ABSTRACT
Case history
Between August and October 2020, following the monsoon, signs of lumpy skin disease (LSD) were recorded and described in 154 oxen, 34 cows, 13 calves (Bos indicus) and two Asian water buffalo (Bubalus bubalis) cows belonging to smallholder farmers in 32 villages located around the Kanha and Bandhavgarh tiger reserves in the state of Madhya Pradesh, central India. Affected animals were subjected to a full clinical examination and detailed findings were recorded in a clinical register. A semi-structured questionnaire was attached to the existing clinical register format to gather information on the clinical disease history and animal husbandry practices relevant to the spread of LSD virus.
Clinical findings
The affected animals were between 4 months and 14 years of age (mean 6.4 (SD 2.5) years). Persistent high temperature, depression, anorexia, and characteristic round nodules (lumps) on the skin were reported. The nodules were 2–5 cm in diameter and spread over the face, ears, neck, back, perineum, scrotum, legs, tail, udder, and nasal and oral mucosa. Secondary complications of myiasis (n = 39), mastitis (n = 16) and ulcerative lesions on legs were noticed. Death was reported for one animal (0.5%). The affected animals’ recovery times were variable (mean 18.4 (SD 2.7) days). There was a significant positive correlation between delay in initiating treatment and the duration of sickness. Reduction in milk yield of 30–55% was reported in Bos indicus cows.
Diagnosis
Clinical findings and treatment responses consistent with lumpy skin disease and its sequelae.
Clinical relevance
The presumptive LSD outbreak caused serious economic loss to the animal keepers. LSD is a new disease for India and in the absence of active immunisation, efficient vector control, animal movement control and stall-feeding practice, it will inevitably become endemic in the country. The severe impacts resulting from the introduction of a new disease to a previously unaffected country highlight the need for iterative improvements in global transboundary disease surveillance. The value of clinical examination and recording of findings is demonstrated in the context of smallholder farming systems with limited access to laboratory diagnosis, which are common around the world. The description of an LSD outbreak in naïve populations of cattle and buffalo illustrates the need for increased awareness of the associated clinical signs and maintenance of high levels of biosecurity in hitherto disease-free countries.
Introduction
Lumpy skin disease (LSD) is a transboundary disease of bovines that can result in variably high morbidity and mortality rates in cattle. Asian water buffalo are susceptible, but morbidity rates are usually reported to be lower (El-Nahas et al. Citation2011). The lumpy skin disease virus (LSDV) is a capripox virus, closely related to, but phylogenetically distinct from the viral causes of sheep and goat pox. LSDV is highly contagious and can be transmitted by close contact with infected or asymptomatic carrier animals, contaminated feed and water, or by many blood-feeding arthropod vectors. Following a short period of viraemia, the LSDV replicates within the skin and mucous membranes, causing skin disease, reduced milk yields, weight loss, abortion, poor reproductive performance, hide damage and loss of draft power (Tuppurainen et al. Citation2017).
In recent years, LSD has spread from its African origins into eastern and Mediterranean Europe and central and southern Asia (see Supplementary Information 1), reaching India in November 2019 (Anonymous Citation2019). High morbidity rates resulting from the introduction of the disease to new areas have had dramatic effects on rural smallholder livelihoods that are dependent on cattle production, in addition to impacts caused by human reaction (Molla et al. Citation2017). Uncontrolled livestock movement has been implicated in the long-range transmission of LSDV (Tageldin et al. Citation2014) and could have been a crucial factor for its spread into and through India (see Supplementary Information 1).
The clinical signs that have been reported in LSD outbreaks are variable (Kumar Citation2011; Ayelet et al. Citation2014; Abutarbush et al. Citation2015). Large (3–8 cm) skin nodules are characteristic, sometimes accompanied by fever, occulonasal discharge and swollen lymph nodes. Nodules have been reported on the neck, nares, muzzle, back, legs, perineum, scrotum, eyelids, ears, nasal mucosa, and tail (Al-Salihi and Hassan Citation2015). A characteristic inverted conical zone of necrosis has been described (Abutarbush et al. Citation2015). Oedema of the face, brisket and limbs is sometimes seen (Hunter and Wallace Citation2001; Salib and Osman Citation2011; Tageldin et al. Citation2014). Insect and tick bites, bovine herpesvirus 2 diseases (pseudo-LSD and bovine herpes mammilitis) (D’Offay et al. Citation2003) and parapoxvirus diseases (bovine papular stomatitis) have been listed as differential diagnoses for LSD, but these can be eliminated on the basis of a thorough, herd-level disease history and clinical examination.
