quality of ‘zero’ formaldehyde (<2 mg formaldehyde per 100 g dry weight).
Materials used in analyses and experiments
Specimens of original showcase materials and chemical reference compounds used in this study were supplied by the showcase building company and chemical vendors and were all used within the expiration date ().
Table 1. Reference compounds, construction materials, and conservation products used in analyses and experiments, including technical information.
Methods
Sampling
Although numerous, the electrostatic crystals were hard to pick up and collect in vials. They were sampled dry with the smallest paintbrush available. Samples therefore included dust and other particles along with the crystals. Five chemically different crystals were numbered chronologically in order of their discovery and named K1 to K5. ‘K’ stands for the Dutch word ‘kristal’ meaning crystal.
(Pyrolysis-)gas chromatography-mass spectrometry ((Py-)GC-MS)
Crystals, sealants, and reference compounds were analysed using a Thermo Scientific Trace1310 GC in combination with an ISQ MS. shows the various conditions used in the chromatographic analyses for each sample, depending on the research question and sample size, with the result that similar compounds appeared at different retention times in the chromatograms. A Frontier Laboratories multi-shot EGA/Py-3030D pyrolyser was used on all samples except on K2 and K5 crystals, for thermal desorption and pyrolysis, and at reduced temperature as a solvent injector. The MTBSTFA silylation agent was chosen for the analyses of crystals K3 and K4 for better detecting volatile acids compared to MSTFA. For the analyses of sealants and reference samples, volatile compounds were trapped and concentrated using the Frontier Laboratories microjet cryogene trap MJT-1030Ex. Analyses of Terostat-9220 specimens were carried out immediately after opening new cartridges. The samples were collected with a knitting needle, from the centre of the cartridges. Xcalibur software was used for mass spectral data acquisition. If peaks in the presented chromatograms are not specified in the text, they derive from the silylation and solvent agents, or are not relevant for this study.
Table 2. Instrument conditions used in the (Py-)GC-MS analyses of crystals K1-K5, Terostat-9220, and Tinuvin-770.
µ-Raman spectroscopy (µ-Raman)
The crystals were analysed using a Renishaw inVia Reflex confocal µ-Raman spectrometer with a green Nd:Yag laser operating at 532 nm, 1800 lines mm−1 grating, a Peltier-cooled CCD detector, and WIRE software. Spectra were acquired between 100 and 3300 cm−1 with a laser intensity of 1–5 mW, exposure time 10 s, and one accumulation. For detecting weak vibrations in the region of interest, static scans were used with a range of 1800 cm−1 and more than one accumulation. Due to high crystallinity of the samples, analyses were carried out with two to four different orientations of the sample. Peak locations were calculated by pre-set slope and threshold values. Values on the Y-axis expressed normalised counts with offset. Spectra were presented without subtraction of baselines.
Ion chromatography (IC)
Ion chromatography was used to identify the anion of the K2 crystal. Analysis was carried out on a Dionex ICS-2100 IC system (Thermo Fisher Scientific) equipped with Ionpac AS17-C 2 × 250 mm analytical and guard columns, a Dionex AERS500 suppressor, a Dionex CR-ATC trap column, a Dionex EGC-III eluent generator cartridge, and a Dionex DS6 conductivity detector. Potassium hydroxide was used as the eluent with a gradient ranging from 1.5 to 40 mM at a flow rate of 0.25 ml min−1 over a runtime of 20 min.
Scanning electron microscopy (SEM)
Scanning electron images of the crystals were taken using a FEI Nova NanoSEM450 (Thermo Fisher Scientific) electron microscope with an ultra-sensitive solid-state directional backscatter detector for low voltage imaging. Samples were not coated, and were photographed under high vacuum at 1 kV acceleration voltage. The working distance was 4.7 mm and the magnification was 1000.
Energy-dispersive X-ray spectroscopy (EDX)
EDX analyses were carried out using a Thermo Scientific liquid-free silicon drift detector and NSS7 software connected to a JEOL JSM5910LV SEM. All spectra were taken in low vacuum at a working distance of 10 mm using 20 kV acceleration voltage.
(Polarised) light microscopy ((P)LM)
A Zeiss Stemi2000 stereo microscope with raking light was used for investigations up to 50×, a Zeiss AxioLabA1 transmitted light microscope for investigations up to 500x in polarised transmitted light, and a Hirox KH8700 digital light microscope for magnifications up to 2500.
Determination of formaldehyde content
Analyses of the formaldehyde content of samples from MDF-WJ and MDF-ZF back panels were carried out in May 2015 by the Fraunhofer Institute for Wood Research, Braunschweig, Germany, using the European normalised Perforator Method DIN EN120:1992-08. The moisture content was determined according to DIN EN 322:1993-08 (Schwab, Marutzky, and Meyer Citation2012).
Laboratory experiments
Approximately 500 experiments were carried out in glass Petri dishes with diameters 6 and 10 cm, to imitate a miniature showcase with moderate airtightness (). Dishes were filled with combinations of construction and conservation materials, chemical reference compounds, bronze, oak wood, and brass with and without waxed surfaces, and were left at 20°C and 50% RH, similar to the conditions in the galleries. The dishes were monitored over time to check crystal growth.
Results
Characterisation and origin
In this section the identification of five crystals is described, including the chemical components and the construction and conservation materials involved.
Crystal K1
Appearance of K1
The first crystals were found in display cases with V-shaped transparent and tubular grey silicone door gaskets. The fragile crystals were light in weight, electrostatic, and aggregated at the surface of the gaskets. They dissolved in demineralised water, while the pH remained constant. Their appearance varied from long white needles (>1 cm) to small transparent sparkling dust (<0.5 µm, a,b). The electron micrograph shows that the crystals consist of bundled flat fibres less than 1 µm thick (a).
