Abstract
Chromium trioxide and N-hydroxyphthalimide (NHPI) supported on activated clay could serve as an efficient and mild oxidant for allylic selective oxidation of Δ5-sterols. Thus, a ketone group could be easily introduced into the allylic position of Δ5-sterols with the existence of a sensitive 3β-hydroxyl group. The oxidant residue can be removed easily from the reaction mixture by filtration and reused after reactivation at 120 °C for 4–6 h.
7-Keto-Δ5-sterols are found in animal tissues, food products, and certain folk medicines and are reported as inhibitors of sterol biosynthesis, HMG-CoA reductates, and cell replication.[ Citation 1 ] Therefore, a great number of oxidants for allylic oxidation of Δ5-sterols are developed to obtain 7-keto-Δ5-sterols. The most common allylic oxidants used are CrO3 complexes, such as Collins reagent,[ Citation 2 ] pyridinium chlorochromate (PCC),[ Citation 3 ] pyridinium dichromate (PDC),[ Citation 4 ] CrO3-3,5-dimethylpyrazole,[ Citation 5 ]pyridinium fluorochromate (PFC),[ Citation 6 ] and so on. However, a large excess of oxidants (about 10–30 equiv.) must be applied in this process, which led to a difficult workup process and production of environmentally hazardous chromium residues. Recently, low molar ratios of the oxidants are realized by using CrO3/NHPI in a cosolvent system of water and organic solvents[ Citation 7 ] however, the complicated separation procedure and difficult disposal of the toxic chromium residues remained unsolved.
Many supported reagents were studied, and some provided particularly environmentally friendly mild procedures in acceptable yields,[ Citation 8 ] Among them, Cr(VI)-supported reagents were used as efficient oxidants for the transformation of hydroxyl compounds to carbonyl compounds;[ Citation 9 ] unfortunately only few are related to the allylic oxidation.[ Citation 10 ] In this article, we report that CrO3 and N-hydroxyphthalimide (NHPI) adsorbed on activated clay could be applied as a new supported reagent for allylic selective oxidation of Δ5-sterols. The reactions were carried out under room temperature, the chromium residues could be easily removed from reaction mixture by filtration and reused by reactivation at 120 °C for 4–6 h. The structures of the products were characterized by infrared (IR) and NMR. The sensitive 3β-hydroxyl groups in the Δ5-sterols remained uninfluenced.
As far as we know, reported oxidants, including CrO3 complexes or t-butyl hydroperoxide (TBHP), combined with different types of catalysts or cooxidations,[ Citation 11 ] when used as allylic oxidants of 3β-hydroxyl Δ5-sterols. In most cases, the 3β-hydroxyl group should be protected before oxidation, usually as the acetate or benzoate ester[ Citation 1 ]; if not, 3β-hydroxyl group would be oxidized to a ketone group. For example, oxidation of steroidal 5-en-3β-ol (via intermediate 5-en-3β-one) yielded 4-en-3,6-diones instead of 7-one-5-en-3β-ol using of Jones reagent[ Citation 12 ] or PCC.[ Citation 13 ]
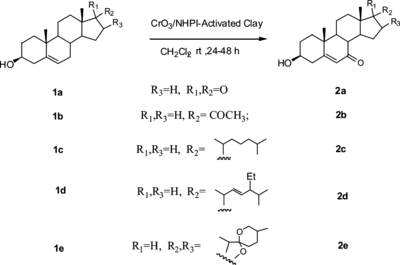
To demonstrate the validity and regioselectivity of the CrO3/NHPI-activated clay system developed herein, some model compounds were tested, and the results are summarized in Table . In all cases, the 7-keto-Δ5-sterols were synthesized successfully in acceptable yields by using this supported oxidizing system; the 3β-hydroxyl group remained uninfluenced as concluded from the IR and NMR spectra of the corresponding oxidized products (compounds 1a–1e) (Scheme ). For Δ5-steroid with the protected 3β-hydroxyl group (compound 1f), the allylic oxidation remained similar, but increasing reaction rate and oxidizing yield were observed (Scheme ). The oxidant residue can be recovered by reactivation at 120 °C for 4–6 h and reused for the new oxidizing reaction. For example, oxidizing compound 1f with the recovered oxidant still gave 2f in good yield (60%). Note that for compound 1g, after the oxidizing reaction, the acetal protecting group was cleaved to 2a (as concluded from 1H NMR and 13C NMR). Probably the oxidizing conditions used here are not mild enough for the acid-sensitive acetal protecting group in 1g to survive the transformation (Scheme ).