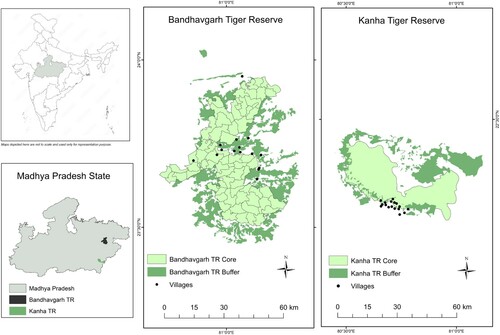
India is a region of immense biodiversity and has a large number of protected areas for wildlife conservation, with 29 parks and sanctuaries >1000 km2 and two parks >5000 km2 (Ghosh-Harihar et al. Citation2019). The mechanism for maintenance of LSDV during inter-epidemic periods is unknown, and in Africa, a low level of persistence in wild animals has not been ruled out (Hunter and Wallace Citation2001). This report documents the investigation of outbreaks of LSD in villages around two important wildlife parks, Kanha Tiger Reserve (KTR) and Bandhavgarh Tiger Reserve (BTR) in the central Indian highlands in the state of Madhya Pradesh, where despite a healthy eco-tourism industry, agriculture and animal husbandry are the mainstays of the rural economy. Government-trained local paraveterinary workers are active within the community, providing animal health care and engaging in discussions and deliberations on animal health issues with the animal keepers (Hopker et al. Citation2018). State-run veterinary dispensaries each serve several distantly located villages and are poorly equipped for laboratory diagnoses. An Indian non-governmental organisation, The Corbett Foundation (TCF) provides veterinary care to the fringe villages in the vicinity of these tiger reserves.
While the movement of people and livestock is controlled in these tiger reserves’ core zones, there is provision for grazing of livestock in the buffer zones. Movements of livestock into the forest and venturing of wild animals into human habitations are common, creating a functional interface where disease transmission is possible between wild and domestic animals. While some of the cattle owners accompany their herds into the forest, many pool their cattle and hire an animal watcher who accompanies several families’ cattle daily to the forest. In the forest, the cattle may come into direct contact with, or share environments with other cattle herds, Indian gaur (Bos gaurus), spotted deer (Axis axis), sambar (Rusa unicolor) and Asian elephants (Elephas maximus).
Most of the cattle in the villages are of native Indian breeds. Poorly ventilated cattle sheds adjoining animal keepers’ residences, and the areas where the cattle are taken to grazing provide suitable conditions for a wide range of blood-feeding arthropod vectors. Weekly cattle markets are held in certain locations for animal keepers to buy and sell their animals. At these markets, animals mix freely without effective biosecurity controls and purchased livestock result in the regular and frequent entry of new animals to the villages.
The livelihood of villagers around these tiger reserves revolves around cattle providing milk and draft power and so is particularly vulnerable to the introduction of production-limiting transboundary diseases. This report describes the clinical signs, timing of recovery, types of treatments, production losses, and other factors such as mixing of animals and animal trading pertaining to the spread of LSDV in Indian cattle. The aims are to highlight the rapid speed at which the disease can spread in animal populations that were previously presumed to be naive and to quantify its impact with reference to subsistence agriculture in rural communities. This information is necessary to inform the need for vaccine development and strategies for their use, and to raise awareness of risks to currently disease-free regions of the world, where the impact of LSDV in European cattle breeds could be devastating. Furthermore, while there are no records of LSD in Indian gaur, it is important to document and control LSDV around their habitats.
