ABSTRACT
Aims
To establish a reference range for the canine C-ACT activated clotting time (ACT) test using a water bath and visual clot assessment technique.
Methods
Healthy, privately owned dogs (n = 48) were prospectively recruited to the study. Blood samples were collected via direct jugular venipuncture for complete blood count, serum biochemistry analysis and measurement of prothrombin time (PT) and activated partial thromboplastin time (aPTT). Five animals with major abnormalities or who became agitated during phlebotomy were excluded. For the 43 remaining animals, 2 mL of blood was collected via the cephalic vein and added directly to a C-ACT tube that was shaken vigorously before being placed in a water bath at 37°C. Tubes were visually assessed for clot formation and C-ACT was recorded in seconds when the magnet within the tube lodged in the clot.
Results
The nonparametric reference interval (capturing the central 95% of the data) was 50–80 seconds, with a 90% CI for the lower limit of 50–55 seconds and a 90% CI for the upper limit of 75–80 seconds. The C-ACT ACT test had a positive correlation with aPTT (0.42; 95% CI = 0.13–0.64). There was no evidence of a correlation between C-ACT ACT and age, weight, PT, haematocrit, white blood cell count, platelet count or total protein.
Conclusions and clinical relevance
The results of this study suggest that the normal reference interval for ACT in dogs using C-ACT tubes in a 37°C water bath is 50–80 seconds. Care should be taken extrapolating the results of this study to the general population, as the smaller study design had less control for confounders than a larger study. However, when using the described analytical methods, C-ACT tube ACT test results >80 seconds should be considered prolonged in dogs and should prompt further investigation.
Introduction
The activated clotting time (ACT) test was first described in 1966 and is typically used in human medicine for anticoagulant monitoring of unfractionated heparin therapy (Hattersley Citation1966). The test is usually performed with the aid of a benchtop analyser that maintains sample temperature and measures clot formation automatically (FitzGerald et al. Citation2009). Manual methods of assessing time to clot formation may also be used where analysers are not available. While some of these manual methods have been validated, reference ranges tend to vary between sample activators and test protocols (Byars et al. Citation1976; Middleton and Watson Citation1978; Glaus et al. Citation1996).
The ACT test is commonly used in many veterinary hospitals to rapidly assess coagulation status in bleeding animals where anticoagulant rodenticide toxicity, elapid envenomation, liver failure, disseminated coagulopathy, haemophilia, or other deficiencies in intrinsic or common pathway coagulation factors are suspected (Topper and Welles Citation2003). The test is an inexpensive, convenient alternative to activated partial thromboplastin time (aPTT), particularly where coagulation analysers are unavailable and turnaround times for external laboratory testing limit their usefulness in the emergency setting.
At the time of writing, the only two types of ACT test tubes available in Australia and New Zealand are MAX-ACT and C-ACT tubes (Helena Laboratories Corporation, Beaumont, TX, USA). Both were initially developed for human use and are designed for use with a point-of-care automated analyser (Actalyke Mini II Activated Clotting Time Test System, Helena Laboratories Corporation). The MAX-ACT tube contains celite (diatomaceous earth), kaolin and glass beads, and has the advantage of needing only 0.5 mL of whole blood for testing, while the C-ACT tube contains a magnet and a small amount of celite and needs a larger 2 mL sample. In Australia, the C-ACT tube is used almost three times more than the MAX-ACT tube by veterinarians, but in New Zealand the C-ACT tube is not commonly used despite being less expensive than the MAX-ACT tube (G. Rallings, pers. comm.Footnote1) and lack of a published reference interval may be a contributing factor. For both tubes whole blood is added to a tube containing negatively charged contact activators, and the result is recorded as the time taken for a clot to form. The test relies on in vitro contact activation of factor XII to stimulate fibrin clot formation. This information gives a general assessment of clotting ability, with prolonged clot formation time suggestive of deficiency (or deficiencies) of intrinsic or common pathway coagulation factor(s).
In an early study comparing ACT test results between healthy dogs and dogs with naturally occurring or experimentally induced coagulopathy, the test was more sensitive in detecting coagulopathy when measured at 37°C than at room temperature (Middleton and Watson Citation1978). ACT test reference intervals (55–80 seconds) have been reported for MAX-ACT tubes using manual clot assessment methods in healthy dogs and cats (See et al. Citation2009), but to our knowledge there is no contemporaneous study establishing normal reference intervals in healthy dogs for the more commonly used C-ACT tube (See et al. Citation2009).
The objective of this study is to establish a reference range for the canine C-ACT activated clotting time in a convenience sample of healthy dogs using a water bath and manual clot assessment technique. We hypothesised that ACT test reference intervals using the C-ACT tubes would be comparable to those previously reported for MAX-ACT tubes in a similar animal population (See et al. Citation2009).
Materials and methods
Animals
A convenience sample of 48 healthy dogs owned by staff and students at the University of Queensland School of Veterinary Medicine (Gatton, QLD, Australia) were enrolled in the study, if they had no history of ill health and had not received medication (other than routine parasite prophylaxis) in the preceding 6 weeks, and the owner was willing. Subjects with abnormal general physical examination findings, a history of significant medical problems, or that became distressed during phlebotomy attempts were excluded. For each subject, age, breed, sex, desexing status and weight were recorded. All study protocols were approved by the University of Queensland Animal Ethics Committee (2021/AE000818) and written consent of owners was obtained prior to enrolment.