Figure 7. (a) Backscattered electron micrograph of crystals K1, sampled from a transparent silicone door gasket. Photograph: (Keriya Mam (FEI). (b) Petri dish with a lump of the black adhesive Terostat-9220 and three samples of MDF-WJ with and without coating. Long white K2 crystals are visible on Terostat-9220, photograph was taken one week after enclosure. (c) Long white K3 crystals growing on Terostat-9220 in a petri dish with uncured transparent UV-adhesive Delo-Photobond-4468. Photograph was taken one week after enclosure. (d) Polarised transmitted light micrograph of K4 crystals sampled from bronze sculpture Minerva and Cupido, BK-1959-2, Girolama Campagna, c.1600. (e) Micrograph of K4 crystals growing on the tip of palmitic acid granules as extensions (white circle). Photograph was taken half a year after enclosure of palmitic acid and TMP-ol (2,2,6,6-tetramethyl-4-piperidinol).
Chemical identification of K1
The crystals were analysed with SEM-EDX to determine their elemental composition. Chlorine, nitrogen, carbon, and oxygen were detected. Two main components 2,2,6,6-tetramethyl-4-piperidinol (TMP-ol) and 2,4-dichlorobenzoic acid (DCBA) were found in the Py-GC-MS chromatogram (a). Minor peaks were attributed to 2,4-dichlo-robenzene and 2,2,6,6-tetramethyl-4-piperidone. A Raman spectrum of the crystals was taken and compared with the spectra taken from the reference compounds of TMP-ol and DCBA. It showed that the crystals consisted of one single compound and that this compound was neither TMP-ol nor DCBA (a–c). The solid reference compounds TMP-ol (a base) and DCBA (an acid) were placed together in a petri dish at room temperature. The powders were not in contact with each other. Without the contribution of a third component or additional energy, white needle-shaped crystals developed, visible under magnification on top of the DCBA granules after three days. The crystals were analysed with µ-Raman and yielded a spectrum identical to the spectrum of the crystals sampled from the silicone gaskets.
Figure 8. (a) Py-GC-MS chromatogram of crystal K1. The crystal sample was taken from the silicone gasket of a hinged door showcase. (b) Py-GC-MS chromatograms of the black structural adhesive Terostat-9220 (brown) and of the reference compound Tinuvin-770 (orange). DCB = 2,4-dichlorobenzene, DCBA = 2,4-dichlorobenzoic acid, sebacic acid = 1,8-octanedicarboxylic acid, Tin-770 diester = bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate, Tin-770 monoester = 2,2,6,6-tetramethyl-4-piperidyl sebacate, TMP-ol = 2,2,6,6-tetramethyl-4-piperidinol, TMP-one = 2,2,6,6-tetramethyl-4-piperidone. Instrument conditions in Table 2.
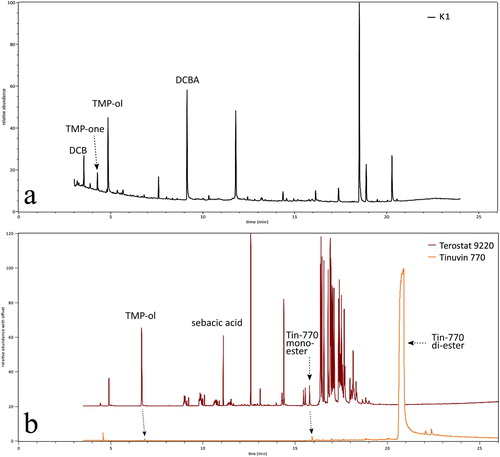
Figure 9. (a) Raman spectra of crystals K1–K5 with relevant vibrations. Samples of crystal K1 and K2 were taken from showcases, K3 from a petri dish experiment, K4 from the Wax seal of Floris, count of Holland NG-KOG-1901-52 anonymous 13th century, and K5 from the Amsterdam chest, AK-RAK-2013-3-1 Kaomi workshop c.1635-1645. (b) Raman spectra of the carboxylic acid reference compounds DCBA (2,4-dichlorobenzoic acid), formic acid (liquid), palmitic and stearic acid in corresponding colours of their associate crystals K1, K2 and K4. Methacrylic acid is not presented (K3). The acid involved in the formation of K5 is unknown. (c) Detailed Raman spectra of K4 and related crystals made with TMP-ol and palmitic acid, TMP-ol and stearic acid, Terostat-9220 and beeswax. Shaded areas indicate the zone of the CH3 and CH2 stretching vibrations, and the region indicative for chain length of long aliphatic compounds. Ar = aromatic, (a)sym = (a)symmetric, def = deformation, str = stretching, TMP-ol = 2,2,6,6-tetramethyl-4-piperidinol.
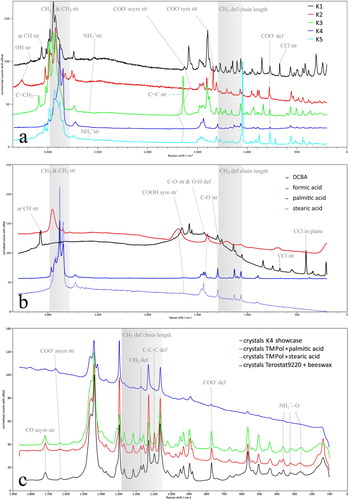
The Raman spectra of the crystals sampled from the silicone gaskets were compared with spectra taken from crystals produced in the laboratory with TMP-ol and other carboxylic acids (acetic acid, oxalic acid, trichloroacetic acid, benzoic acid). Apparently, other acids were able to form crystals with the base TMP-ol as well. A similarity between these spectra was observed: they all showed characteristic peaks of functional groups of organic salts. In this was demonstrated for the spectrum of the crystals taken from the silicone gaskets. It showed the N–H stretching bond of the secondary amine cation R2- as a broad vibration at 2540 cm−1. This peak was absent from the spectrum of the reference compound TMP-ol. The anion salt peaks were visible at 1627, 1378–1405, and 772 cm−1, respectively attributed to the weak band of the carboxylic acid anion COO− asymmetric stretching, to the strong bands of the COO− symmetric stretching, and to the COO− deformation vibration. That the crystals were neither an amine nor a carboxylic acid, was concluded from the missing N–H stretching bond (in TMP-ol strong at 3256 cm−1) and from the missing C=O symmetric stretching vibration typical for carboxylic acids (in DCBA strong at 1655 cm−1).