Case history
Study population
This study included 203 cattle from 32 villages around BTR and KTR that received TCF veterinary and extension services () and had reported signs consistent with LSD between the start of August 2020 when signs were first reported and the end of October 2020 when new reports of the disease stopped. Animal husbandry in these villages is primarily based on smallholdings, with an estimated mean herd size of four cattle. There are around 13,000 ruminant livestock animals in 32 villages, but precise cattle numbers are unavailable. The cases included in the study had been presented to the veterinary services teams of TCF during village visits, but some animal keepers may not have sought veterinary care or may have used a different service.
Figure 1. Location of 32 villages affected with lumpy skin disease (LSD) located around the Kanha Tiger Reserve (KTR) and Bandhavgarh Tiger Reserve (BTR) in the state of Madhya Pradesh, India. KTR is spread over 2051 km2 and includes a 1134-km2 multiple-use buffer zone (dark green) surrounding a 917-km2 core zone (light green) of restricted human activity. There are 20 villages (black dots) in the core zone and 161 villages in the buffer zone. BTR is spread over an area of 1598 km2 with 11 villages in the core zone (716 km2) and 140 villages in the buffer area (820 km2). The inhabitants belong predominantly to the Baiga and Gond tribes.
Disease history
A febrile nodular disease accompanied by swelling of the brisket and legs was reported to TCF animal healthcare professionals on 10 August 2020 in the Bijharia village of Umaria district near Bandhavgarh. More cases with similar clinical signs were reported from six adjoining villages in the BTR during the following 2 weeks. During this period, similar cases were also reported from villages in the Balaghat district around the KTR, about 250 km distant.
Household visits and collection of data
The 32 villages in the study are routinely provided with veterinary care by the TCF veterinary team. Smallholder household animal keepers contacted the TCF veterinary team to seek healthcare advice and treatment upon noticing signs in their livestock of febrile, nodular disease accompanied by swelling of the brisket and legs. All such requests were promptly attended to by a paraveterinary worker visiting each affected animal. All of the cases presented were subjected to a full clinical examination to ensure that primary signs of LSD, as well as any secondary signs and sequelae were identified. Sick animals that had multiple nodules anywhere on the body, accompanied by fever and/or swelling of the face, brisket or legs were defined as cases of LSD for inclusion in the study. Data for the animal's age, type, sex, body condition score (using a 1–6 scale), rectal temperature, size and location of nodules, swelling of face, brisket, legs and presence of ticks and flies were recorded in the clinical register of TCF. In this instance and for an outbreak of a disease not previously seen in the region, the clinical register was modified to include a detailed description of the clinical signs, including lesions and their distribution, production losses, duration of sickness and recovery periods. Estimation of morbidity was not attempted as the precise numbers of cattle kept in the villages could not be determined, and some of the animal keepers might have accessed veterinary services of other rural animal health workers, and thus unknown numbers of affected animals might have been beyond the coverage of this study.
A semi-structured questionnaire (see Supplementary Information 2) was attached to the existing clinical register to gather information on the clinical disease history, animal husbandry practices including the sending of cattle to the nearby forests for grazing, and attitudes such as the willingness of animal keepers to vaccinate their animals against LSD should a vaccine become available to the farmers in the regions. The duration of clinical signs and lesions was also recorded. Good veterinary clinical practice dictated that vector control measures, animal movement control, and therapeutic guidelines were explained to each animal keeper.
In the absence of vaccination, the farmers were advised to treat affected animals with broad-spectrum antibiotics and non-steroidal anti-inflammatory drugs. The treatment protocol was recommended to control complications due to secondary bacterial infections and inflammation. Wound dressings were applied using antiseptic sprays, antibiotic sprays and fly repellent sprays. There were delays in accessing treatment during the initial phase of the outbreak, but as animal keepers later learned more about the disease, veterinary care was sought as soon as they noticed lesions.
Data management and analysis
The collected information was entered into an Excel 2007 spreadsheet (Microsoft Corporation, Redmond, WA, USA). The data were coded before they were analysed. The data were anonymised by avoiding identification marks for households, animals and animal keepers. Data analyses were performed using SPSS software, version 17 (SPSS Inc., Chicago, IL, USA) to describe percentile values, central tendency and dispersion. Descriptive statistics were used to find mean and SD. Bivariate correlation was used to find the tendency between delay in initiating treatment and time taken to recover.