Scheme 2 Allylic selective oxidation of compound 1f with protected 3β-hydroxyl group to compound 2f.
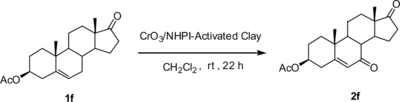
Scheme 3 Allylic selective oxidation of compound 1g to compound 2a accompanied by cleavage of the acetal protecting group.
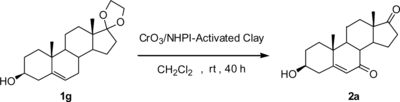
Table 1. Allylic oxidations with CrO3/NHPI-activated clay
In summary, CrO3/NHPI-activated clay in CH2Cl2 proved to be an effective oxidant for allylic oxidation of Δ5-sterols. In this way, allylic hydrogen atoms of Δ5-sterols could be oxidized to a ketone group with the existence of the sensitive 3β-hydroxyl group, and the chromium residues can be removed easily by filtration after the reaction and reused after reactivation at 120 °C for 4–6 h.
The reactions can be carried out under mild condition with moderate yields. This procedure expands the utility of supported reagents as an efficient method for allylic selective oxidation of Δ5-sterols.
EXPERIMENTAL
Preparation of CrO3/NHPI-Activated Clay-Supported Oxidant
Solid chromium trioxide (10 g, 100 mmol) was dissolved in water (15 ml) at 40 °C; NHPI (5 g, 260 mmol) was added. The mixture was stirred for 20 min; then water (15 ml) and activated clay (φ < 75 µm) (45 g) were added. The mixture was further stirred for 10 min and then dried in vacuum. The dry mixture was ground with a mortar and obtained finally as a kind of brown powder.
Oxidation of 3β-Hydroxyl-Δ5-Sterols: Typical Procedure
To a solution of 3β-hydroxyl-Δ5-sterols (5 mmol) in CH2Cl2 (120 ml), the CrO3/NHPI-activated clay-supported oxidant (5.8 g, CrO3 content, 8 mmol) was added at rt with stirring. After 10 h to 24 h, an additional amount of CrO3/NHPI-activated clay-supported oxidant (5.8 g, CrO3 content, 8 mmol) was added portionwise during a period of 10 h. After that, the reaction mixture was further stirred until the reaction completed (thin-layer chromatography, TLC, control) (about 24 h to 48 h). The mixture was filtered through a bed of activated clay and washed with dichloromethane. The combined filtrate was washed repetitively with saturated Na2CO3 solution until no orange color was observed in the organic layer (at this moment, almost all the NHPI residues were removed). The organic layers were then washed with brine and water and dried over MgSO4; the solvent was evaporated in vacuum. Purification of the product was done by column chromatography (eluent: petroleum/ethyl acetate 3/1).
ACKNOWLEDGMENTS
Thanks are due to Prof. Fu-Chu Liu (Yunnan University) for helpful criticism of the manuscript. This work was supported by the National Natural Science Foundation of China (No. 20472070) and the Yunnan Science Foundation (No. 2005E008M).
Notes
a All substrates are commercially available.
b 1H-NMR and 13C-NMR spectra were recorded on a Bruker–DRX-500 spectrometer in CDCl3.
c IR was recorded on Thermo Nicolet Avatar 360.
d Isolated.
e Yield with the recovered oxidant.
REFERENCES
- Parish , E. J. ; Kizito , S. A. ; Qiu , Z. Review of chemical syntheses of 7-keto-Δ5-sterols . Lipids 2004 , 39 ( 8 ), 801 – 804 .
- Fullerton , D. S. ; Chen , C.-M. In situ allylic oxidation with Collins reagent . Synth. Commun. 1976 , 6 ( 3 ), 217 – 220 .
- Parish , E. J. ; Chitrakorn , S. ; Wei , T-Y . Pyridinium chlorochromate–mediated allylic and benzylic oxidation . Synth. Commun . 1986 , 16 ( 11 ), 1371 – 1375 .
- Parish , E. J. ; Wei , T-Y . Allylic oxidation of Δ5-steroids with pyridinium chlorochromate (PCC) and pyridinium dichromate (PDC) . Synth. Commun. 1987 , 17 ( 10 ), 1227 – 1233 .
- Salmond , W. G. ; Barta , M. A. ; Havens , J. L. Allylic oxidation with 3,5-dimethylpyrazloe chromium trioxide complex: Steroidal Δ5-7-ketones . J. Org. Chem. 1978 , 43 ( 10 ), 2057 – 2059 .