Based on the similarity of the Raman spectra of the crystals from the silicone gasket and those of the lab-produced crystals with TMP-ol and DCBA, it was confirmed that the unknown crystal from the silicone gasket was the result of a reaction between the compounds TMP-ol and DCBA, without the interference of another compound. Based on the occurrence of the ammonium cation and the carboxylate anion, demonstrated from the Raman spectrum, it was concluded that the crystal on the silicone gaskets is the organic ammonium salt of the acid–base reaction between the base TMP-ol and the acid DCBA. The crystal is named 2,2,6,6-tetramethyl-4-piperidyl 2,4-dichlorobenzoate, referred to as K1 in further text.
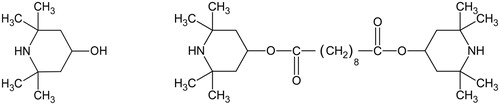
Origin of TMP-ol
The base 2,2,6,6-tetramethyl-4-piperidinol is a molecular fragment in the hindered amine light stabiliser Tinuvin-770, which is bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate, an alkaline diester. One molecule of Tinuvin-770 includes two molecules of TMP-ol, one at each end of the chain (). Tinuvin-770 inhibits the photo-oxidative degradation of polymers in powder coatings, adhesives, inks, and sealants (BASF product brochure EDC Citation2811e). For that reason, it is applied in many of those products. Tinuvin-770 is made industrially by esterification, using TMP-ol, sebacic acid, a catalyst, and heat (CitationPatent no. CN103980185A). In relation to the TMP-ol-containing crystals in the Rijksmuseum showcases, Py-GC-MS analyses were carried out on tapes and coatings in an attempt to find Tinuvin-770, the supposed source for TMP-ol. Surprisingly no Tinuvin-770 was found, while there was a high TMP-ol peak observed in the chromatogram of the black MS®-polymer Terostat-9220 (b). Other compounds were attributed to 1,8-octanedicarboxylic acid (sebacic acid) and 2,2,6,6-tetramethyl-4-piperidyl sebacate (the monoester of Tinuvin-770). Phenol, phenol-carbonic acid plasticisers, benzotriazole UV328, hindered phenol antioxidant, and Cenwax (methyl 12-hydroxyoctadecanoate) were also detected. The high amount of TMP-ol measured in Terostat-9220, in the absence of the diester Tinuvin-770, was unexpected because TMP-ol is not known as a light stabiliser by itself. In order to check whether this result was due to an analytical artefact (Tinuvin-770 converts into TMP-ol and sebacic acid by hydrolysis), a similar analysis was carried out with the reference compound of Tinuvin®-770 DF, using the same instrument conditions. This chromatogram showed, besides a high peak of Tinuvin-770, only traces of TMP-ol, the monoester, and sebacic acid ( and , b). This confirmed that the high amount of TMP-ol in the previous analysis of Terostat-9220 was not due to hydrolysis of Tinuvin-770 during the analysis, but that TMP-ol was present already in the cartridge.
Figure 10. Structural formulae for 2,2,6,6-tetramethyl-4-piperidinol (TMP-ol, left) and bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate (Tinuvin-770, right).
Relative percentages of the four relevant components in Terostat-9220 and Tinuvin-770 are given in . They show that TMP-ol was present in Terostat-9220 with a relative weight percentage of 41.3. Because TMP-ol was not found in other construction materials used for the affected Rijksmuseum showcases, it was concluded that the adhesive Terostat-9220 was the source of TMP-ol, the alkaline reagent in the crystal formation.
Table 3. Relative mole and weight percentages (rel. mole %, rel. wt.%) of four compounds in Terostat-9220 and Tinuvin-770.
Origin of DCBA
K1 crystals were found in all display cases with peroxide-cured silicone door gaskets. Py-GC-MS analyses were carried out on the two types of silicone gaskets present: the transparent V-shaped and the grey tubular gasket. A small amount of DCBA was detected in both types. DCBA is known as a by-product of the curing process of peroxide-cured silicone elastomers, where di(2,4-dichlorobenzoyl) peroxide is the catalysing agent in the production of polysiloxane (Colas, Malczewski, and Ulman Citation2004; Arlon product brochure Citation2004). Although the by-product usually volatilises during post curing, a small part may remain, which could migrate to the surface, sublimate, or deposit as a bloom.
Products involved in K1 formation
The confirmation that crystal K1 was formed by the reaction between DCBA and TMP-ol, sublimating from respectively the peroxide-cured silicone gaskets and the structural adhesive Terostat-9220, was demonstrated by bringing together specimens of the gasket and the adhesive in a glass petri dish. After less than a week, crystals were visible (under magnification) on the gasket and identified by µ-Raman as being K1. A similar experiment was carried out as a control, using custom-made Terostat-9220 without the addition of Tinuvin-770. In this experiment no crystals were formed.
Crystal K2
Appearance of K2
A second type of crystal (K2) was found on seals of the black adhesive Terostat-9220 (c,d). Terostat-9220 was applied in the top and bottom parts of the showcases, where glass windows meet metal parts (, white arrows). K2 crystals appeared as long white fragile needles. Based on their physical appearance, they were indistinguishable from K1 crystals. The crystals easily dissolved in demineralised water, while the pH remained constant.
Chemical identification of K2
The crystals from the seals were different from the crystals on the silicone gaskets (K1), as chlorine was lacking in the seal crystals. The elements C, O, and N were detected using SEM-EDX. TMP-ol was identified by GC-MS without pyrolysis as the key component. Ion chromatography identified the presence of a formate anion. The Raman spectrum showed peaks representative for the formate anion at frequencies 1572, 1342, and 778 cm−1, respectively attributed to the weak asymmetric and strong symmetric stretching vibrations of COO−, and to the deformation vibration of COO− (a). A broad and weak peak at c.2550 cm−1 represented the vibration of the piperidyl cation. The K2 crystal was synthesised in the laboratory by placing TMP-ol and formic acid (1M in water) in a petri dish at room temperature. The results led to the conclusion that crystal K2 was formed by the acid–base reaction of TMP-ol and formic acid. K2 is the organic ammonium salt named: 2,2,6,6-tetramethyl-4-piperidyl formate.
Origin of formic acid
The black adhesive Terostat-9220 was combined with various construction and conservation materials in petri dishes, to find the product responsible for the acid outgassing. Several combinations gained crystals, but only crystals formed in the dish with medium-density fibreboard of the type MDF-WJ, resulted in a spectral match with K2 crystals from the showcases. The synthesised K2 crystals had deposited on the black adhesive, similar to what was observed in the showcases, and were visible already after several days under magnification at 5× (Figures 1c,d and 7b).