Ethical approval
The aims and scope were explained to each animal keeper respondent, by a local paraveterinary worker known to them and using local language. Verbal consent was recorded, and it was made clear to each respondent that they could withdraw from the study at any point until their data was anonymised and entered into the database. Ethical oversight was provided by the Royal (Dick) School of Veterinary Studies Human Ethical Review Committee (HERC 47/17).
Clinical findings
Clinical features
Clinical signs consistent with the case definition of LSD were first reported between 1 and 28 September in 17 villages around the KTR and between 21 August and 22 September in 15 villages around the BTR. LSD was diagnosed in 154 oxen (bullocks used for their draft power), 34 cows, 13 calves (all Bos indicus) and two Asian water buffalo (Bubalus bubalis) cows, in the 32 villages. Body condition scores (BCS) ranged between 1 and 4 on a 1–6 scale with 52% of the affected cattle having a BCS of 2 and 15% having a BCS of 1 (most of the cattle were considered to be in poorer condition than expected, but there are no contemporaneous reports of BCS in non-descript cattle (i.e. non-purebred native or European breeds) in central India for comparison). Signs of LSD were seen in all age groups and the mean age of the affected animals was 6.4 (SD 2.5) years (min 4 months, max 14 years; mode, 6.0 years).
Multiple urticaria lesions were observed over the skin of each affected animal. The animal keepers had not previously seen similar lesions. All of the animals showed inappetence and were depressed. Rectal temperatures were 37.8–40.3°C (normal ∼38.0 to ∼39.0°C) at the time of first clinical examination. The nodules measured 2–5 cm in diameter and were distributed on face, neck, back, legs, perineum, scrotum and ears, but primarily concentrated on neck and back ().
Swelling of limbs was recorded in 84% of the affected oxen, which was higher than observed in Bos indicus cows and calves, albeit the number of animals in each group were too low to draw any statistical inference. Swelling of superficial lymph nodes was observed in all types of animals affected. Secondary complications of mastitis, myiasis, ulcerations of hock joints, abortion, and lameness were observed (). Salivation and lameness were less pronounced in calves, but nodules were seen all over the body, with the highest concentration on the head ().
Figure 2: Clinical lesions typical of the cases seen in an outbreak of presumptive lumpy skin disease (LSD) in native cattle (Bos indicus) and Asian water buffaloes (Bubalus bubalis) around the tiger reserves of the central Indian highlands. (a) Affected ox showing the spread of nodules on the skin; (b) excoriated wound on leg of an affected ox; (c) swelling of the brisket; (d) lesions on the head of a calf.
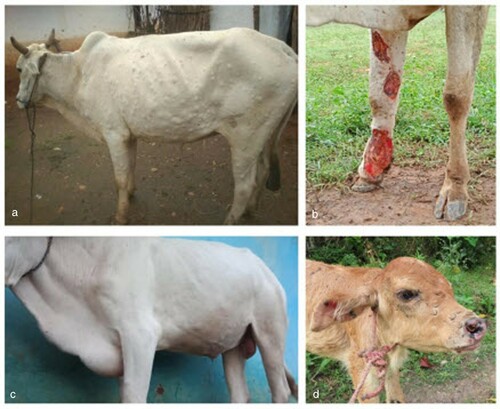
Table 1. Number (%) of native cattle (Bos indicus) and Asian water buffaloes (Bubalus bubalis) recorded as having clinical signs and lesions associated with lumpy skin disease (LSD) during an outbreak of presumptive LSD in and around the tiger reserves of the central Indian highlands.