Screening tests
For each subject, 9 mL of blood was collected via direct jugular venipuncture using a 23 gauge needle and 10 mL syringe. The needle was removed from the syringe and the blood sample was aliquoted into opened sterile vacutainer tubes containing either citrate, ethylenediaminetetraacetic acid (EDTA) or no anticoagulant (Greiner Bio-One, Kremsmünster, Austria), with care being taken not to cross-contaminate samples with tube additives. Samples from each subject were labelled with a unique subject identifier.
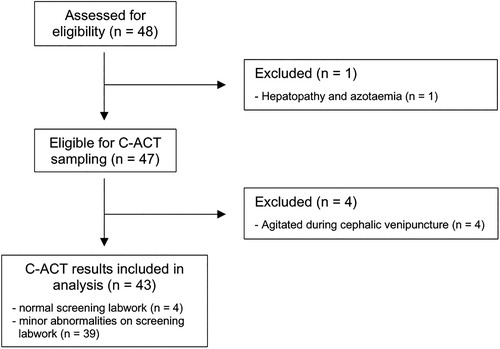
Serum and EDTA tubes were submitted to the University of Queensland Veterinary Laboratory Service (Gatton, QLD, Australia) for complete blood count (Sysmex XN-1000 Hematology Analyzer; Sysmex Corporation, Kobe, Japan) and serum biochemistry profiles (AU480 Chemistry Analyzer; Beckman Coulter Ireland Inc., Lismeehan, Ireland). Citrate samples were used for in-house prothrombin time (PT) and aPTT testing (Coag Dx Analyser; IDEXX Laboratories, Westbrook, ME, USA) as per manufacturer instructions. All samples were tested within 2 hours of collection. Subjects with mildly decreased platelet counts but evidence of clumping on evaluation of a blood smear remained eligible for inclusion. Dogs with other minor abnormalities on any screening test were assessed on a case-by-case basis and included if changes were deemed clinically irrelevant and unlikely to affect haemostasis. Dogs with test abnormalities that did not meet these criteria were excluded from further participation in the study. The flow of subjects from the initial source population to the study sample are shown in .
ACT test with C-ACT tube
Dogs that remained eligible for inclusion underwent a second phlebotomy. Two millilitres of blood were taken from the cephalic vein using a 23-gauge needle and 3 mL syringe. The needle was removed from the syringe and the sample was added directly to an opened C-ACT tube. The lid was secured and the tube was then vigorously shaken and immediately placed in a commercial water bath (Optima T100 Heated Circulating Bath; Grant Instruments (Cambridge) Ltd, Shepreth, UK) set to 37°C. Tubes were visually assessed for clot formation after 30 seconds, then every 5 seconds, and the ACT recorded as the time (seconds) from the addition of the sample to the tube to visible clot formation with the magnet lodged within.
Statistical analyses
Statistical analyses were performed using a commercial statistical software programme (SAS 9.4; SAS Institute Inc., Cary, NC, USA). Normal distribution of data was assessed using Q-Q plots, histograms, and the Anderson–Darling test. A reference interval for ACT using the C-ACT tubes was calculated following American Society for Veterinary Clinical Pathology guidelines (Friedrichs et al. Citation2012) and using the Reference Value Advisor v2.1 Excel Add-in (École Nationale Vétérinaire de Toulouse, Toulouse, France).
Differences in ACT between groups with respect to gender and neutering status were assessed using the Kruskal–Wallis test, and Spearman correlation coefficients comparing ACT with age, weight, PT, aPTT, haematocrit (Hct), white blood cell count, platelet count and total protein concentration were estimated. Ninety-five percent CI for the Spearman correlation coefficients were calculated by performing a Fisher's z-transformation and substituting Bonett and Wright’s (Citation2000) estimate of the standard error of z. Significance of all analyses was set at a value of p < 0.01.
Results
Forty-eight clinically healthy dogs were enrolled in the study. One dog was excluded due to evidence of hepatopathy and azotaemia on screening tests. A further four dogs were excluded due to agitation during phlebotomy attempts. Forty-three dogs were included in our analysis cohort (). Of these, 39/43 dogs had very minor abnormalities on screening tests that were deemed clinically irrelevant and unlikely to impact ACT, and these dogs were included in ACT analysis. The abnormalities seen, number of dogs affected, most aberrant value, and reference interval for these abnormalities are listed in Supplementary Table 1.
The study population consisted of 18 neutered females, 17 neutered males, five entire males and three entire females. Median age was 4 (min 0.33, max 11) years and median body weight was 20 (min 3.1, max 46.1) kg. Breeds included mixed breed (17), Miniature Dachshund (4), Border Collie (3), Labrador Retriever (3), Australian Kelpie (2), Australian Cattle Dog (2), Jack Russell Terrier (2), Siberian Husky (2) and one each of the following: Australian Koolie, German Shepherd Dog, German Shorthaired Pointer, Greyhound, Miniature Fox Terrier, Old English Sheepdog, Pomeranian and Pug.