Medium-density fibreboard MDF-WJ was used in 57 of the 180 polluted showcases for pedestals and back panels. In all these 57 showcases K2 crystals were found. MDF is known to emit formaldehyde, which is converted into formic acid in contact with water vapour from the air. Most of the formaldehyde emitted by MDF boards originates from the formaldehyde resin used as a binder for the wood fibres. Since formaldehyde is harmful to living organisms, MDF boards are available in different types for different applications, distinguished by formaldehyde content. According to the International Composite Board Emission Standards (ICBES), two European formaldehyde classes are distinguished, E1 and E2. For application in museums a third type of board was developed, using resin made of natural lignin instead of formaldehyde, named MDF-ZF (zero formaldehyde). For this type of board, the class E0 is used. However, since wood fibres are also a source of formaldehyde on their own, the formaldehyde content of MDF-ZF is never as low as zero, but between 0 and 2 mg per 100 g dry weight.
MDF-ZF was meant to be ordered for the 57 cases, but MDF-WJ (Novolegno) was unintentionally used instead. A specimen of this MDF and a specimen of MDF-ZF used in showcases built by another company were analysed by the Fraunhofer Institute for Wood Research, to determine the formaldehyde content using the perforation method () (Schwab, Marutzky, and Meyer Citation2012). A concentration of 10.6 mg per 100 g dry weight at moisture 6.5% was measured for MDF-WJ, which is higher than the limit accepted for MDF-ZF (2 mg per 100 g dry weight). MDF with that high concentration of formaldehyde is equivalent to the lower quality MDF-E1 with a maximum of 10 mg per 100 g dry weight. Such a high concentration is not regarded as suitable for use in showcases without air exchange. The control specimen of MDF-ZF contained 1.7 mg formaldehyde per 100 g dry weight.
Products involved in K2 formation
A final test in Petri dishes was carried out, using Terostat-9220 with both MDF-WJ and MDF-ZF. K2 crystals were formed only in the dish with MDF-WJ, not in the dish with MDF-ZF. Apparently in the first dish the concentration of formic acid – resulting from the reaction of formaldehyde and water from the air – was high enough to form K2 crystals. Based on this fact and the fact that K2 crystals did not form in any of the other dishes with construction materials, it was concluded that MDF-WJ was the main source of formic acid (from formaldehyde) in the formation of crystal K2.
Crystal K3
Appearance and products involved in K3 formation
Transparent UV-adhesive Delo-Photobond-4468 was applied in the showcases for connecting vertical edges of glass windows. In a Petri dish with Terostat-9220 (black) and the UV-adhesive (transparent), crystal K3 developed (c). Crystal K3 was the reaction product of TMP-ol, a component in Terostat-9220, and methacrylic acid (MAA), a component in the UV-adhesive. This was confirmed by the spectral match of crystals in the dish with reference compounds TMP-ol and MAA. The water-soluble crystals appeared as long white needles on the black adhesive Terostat-9220 and on TMP-ol within a week. K3 deposits were not found in the showcases of the galleries. It was believed that this was related to limited possibilities for inspection at locations where the crystals would have formed. They were expected to form under the bottom deck, where spilled adhesive could have ended up, out of the reach of the UV-lights, leaving the uncured carboxylic acid MAA behind. Though not found in the galleries, it was important to include K3 in this study, as the construction materials needed for its formation were used in the affected cases.
Chemical identification of K3
In the Py-GC-MS chromatogram of crystal K3, TMP-ol appeared as a main component. The Raman spectrum showed a weak asymmetric and strong symmetric stretching vibration of the COO− carboxylate ion at respectively 1672 (shoulder) and 1401 cm−1, and a strong COO− deformation vibration at 771 cm−1 (a). A weak vibration was detected at 2582 cm−1. The peak at 1644 cm−1 was attributed to the C = C bond. The results indicated that K3 was formed in the same way as described for K1 and K2, by an acid–base reaction. The organic salt K3 is named 2,2,6,6-tetramethyl-4-piperidyl methacrylate.
Crystal K4
Appearance of K4
The fourth crystal appeared on surfaces of bronze sculptures and on objects made of or treated with wax, such as wax seals, puppets, leather belts, tin vases, shoes, and guns. Within six months after installation approximately 150 objects were covered with a whitish haze of K4 crystals (a–c). Samples were taken from bronze sculptures, wax seals, and from a pewter vase. Macroscopically K4 crystals resembled sparkling dust (). Using a light microscope, the particles appeared as stacked crystalline sheets with flat ends (d). K4 was not soluble in demineralised water.
Chemical identification of K4
TMP-ol was detected with Py-GC-MS. The elements C, O, and N were distinguished using SEM-EDX. The Raman spectrum showed features characteristic for molecules with long saturated aliphatic hydrocarbon chains; weak vibrations at frequencies below 1000 cm−1, dominant CH3 and CH2 stretching vibrations around 2900 cm−1, and no C=C double bond vibration between 1630 and 1680 cm−1 (a).
Based on the strong resemblance of the K4 spectrum and that of saturated carboxylic acids palmitic and stearic acid in the high wavenumber region, the characterisation of K4 was begun by the attempt to synthesise the crystals in parallel experiments with TMP-ol and both palmitic (Sigma-Aldrich) and stearic acid (Stearin, Kremer Pigmente) (Hu, Hsu, and Wang Citation1992; Robinet and Corbeil Citation2003; Czamara et al. Citation2015) (b). After six months, tiny sheet-shaped crystals had grown on top of both acidic granules, visible at a magnification of 400 (e, for palmitic acid). The Raman spectra of crystals made with TMP-ol and palmitic acid were similar to that of K4 (c). The spectrum of the crystals made with stearic acid slightly differed in the region characteristic for chain length (1050–1300 cm−1). Due to the longer chain length of stearic acid compared to palmitic acid, the CH2 deformation vibration, and a C–C stretching or C–C–C deformation vibration of the piperidyl stearate salt were found at higher wavenumbers than in the piperidyl palmitate salt, at 1177 and 1104 cm−1 respectively (). The vibration at 1104 cm−1 was also significantly weaker in the piperidyl stearate salt than in the piperidyl palmitate salt (K4).