Epidemiological features
In some cases, goats and cattle shared the same area for housing and feeding; but as expected for the presumptive diagnosis of LSD, no sheep or goats were recorded as having similar clinical signs in the households where cattle were affected by LSD. Around 60% of the affected animals had recovered enough to walk to the forest to feed and return to work by 18 days after the onset of clinical signs (Supplementary Table 1). The mean time taken to seek veterinary care for sick animals fell from 1.8 days during the first half of the study period to 0.9 days during the second half of the study period. There was a significant positive correlation between the delay in being treated and the duration of the illness (p < 0.001). All of the affected cows showed a fall in milk production, while abortion was reported for only one cow. During the initial stage of sickness, the mean fall in the milk yield was 30% which progressed to 55%, corresponding in many cases with the onset of secondary complications of mastitis and myiasis. No mortality was observed in the affected cattle of the KTR but an ox (BCS = 1, age 13 years) died in the BTR. In this study, 72% of the animal keepers routinely immunised their livestock against foot and mouth disease, and 85% expressed their willingness to immunise their cattle and buffalo against LSD should a vaccine become available. Summary statistics describing epidemiological features are shown in Supplementary Information 3. Some of the relevant quotes and comments made by animal keepers are shown in Supplementary Information 4.
Discussion
Due to the remoteness of the affected villages from government veterinary centres, laboratory confirmation of LSDV was not available; nevertheless, the disease's pathognomic clinical signs were observed in each of the sick animals. The clinical signs and lesions were consistent with those reported from other LSD outbreaks (Hunter and Wallace Citation2001; Tuppurainen and Oura Citation2012; Kumar et al. Citation2021) including those in India (Sudhakar et al. Citation2020; Kumar et al. Citation2021). The main differential diagnoses of pseudo-LSD (which has not been reported in Asia), bovine papular stomatitis and reactions to arthropod bites were discounted on the basis of inconsistent disease history and clinical signs. This, coupled with the comprehensive affirmation by animal keepers and healthcare professionals that these lesions and clinical signs had not been seen before, supports the conclusion the disease outbreak was caused by the recent introduction of LSDV. Ancillary diagnostic tools, such as laboratory confirmation of causative agent and its isolation, are helpful in epidemiological investigations; however, such facilities are not readily available or affordable in rural India, as was the case with this outbreak. This emphasises the importance of rural animal health workers collecting necessary clinical and epidemiological data quickly and reliably while offering immediate advice to animal keepers.
Oxen are critical to the economy of tribal people. The high incidence of LSD in bullocks could be attributed to their inability to escape biting flies while harnessed in yokes. The Indian Meteorological Department in its monthly weather bulletin for August 2020, recorded an actual rainfall of 495.2 mm in central India against a mean rainfall of 307.3 mm between 1951 and 2001, indicating a 61% departure from the long period mean (Anonymous Citation2020). The first appearance of LSD around the KTR and BTR coincided with moderate to hot weather in central India, enhancing the populations and activity of biting fly, tick (Tuppurainen et al. Citation2017) and mosquito (Chihota et al. Citation2001) vectors. The outbreak started to taper off during the second week of October when it became dry and cooler.
This observation linking a declining incidence to a declining blood-feeding arthropod vector population is similar to previous findings (Al-Salihi and Hassan Citation2015) and might imply a major role of arthropods in transmitting LSDV in the region. It is also likely that this tapering was because most of the susceptible animals had already been affected. Future LSD outbreaks will need to be closely monitored against climatic patterns to inform predictions of the onset of disease and aid in vaccination programmes, should they become available.
The risk of vector spread of LSDV was high due to the nature of the animal husbandry practised in the tribal villages. The design and nature of walls (wooden planks) and floors (uneven and earthen) in overnight accommodation, made the use of insecticidal sprays difficult. A high proportion of the affected cattle (91%) had been regularly sent to the forest for grazing (described in Supplementary Information 1). The animal keepers have been sending their cattle to the forest for grazing for many generations and do not practice stall feeding. All the infected cattle that could walk were sent for grazing even when signs of sickness were still apparent, while those cattle that stayed back were sent with the herd as soon as their lameness was reduced. Farmers’ insistence on sending their cattle to graze on forest land may have facilitated vector spread of LSDV and is a potential risk factor for disease transmission between cattle or buffaloes and susceptible wildlife, as noted in Ethiopia (Gari et al. Citation2010). Communal grazing and sharing water resources in the forest may have further facilitated the transmission. Isolation of sick animals and vector control would necessitate animal keepers staying at home to look after the ill and segregated animals. In tribal societies, both men and women share outdoor work in agricultural fields and forest produce collection (Rajiv and Vijendra Citation2006; Marawi and Modi Citation2017; Lakra Citation2019). Tribal animal keepers tended to avoid holding animals at home during the day when both men and women go out for work.