- Parish , E. J. ; Sun , H. ; Kizito , S. A. Allylic oxidation of Δ5-steroids with pyridinium fluorochromate . J. Chem. Res., Synop. 1996 , 12 , 544 – 545 .
- Marwah , P. ; Lardy , H. A. Process for effecting allylic oxidation using dicarboxylic acid imides and chromium reagents . US Patent 6,384,251, May 7, 2002 .
- (a) Smith , K. (Ed.). Solid Supports and Catalysts in Organic Synthesis ; Ellis Horwood and PTR Prentice Hall : New York , 1992 ; (b) Varma, R. S. Clay and clay-supported reagents in organic synthesis. Tetrahedron 2002, 58, 1235–1255 .
- ( a ) Khadilkar , B. ; Chitnavis , A. ; Khare , A. An easy preparation of a silica gel supported chromium trioxide oxidant and its use in the selective oxidation of alcohols . Synth. Commun. 1996 , 26 ( 2 ), 205 – 210 ; (b) Varma , R. S. ; Saini , R. K. Wet alumina supported chromium(VI) oxide: Selective oxidation of alcohols in solventless system . Tetrahedron Lett . 1998 , 39 ( 12 ), 1481 – 1482 ; ( c ) Ali , M. H. ; Wiggin , C. J. Sillica gel supported Jones reagent (SJR), a simple, versatile, and efficient reagent for oxidation of alcohols in monaqueous media . Synth. Commun . 2001 , 31 ( 21 ), 3383 – 3393 ; ( d ) Gruttadauria , M. ; Liotaa , L. F. ; Deganello , G. ; Noto , R. Chromium(VI) supported and entrapped on silica and zirconia as recyclable materials for oxidation of alcohols . Tetrahedron 2003 , 59 ( 27 ), 4997 – 5002 , and references therein .
- Baptistella , L. H. B. ; Sousa , I. M. O. ; Gushikem , Y. ; Aleiox , A. M. Chromium(VI) adsorbed on SiO2/ZrO2, a new supported reagent for allylic oxidations. Tehrahedron Lett. 1999, 40, 2695–2698.
- (a) Salvador , J. A. R. ; Clark , J. H. The allylic oxidation of unsaturated steroids by tert-butyl hydroperoxide using homogeneous and heterogeneous cobalt acetate . Chem. Commun. 2001 , 1 , 33 – 34 ; (b) Miller , R. A. ; Li , W. ; Humphrey , G. R. A ruthenium catalyzed oxidation of steroidal alkenes to enones . Tehrahedron Lett . 1996 , 37 ( 20 ), 3429 – 3432 ; ( c ) Arsenou , E. S. ; Koutsourea , A. I. ; Fousteris , M. A. ; Nikolaropoulos , S. S. Optimization of the allylic oxidation in the synthesis of 7-keto-Δ5-steroidal substrates . Steroids 2003 , 68 ( 5 ), 407 – 414 ; ( d ) Muzart , J. Synthesis of unsaturated carbonyl compounds via a chromium-mediated allylic oxidation by 70% tert-butyl hydroperoxide . Tetrahedron Lett . 1987 , 28 ( 40 ), 4665 – 4668 ; ( e ) Shing , T. K. M. ; Yeung , Y. Y. ; Su , P. L. Mild manganese(III) acetate catalyzed allylic oxidation: Application to simple and complex alkenes . Org. Lett . 2006 , 8 ( 14 ), 3149 – 3151 ; ( f ) Marwah , P. ; Marwah , A. K. ; Lardy , H. A. An economical and green approach of the oxidation of olefins to enones . Green Chem . 2004 6 ( 11 ), 570 – 577 ; ( g ) Choi , H. ; Dyle , M. P. Optimal TBHP allylic oxidation of Δ5-steroids catalyzed by dirhodium caprolactamate . Org. Lett . 2007 , 9 ( 26 ), 5349 – 5352 .
- Solaja , B. A. ; Milic , D. R. ; Dosen-Micovic , M. L. Oxidation of steroidal 5-en-3 beta-ols with Jones reagent in ether . Steroids 1994 , 59 ( 5 ), 330 – 334 .
- Nangia , A. ; Anthony , A. Facile synthesis of steroidal Δ4-3,6-diones from Δ5-3-ols using pyridinium chlorochromate . Synth. Commun. 1996 , 26 ( 2 ), 225 – 230 .