Table 4. Raman frequencies (cm−1) and their attributions relevant for this study of K4 crystals, related synthesised crystals and reference compounds.
Vibrations characteristic for carboxylate salts were mostly weak in K4. Therefore, interpretation of bands was supported by earlier studies of carboxylic acids and carboxylates, and by spectra of the reference compounds TMP-ol, palmitic and stearic acid (Freeman Citation1958; Visser and Van der Maas Citation1978; Strommen et al. Citation1987; Ahn and Franses Citation1992). The spectra of 2,2,6,6-tetramethyl-4-piperidyl stearate (TMP-yl stearate) and Tinuvin-770 were added to understand peak differences in esters and organic salts. Wavenumbers of bands and their attribution were given in and indicated in b,c. The clearest salt vibration was recovered at 771 cm−1, deriving from the COO− deformation vibration. The weak asymmetric stretching vibration of COO− occurred at 1631 cm−1. The stretching vibration and the COO− symmetric stretching were too weak to be determined. Three vibrations of the ion bond of
-O showed at 266, 324, and 369 cm−1. The clearest indication that K4 was not the ester of TMP-ol and palmitic acid was the missing NH band, present in the esters TMP-yl stearate and Tinuvin-770 at respectively 3319 and 3322 cm−1. Other esters vibrations in TMP-yl stearate and Tinuvin-770 did not appear as well; the C–O–C asymmetric stretching at 1233 and 1234 cm−1, and the C–O–C deformation vibration at 799 and 794 cm−1. The weak peak at 1717 cm−1 was believed to reflect the asymmetric stretching vibration of the carbonyl group in a large complex of K4 salt molecules attached to each other by van der Waals forces (Karvas et al. Citation1988). According to Karvas et al. (Citation1988) piperidyl salts made with aliphatic mono-carboxylic acids with more than eight CH2 groups gain large sheet-shaped complexes – connected by hydrogen bonds – with an acid to base ratio of on average 2:1. Based on the salt vibrations in the Raman spectrum and the information in this paper, it was proposed that the K4 crystal was a complex organic salt formed by the reaction of one molecule TMP-ol and two molecules of palmitic acid, bridged by hydrogen bonding. The K4 crystal is named bis(2,2,6,6-tetramethyl-4-piperidyl) palmitate.
Origin of palmitic acid
Palmitic acid is one of the most common saturated fatty acids found in animals, plants, and micro-organisms. In conservation materials it occurs in vegetable oils and waxes made of natural products, which often contain stearic acid as well. Beeswax is one of those products; it contains a relatively high percentage of palmitic acid (8%), although the content can vary depending on the type (Tulloch Citation1970).
Products involved in K4 formation
In a Petri dish with Terostat-9220 and granular beeswax (Verfmolen de Kat) crystals formed after half a year. The spectrum of these crystals resembled to a large extent that of K4, although peaks were less pronounced (c). Peaks were weaker because beeswax is a mixture of compounds, and mixtures in general show less pronounced and wider peaks. Although weak, the peaks at frequencies below 900 cm−1 were matching those of K4 in every detail. This result supported the conclusion that beeswax is capable of forming K4 crystals when enclosed with Terostat-9220. As beeswax is often mixed with solvents and other compounds in a wide variety of materials, the source of palmitic acid responsible for the formation of K4 crystals is assumed to come from other materials in addition to beeswax. In this study other products with palmitic acid are not specified further.
Crystal K5
Appearance of K5
The fifth crystal (K5) deposited on surfaces of wooden frames and furniture, veneered panels, lacquered chests, polychromes, gilded objects, panel paintings with tempera, and on two oil paintings on copper ( and ). About 300 objects were covered with K5 crystals. The crystals mainly appeared on decorated front sides. The whitish deposits were visible five months after installation and gave the objects a dull appearance. Samples were taken from surfaces of a wooden cabinet, a lacquered chest, an armchair, tempera on panel, a gilded vase, a polychrome wooden sculpture, and an oil painting on copper. Similar to K1-K3, K5 was soluble in demineralised water.
Chemical identification of K5
TMP-ol was found in all K5 samples using GC-MS without pyrolysis. The Raman spectrum showed a pattern comparable to the patterns found in spectra of the other crystals, with a COO− deformation vibration at 715 cm−1 attributed to a carboxylic acid salt (a). Different from the other crystal spectra is the pronounced peak at 1046 cm−1. No carboxylic acid was found in any of the GC-MS chromatograms. Attempts to identify the unknown carboxylic acid using experimental syntheses were unsuccessful. The acidic materials tested with TMP-ol and Terostat-9220 were listed in . The selection of the materials was based on several criteria. (1) The unknown carboxylic acid is expected to derive from a commonly used conservation product applied as finishing layers on wood, lacquer, tempera and oil paint, or from a commonly used binding medium; many objects showed crystals on treated or decorated front sides, not on untreated back sides. (2) Disinfectants were excluded from the experiments because they were assumed to be applied to all sides of objects. (3) Acids with a higher vapour pressure than that of TMP-ol were excluded (Discussion). (4) The last selection criterium was directed by the pronounced Raman peak at 1046 cm−1. Such a strong peak could derive from a vibration in (hydrogen) carbonate salts and esters (1090–1020 cm−1) but could also derive from salts synthesised from carboxylic acids with a mono-substituted benzene ring, like phenylalanine. Phenylalanine is an amino acid which occurs in the proteinaceous binding medium casein, showing a prominent peak at 1002 cm−1 which is attributed to the aromatic breathing of the benzene ring (Vandenabeele et al. Citation2000).
Table 5. Listing of carboxylic acids well-known in conservation, (hydrogen) carbonates, (aromatic) amino acids, and common conservation products and binding media, used with TMP-ol or Terostat-9220 in order to test the possible synthesis of K5 crystals.
Products involved in K5 formation
Although the acid related to the K5 formation was not identified, based on the COO− and vibrations in the Raman spectrum, and the timing and manner in which the crystals were formed, K5 is believed to be an organic piperidyl salt, possibly a piperidyl carbonate complex. Because many objects were covered with K5 crystals on decorated front sides only, it was concluded that the unidentified carboxylic acid is likely to have derived from a common acidic conservation product used for finishing layers or from a binding medium.