There was a significant positive correlation between the delay in being treated and the duration of the sickness, and secondary complications were less severe for the animals affected later during the outbreak, as treatments were initiated promptly. These observations highlight the need for increased awareness of the clinical signs and impact of LSD in currently disease-free countries and regions.
No mortality was observed in the affected cattle of the KTR which is similar to the observation in the eastern Indian state of Odisha (Sudhakar et al. Citation2020), but one ox died in the BTR being thin, old and weak. A low mortality rate of 0.51% has also been reported in Iraq (Al-Salihi and Hassan Citation2015). The low mortality could be related to a higher level of innate resistance in native cattle, or lower susceptibility to blood-feeding arthropod vectors when compared to European breeds, in which the impact of LSD is much higher (Kiplagat et al. Citation2020).
It was not possible to assess the precise origin of the outbreaks around the two tiger reserves, because the animal husbandry practices allowed massive and regular movement of cattle from villages to the nearby forests for grazing. The entry of new animals took place by buying and selling livestock between villages, despite the closure of traditional cattle markets under COVID-19 regulations. It would be challenging to identify the source of infection to India at the national level because of the vast international boundaries with Pakistan, China, Bhutan, Nepal, Bangladesh and Myanmar, allowing movement of affected animals as described in Iraq (Al-Salihi and Hassan Citation2015) and Egypt (Yeruham et al. Citation1995) and windborne spread of biting fly vectors, as concluded from a 1989 LSD outbreak in Israel that traced the origin of the insects to Egypt (Kumar Citation2011).
The animal keepers in the villages around the KTR and BTR suffered severe economic losses due to the first outbreak of lumpy skin disease. All age groups of cattle were affected, and the signs of LSD were evident through skin nodules on most parts of the body of animals. The secondary complications of mastitis, myiasis, ulcerations of hock joints, abortion, and lameness made the situation worse. The reduction in milk production and the loss of draft power were the most apparent impacts, albeit losses were difficult to compute due to poor record-keeping by the local animal keepers. There would also have been associated costs of the missed opportunities for breeding and trade. These observations further highlight the need for enhanced knowledge of the epidemiology and global spread of LSDV and maintenance of biosecurity in currently disease-free countries and regions.
Supplemental Material
Download PDF (482.7 KB)References
- Abutarbush SM, Ababneh MM, Al Zoubi IG, Al Sheyab OM, Al Zoubi MG, Alekish MO, Al Gharabat RJ. Lumpy skin disease in Jordan: disease emergence, clinical signs, complications and preliminary associated economic losses. Transboundary and Emerging Diseases 62, 549–554, 2015
- Al-Salihi KA, Hassan IQ. Lumpy skin disease in Iraq: study of the disease emergence. Transboundary and Emerging Diseases 62, 457–462, 2015
- Anonymous. Lumpy Skin Disease, India. https://wahis.oie.int/#/events (accessed 17 September 2021) World Organisation for Animal Health, Paris, France, 2019
- Anonymous. All India Monthly Weather Report. https://internal.imd.gov.in/pages/aimonthlywx_mausam.php (accessed 8 April 2021). India Meteorological Department, Government of India, New Delhi, India, 2020
- Ayelet G, Haftu R, Jemberie S, Belay A, Gelaye E, Sibhat B, Skjerve E, Asmare K. Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of the virus. OIE Revue Scientifique et Technique 33, 877–887, 2014
- Chihota CM, Rennie LF, Kitching RP, Mellor PS. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology and Infection 126, 317–321, 2001