Summary
The characterisation and origin of the crystals are summarised in the overview presented in and .
Table 6. Overview of compounds involved in the formation of the five deposits found in the Rijksmuseum display cases, their (proposed) structural formulas and reaction equations.
Table 7. Overview of construction and conservation materials involved in the formation of the five deposits found in the Rijksmuseum display cases, the location of the deposits and the time needed for their formation in petri dishes.
Discussion
Adhesive
Research on the origin of the crystalline deposits in Rijksmuseum showcases led to the conclusion that the deposits were related to the alkaline amine TMP-ol, a component in the structural adhesive Terostat-9220. The presence of a small quantity of TMP-ol in the adhesive was explained as the residual of unreacted TMP-ol in the synthesis of the light stabiliser Tinuvin-770 by the manufacturer (personal communication). An analysis of the chemical reference compound Tinuvin-770 from CIBA in this study, indeed showed that a very small amount of TMP-ol was present in the light stabiliser (). However, such a small quantity of TMP-ol was inconsistent with the large quantity of deposits formed in the showcases. Measurements demonstrated that there was more TMP-ol available in the adhesive than there could have been if its occurrence was only an impurity of Tinuvin-770 (, Supplemental Tables 1 and 2, Supplemental Data). From a weight experiment the conclusion was drawn that already 9–82 times more crystals were formed after three years, than could have been formed when regarding TMP-ol as impurity. Two hypotheses were formulated to explain the unexpected high TMP-ol content. The first hypothesis suggests that TMP-ol was formed by degradation of the light stabiliser inside the adhesive, before using the cartridge. The second hypothesis suggests a change in the production of the adhesive. Instead of adding Tinuvin-770 from a chemical vendor, TMP-ol and sebacic acid could have been added separately in order to produce Tinuvin-770 during the production process of the polymer; incomplete mixing or an imbalance could have resulted in the components remaining separate.
In the analyses performed at Rijksmuseum, no or very little Tinuvin-770 molecules were detected in the adhesive Terostat-9220. This shortcoming did not affect the adhesion strength but would imply a (partial) lack of protection from photodegradation in an environment prone to sunlight. In the museum this was not an issue due to the UV-free illumination.
Reaction mechanism and vapour pressure
The reagents involved in the deposit formation were physically separated inside the museum cases. It could be deduced that the acidic and alkaline reagents all had a strong evaporation capacity which caused them to migrate to the surface of the source products, where they subsequently turned into the gas phase. If a gas is readily emitted from its source material (high vapour pressure), it more easily becomes distributed throughout the showcase. The reagent with the highest vapour pressure filled the micro-environment with concentrations high enough to react with the less volatile reagent at substantial distances, where – in this case – it formed organic salts by crystallisation. It was observed that the crystalline deposits were formed primarily on or near the compound with the lowest vapour pressure (Supplemental Table 3).
Airtight showcases
Airtight showcases explain why so many deposits formed inside the showcases, while nothing happens when the adhesive is used in an open environment. Air exchange rates are low in all Rijksmuseum showcases; there is no active or passive ventilation and gaskets tightly seal spaces around the doors. This led to an unintentional build-up of emitted gasses, supporting the formation of the deposits. The concentration of gaseous TMP-ol in the showcases was not measured, but estimations showed that the concentrations of TMP-ol could have been very high, in the hypothetical situation that all TMP-ol had accumulated till exhaustion of Terostat-9220 without intervention, crystal formation or air leakage (Supplemental Data, values in red).
Impact and treatment
The formation of deposits did not stop after three years; each time a cleaned object was reinstalled in a cleaned (but not treated) showcase, deposits developed at the same rate as before. When regarding a constant rate of crystal formation, it was estimated that the crystal formation would continue for over half a century, due to the airtightness of the Rijksmuseum cases and the excessive use of the adhesive in deep narrow seals in the bottom parts as a consequence of the elegant design (Supplemental Table 2). Taking this long-lasting emission of TMP-ol into consideration, the museum aimed for a solution that would be effective in the long term, even if this would imply a major intervention. A solution that absorbs or filters the harmful component, one that other museums were looking into (Hatchfield et al. Citation2015; Diehl et al. Citation2016), was no option for the Rijksmuseum, because this strategy would require years of attention and concern due to the design of the cases and the amount of adhesive used. In addition, the showcases are not made to hold scavengers and/or filter systems. Therefore, a solution was chosen that would prevent the adhesive from releasing TMP-ol into the display cases.
Sealing Terostat-9220 with the two-component epoxy Araldite-4859 was found the best method to block the emission of TMP-ol from the adhesive (see Supplemental Table 4). The Araldite adhered well to Terostat, it is hard and strong, it is flexible enough (showing no rupture during or after application), drying time was within application time limits, lifetime was presumed to be decades, it has a good chemical resistance, it contains no acids and no TMP-ol, and it behaved well in compatibility tests and aging test on Cu, Pb, and Ag coupons and on glass. Laboratory tests showed that no new crystals grew on fresh peroxide-cured silicone gaskets when Terostat was covered with an Araldite-4859 seal in a Petri dish (tested over a period of two years). Similar results were gained from test showcases where Araldite-4859 was applied on top of the original Terostat seals (tested over a period of one year). Four pilot studies were carried out to test logistics and to develop the application of Araldite-4859 in the galleries on large scale. To obtain smooth aesthetic and airtight seals, the Araldite-4859 was heated at 60°C for five hours.
Although it was unknown how long Araldite-4859 would prevent new crystal growth, after nearly three years of research and testing, the museum decided to start the treatment in all 180 affected showcases. It was a major intervention: not only was the treatment complicated, but 473 objects also had to be taken out of the cases, cleaned, and repositioned. Acidic materials were replaced by non-acidic materials where possible: MDF-WJ back panels by Dibond® panels (aluminium and polyethylene) and peroxide-cured silicone door gaskets by platinum-cured silicone gaskets. At the time of writing, four years after the intervention, the crystals did not return in 176 of the 180 display cases treated. In only four display cases traces of deposits were observed recently. We hypothesised this is due to cracking of the Araldite seals caused by recent handling of the showcases.
Lessons learned and future strategy
In the field of preventive conservation, TMP-ol is an unusual compound. The compound has not been included in standard emission tests, and if it had, by the lack of product compatibility tests no concerns would have been raised. The crystal formation described in this paper and known from some other museums, is an unfortunate example illustrating that the standard tests available for preventive conservation are insufficient and do not cover all phenomena that could possibly occur. This is especially true because nowadays airtight showcases are available, with the result that material emission has become increasingly critical, and threshold values continue to drop. To avoid future complications related to TMP-ol crystal formation, it is important to add a TMP-ol test to the standard test methods, as well as a compatibility test. This is especially true because TMP-ol appeared not to be limited to one type and brand of adhesives (unpublished results).
Conclusions
Five different crystalline deposits, observed in 180 showcases on artworks as well as construction materials, were characterised mainly by (Py-)GC-MS and µ-Raman. All crystals were formed by the same mechanism and were classified as organic piperidyl salts. The crystals were regarded to be the result of an acid–base reaction; they all contained a carboxylate anion (COO−) and a secondary ammonium cation (). The ammonium cation derived from the base compound 2,2,6,6-tetramethyl-4-piperidinol (TMP-ol). This compound was the common factor in all crystal formations and known as part in the light stabiliser Tinuvin-770. The carboxylate anions derived from five different carboxylic acids, respectively 2,4-dichlorobenzoic acid, formic acid, methacrylic acid, palmitic acid, and an unknown carboxylic acid. The product responsible for the emission of the base TMP-ol was the black structural adhesive Terostat-9220. It was used in large quantities to adhere glass windows to metal structural elements of the cases. The materials emitting the carboxylic acids were construction products, as well as materials used in or on works of art. The products involved were peroxide-cured silicone gaskets (K1), Medium-density fibreboards (K2), UV-adhesive (K3), beeswax-containing products (K4), and an unknown but common acidic conservation product or binding medium used on (finishing layers of) wood, lacquer, tempera, and oil paint (K5). Crystal K3 was not found in the galleries but included in this study because it was expected to occur. No component other than TMP-ol and carboxylic acids was required for synthesising the crystalline deposits in the laboratory. The emission of the base TMP-ol from the adhesive Terostat-9220 was a phenomenon inherent in the product itself, as demonstrated by Py-GC-MS analysis. The high amount of TMP-ol which is harmful in a tightly sealed environment appeared to be an unintentional occurrence. The epoxy Araldite-4859 was used to cover the original Terostat-9220 seals to stop the release of TMP-ol into the showcases.
Supplemental Material
Download Zip (2 MB)Acknowledgements
The authors are grateful for the organisational support of Raphael van Amerongen from the Rijksmuseum Projectbureau and for the great collaboration with all parties from the display case company Goppion, in particular Sandro Goppion, Patrizia Venturini, Peter Hohenstatt, and Camillo Pezzini. They made it possible to bring the project to a favourable end. We thank Ken Sutherland and Rachel Sabino (AIC, Chicago), Sabine Stanek and Johanna Diehl (KHM, Vienna), Gerhard Schottner and Gabriele Maas-Diegeler (Fraunhofer ISC, Würzburg), Oscar Chiantore and Tomasso Poli (University of Turin), and Peter de Peindert (VibSpec) for the fruitful discussions and sharing of knowledge. We thank Luc Megens and Suzan de Groot (RCE) for their analytical support in the beginning of the research, and Sara Creange and Judith van der Brugge-Mulder (Rijksmuseum, Amsterdam) for their dedication to the project and their useful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahn, D. J., and E. I. Franses. 1992. “Orientations of Chain Axes and Transition Moments in Langmuir-Blodgett Monolayers Determined by Polarized FTIR-ATR Spectroscopy.” Journal of Physical Chemistry 96 (24): 9952–9959. doi: 10.1021/j100203a068
- Arlon product brochure. 2004. “Outgassing of Silicone Heater Compounds.” White-paper of Silicone Technologies Division, 8. Accessed August 27, 2020, 1–8. Accessed August 27, 2020. https://www.thomasnet.com/knowledge/white-paper/outgassing-of-silicone-heater-compounds.
- Ashley-Smith, J., A. Burmester, and M. Eibl. 2013. “Climate for Collections-Standards and Uncertainties.” Postprints of the Munich Climate Conference 7 to 9 November 2012, Doerner Institut, 1-452. Accessed October 23, 2013. https://www.doernerinstitut.de/downloads/Climate_for_Collections.pdf.
- BASF product brochure EDC. 2811e. “Coatings that Stay Looking Good: BASF Performance Additives.” BASF Product Guide.
- Colas, A., R. Malczewski, and K. Ulman. 2004. “Silicone Tubing for Pharmaceutical Processing.” Dow Corning Form 52-1067-01: 1–9.
- Czamara, K., K. Majzner, M. Z. Pacia, K. Kochan, A. Kaczor, and M. Baranska. 2015. “Raman Spectroscopy of Lipids: A Review.” Journal of Raman Spectroscopy 46 (1): 4–20. doi: 10.1002/jrs.4607
- Diehl, J., S. Stanek, H. Hanzer, M. Griesser, B. Goldmann, and V. Pitthard. 2016. “Preventive Conservation Strategies in the Re-opened Collection of the Kunstkammer of the Kunsthistorisches Museum Vienna: Theory versus Practice.” Presented by S. Stanek at Indoor Air Quality 2016: Heritage Research to Conservation Practice, 12th International Conference, 3–4 March 2016, Birmingham. Accessed January 28, 2019. https://iaq.dk/iap/iaq2016/Diehl_IAQ2016.pdf.
- Dzullkiflli, S. N. M., A. H. Abdullah, L. Y. Yong, A. M. Leman, and S. Sohu. 2018. “A Study of Indoor Air Quality in Refurbished Museum Building.” Civil Engineering Journal 4 (11): 2596–2605. doi: 10.28991/cej-03091184
- Engemann-Wendt, C., and T. Waurick. 2010. “Chronologie einer Schatzkammer: vom Barocken Gesamtkunstwerk zu Hightech-Vitrinen.” Beiträge zur Erhaltung von Kunst- und Kulturgut 1: 14–25.