- D’Offay JM, Floyd JG, Eberle R, Saliki JT, Brock KV, D'Andrea GH, McMillan KL. Use of a polymerase chain reaction assay to detect bovine herpesvirus type 2 DNA in skin lesions from cattle suspected to have pseudo-lumpy skin disease. Journal of the American Veterinary Medical Association 222, 1404–1407, 2003
- El-Nahas EM, El-Habbaa AS, El-Bagoury GF, Radwan MEI. Isolation and identification of lumpy skin disease virus from naturally infected buffaloes at Kaluobia, Egypt. Global Veterinaria 7, 234–237, 2011
- Gari G, Waret-Szkuta A, Grosbois V, Jacquiet P, Roger F. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiology and Infection 138, 1657–1666, 2010
- Ghosh-Harihar M, An R, Athreya R, Borthakur U, Chanchani P, Chetry D, Datta A, Harihar A, Karanth KK, Mariyam D, et al. Protected areas and biodiversity conservation in India. Biological Conservation 237, 114–124, 2019
- Hopker A, Pandy N, Dhamorikar A, Hopker S, Gautam P, Pandy S, Kumar S, Rahangadale N, Mehta P, Marsland R, et al. Delivery and evaluation of participatory education for animal keepers led by veterinarians and para-veterinarians around the Kanha Tiger Reserve, Madhya Pradesh, India. PLoS ONE 13, e0200999, 2018
- Hunter P, Wallace D. Lumpy skin disease in Southern Africa: A review of the disease and aspects of control. Journal of the South African Veterinary Association 72, 68–71, 2001
- Kiplagat SK, Kitala PM, Onono JO, Beard PM, Lyons NA. Risk factors for outbreaks of lumpy skin disease and the economic impact in cattle farms of Nakuru county, Kenya. Frontiers in Veterinary Science 7, 259, 2020
- Kumar SM. An outbreak of lumpy skin disease in a Holstein dairy herd in Oman: a clinical report. Asian Journal of Animal and Veterinary Advances 6, 851–859, 2011
- Kumar N, Chander Y, Kumar R, Khandelwal N, Riyesh T, Chaudhary K, Shanmugasundaram K, Kumar S, Kumar A, Gupta MK, et al. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS ONE 16, e0241022, 2021
- Lakra SJS. Sustainable resource management through indigenous knowledge and practices – a case of food security among the Baiga tribe in India. European Journal of Sustainable Development 8, 233, 2019
- Marawi PS, Modi S. Changing status of tribal women in Anuppur district of Madhya Pradesh through minor forest products (MFPS). Abhinav-National Monthly Refereed Journal of Research in Commerce and Management 6, 53–59, 2017
- Molla W, de Jong MCM, Gari G, Frankena K. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Preventive Veterinary Medicine 147, 100–107, 2017
- Rajiv R, Vijendra N. Socioeconomic and livelihood pattern of ethnic group Baiga in Achanakmar reserve forest in Bilaspur Chhattisgarh. Journal of Tropical Forestry 22, 62–70, 2006
- Salib FA, Osman AH. Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate. Egypt. Veterinary World 4, 162–167, 2011
- Sudhakar SB, Mishra N, Kalaiyarasu S, Jhade SK, Hemadri D, Sood R, Bal GC, Nayak MK, Pradhan SK, Singh VP. Lumpy skin disease (LSD) outbreaks in cattle in Odisha state, India in August 2019: epidemiological features and molecular studies. Transboundary and Emerging Diseases 67, 2408–2422, 2020
- Tageldin MH, Wallace DB, Gerdes GH, Putterill JF, Greyling RR, Phosiwa MN, Al Busaidy RM, Al Ismaaily SI. Lumpy skin disease of cattle: an emerging problem in the sultanate of Oman. Tropical Animal Health and Production 46, 241–246, 2014
- Tuppurainen ESM, Oura CAL. Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases 59, 40–48, 2012
- *Tuppurainen E, Alexandrov T, Beltrán-Alcrudo D. Lumpy Skin Disease. A Field Manual for Veterinarians. FAO Animal Production and Health Manual No. 20. Food and Agriculture Organization of the United Nations, Rome, Italy, 2017
- Yeruham I, Nir O, Braveman Y, Davidson M, Grinstein H, Haymovitch M, Zamir O. Spread of lumpy skin disease in Israeli dairy herds. Veterinary Record 137, 91–93, 1995
- *Non-peer-reviewed.