- Freeman, J. P. 1958. “The Carbonyl Stretching Frequencies of Certain Carboxylic Acid Derivates.” Journal of American Chemical Society 80 (22): 5954–5950. doi: 10.1021/ja01555a017
- Grøntoft, T., P. Lankester, and D. Thickett. 2015. “Reduction of Acidic Pollutant Gases Inside Showcases by the Use of Activated Carbon Absorbers.” e-Preservation Science 12: 28–37.
- Grøntoft, T., D. Thickett, P. Lankester, S. Hachkney, J. H. Townsend, K. Ramsholt, and M. Garrido. 2016. “Assessment of Indoor Air Quality and the Risk of Damage to Cultural Heritage Objects Using MEMORI® Dosimetry.” Studies in Conservation 61 (S1): 70–82. doi: 10.1080/00393630.2015.1131477
- Grzywacz, C. M. 2006. “Monitoring for Gaseous Pollutants in Museum Environments.” Tools for Conservation, 190. Los Angeles: Getty Conservation Institute. Accessed August 27, 2020, http://hdl.handle.net/10020/gci_pubs/monitoring_gaseous.
- Gustavsson, J. 1996. “New Developments in Air Filter Test Methods and Classification.” Filtration & Separation 33 (2): 150–160. doi: 10.1016/S0015-1882(97)84207-3
- Hackney, S. 2016. “Colour Measurement of Acid-Detector Strips for the Quantification of Volatile Organic Acids in Storage Conditions.” Studies in Conservation 61 (S1): 55–69. doi: 10.1080/00393630.2016.1140935
- Hatchfield, P., S. Goppion, O. Chiantore, T. Poli, C. Riedo, K. Suslick, and M. Abraham. 2015. “Strange Events Inside Display Cases at the Museum of Fine Arts, Boston, and Lessons to Be Learned From Them – Part 2. Beyond the Oddy Test – the Way Forward.” Presented at Conservation and Exhibition Planning: Material Testing for Design, Display, and Packing, 19-20 November 2015, Lunder Conservation Center, Washington, DC. Accessed January 14, 2017. https://www.conservation-us.org/docs/default-source/education/materialtestingconference-2015-abstractbooklet2FEA0168725A.pdf.
- Hu, Z. S., S. M. Hsu, and P. S. Wang. 1992. “Tribochemical Reaction of Stearic Acid on Copper Surface Studied by Surface Enhanced Raman Spectroscopy.” Tribology Transactions 35 (3): 417–422. doi: 10.1080/10402009208982137
- Karvas, M., M. Göghová, J. Durmis, and V. Vrábel. 1988. “Structural Peculiarities of Salts of 2,2,6,6-Tetramethyl-4-Piperidinol with Aliphatic Monocarboxylic Acids.” Chemical Papers 42 (3): 355–363.
- Leyva Pernia, D. 2019. “Development of an Indoor Air Quality Index for Heritage Conservation: An Exploratory Study.” PhD thesis, University of Antwerp, 1-105.
- Newman, R., M. Derrick, E. Byrne, M. Tan, O. Chiantore, T. Poli, and C. Riedo. 2015. “Strange Events Inside Display Cases at the Museum of Fine Arts, Boston, and Lessons To Be Learned From Them – Part 1.” Presented at Conservation and Exhibition Planning: Material Testing for Design, Display, and Packing, 19–20 November 2015, Lunder Conservation Center, Washington, DC. Accessed January 14, 2017. https://www.conservation-us.org/docs/default-source/education/materialtestingconference-2015-abstractbooklet2FEA0168725A.pdf.
- Patent no. CN103980185A. 2014. Synthesis of Tinuvin-770, Summary Translated from Chinese.
- Robinet, L., and M. C. Corbeil. 2003. “The Characterization of Metal Soaps.” Studies in Conservation 48 (1): 23–40. doi: 10.1179/sic.2003.48.1.23
- Schieweck, A., D. Markewitz, and T. Salthammer. 2007. “Screening Emission Analysis of Construction Materials and Evaluation of Airborne Pollutants in Newly Constructed Display Cases.” In Museum Microclimates. edited by T. Padfield and K. Borchersen, 67–72. Copenhagen: National Museum of Denmark.
- Schieweck, A., and T. Salthammer. 2009. “Emissions from Construction and Decoration Materials for Museum Showcases.” Studies in Conservation 54 (4): 218–235. doi: 10.1179/sic.2009.54.4.218
- Schieweck, A., T. Salthammer, and S. F. Watts. 2009. “Indoor Pollutants in the Museum Environment.” In Organic Indoor Air Pollutants Occurrence, Measurement, Evaluation, edited by T. Salthammer, and E. Uhde, 2nd ed., 273–300. Weinheim: Wiley-VCH. https://doi.org/10.1002/9783527628889.ch12
- Schwab, H., R. Marutzky, and B. Meyer. 2012. “European Regulations for Formaldehyde.” Presentation Fraunhofer Institute for Wood Research. Braunschweig: Wilhelm-Klauditz-Institut. Accessed August 27, 2020. http://owic.oregonstate.edu/sites/default/files/pubs/Schwab.pdf
- Strommen, D. P., A. M. Giroud-Godquin, P. Maldivi, J. C. Marchon, and B. Marchon. 1987. “Vibrational Studies of Some Dicopper Tetracarboxylates Which Exhibit a Thermotropic Columnar Mesophase.” Liquid Crystals 2 (5): 689–699. doi: 10.1080/02678298708086327
- Tulloch, A. P. 1970. “The Composition of Beeswax and Other Waxes Secreted by Insects.” Lipids 5 (2): 247–258. doi: 10.1007/BF02532476
- Vandenabeele, P., B. Wehling, L. Moens, H. Edwards, M. De Reu, and G. Van Hooydonk. 2000. “Analysis with Micro-Raman Spectroscopy of Natural Organic Binding Media and Varnishes Used in Art.” Analytica Chimica Acta 407 (1–2): 261–274. doi: 10.1016/S0003-2670(99)00827-2
- Visser, T., and J. H. Van der Maas. 1978. “Systematic Interpretation of Raman Spectra of Organic Compounds.” Journal of Raman Spectroscopy 7 (3): 125–129. doi: 10.1002/jrs.1250070304