Untransformed and Box–Cox transformed ACT data were not normally distributed, thus a nonparametric reference interval was calculated with reference interval limit CI calculated using a bootstrap method. The nonparametric reference interval (including the 2.5th–97.5th percentile) was 50–80 seconds. The 90% CI for the lower limit was 50–55 seconds and the 90% CI for the upper limit was 75–80 seconds. For comparison, the parametric CI rounded to the nearest 5 seconds was the same: 50–80 seconds, with the 90% CI lower limit 45–50 seconds and the 90% CI upper limit 75–80 seconds. There was no evidence (p = 0.59) for a difference in the ACT test results between male (median 65, min 50, max 80) and female (median 60, min 50, max 75) dogs. There were too few observations per breed for inferential statistical analysis.
Using Spearman correlations, the ACT had a positive correlation with aPTT (rho (95% CI) 0.42 (0.12–0.65); p = 0.005). There was no evidence for a correlation between C-ACT ACT test results and age, weight, PT, Hct, white blood cell count, platelet count or total protein (see ).
Table 1. Spearman correlation statistics (with 95% CI calculated using Fisher's z-transformation) for activated clotting time, measured using C-ACT tubes (Helena Laboratories Corporation, Beaumont, TX, USA) from single venous samples, and the variables age, weight, prothrombin time (PT), activated partial thromboplastin time (aPTT), haematocrit, white blood cell count (WBCC), platelet count and total protein in a convenience sample of healthy dogs (n = 43).
Discussion
To the authors’ knowledge, this study is the first to describe normal reference intervals for the ACT test measured using C-ACT tubes in dogs. The cephalic vein was selected for C-ACT sampling to mimic vessel selection in clinical patients with suspected coagulopathy, where sampling from a more peripheral vein is preferred and jugular venipuncture is not recommended due to concerns for increased risk of haemorrhage (Atkins Citation2012). ACT can be affected by factors that promote activation of platelets, coagulation, or fibrinolysis, such as traumatic or difficult venipuncture or increased turbulence during aspiration (Lubas et al. Citation2010). We standardised vessel selection, needle size, needle length and syringe size for blood sampling for ACT in this study although cephalic vein size would have varied. While there was no evidence of a correlation between ACT result and weight of dog in this study, the results are intended to be applied to samples directly aspirated from cephalic veins only.
The reference interval generated from this study is intended to provide cut-offs that can be used in dogs with potential coagulation disorders in clinical practice. Inclusion criteria were applied to ensure that samples were taken from healthy animals only, so that animals with a history of medications that might interfere with results or that had significant abnormalities on pre-analytical testing were not included in the study. In addition, dogs that were agitated during venipuncture were excluded due to concerns about traumatic venipuncture, which may have affected results. Statistical analyses were based on American Society of Veterinary Clinical Pathology guidelines for the generation of de novo reference intervals in veterinary patients for sample sizes <120 but >40 (Friedrichs et al. Citation2012). Despite these precautions, it is important to note that the convenience sampling method and smaller sample size may have introduced a higher degree of uncertainty that should be considered when extrapolating results to the general population. In addition, samples were not repeatedly assessed from any individual, so no comment can be made on test precision.
The technique employed in the study used visual clot assessment and a 37°C water bath and was intended to be easily reproducible and affordable in clinical practice. The testing methods and results were similar to those reported previously for MAX-ACT tubes in dogs, with that study generating a reference interval of 55–80 seconds (See et al. Citation2009).
The upper limit of normal of 80 seconds found in this study is faster than that of two similiar studies of celite-based ACT in dogs, which reported upper limits of normal of 95 and 125 seconds using heating blocks held at 37°C and 38°C, respectively (Byars et al. Citation1976; Middleton and Watson Citation1978). Both of those studies used ACT tubes that have since been discontinued. As many steps in the coagulation pathways being evaluated are enzymatic, running the test at a consistent temperature is important for an accurate result, and ACT test results have been shown to be significantly influenced by temperature (Middleton and Watson Citation1978). It has been repeatedly demonstrated that ACT reference intervals also vary significantly by tube manufacturer and technique, and it is recommended that each laboratory establish their own reference interval for this reason (Thenappan et al. Citation2012; Maslow et al. Citation2018; Dirkmann et al. Citation2019).
In this study, the lower reference limit was 50 seconds. This is also more rapid than the two similar studies of celite-based ACT in dogs, which found lower limits of 60 and 64 seconds respectively (Byars et al. Citation1976; Middleton and Watson Citation1978). The clinical relevance of ACT times shorter than the reference limit is unknown. Traumatic venipuncture technique may lead to accelerated results due to premature sample activation, so a spurious result should first be excluded (Lubas et al. Citation2010). Animals that became distressed during venipuncture were excluded from our study, and no included animal had signs of traumatic venipuncture such as protracted or difficult sample collection or post-collection haematoma formation. There is currently no evidence to guide the interpretation of shortened ACT after a traumatic collection in veterinary or human medicine, although shortened aPTT times were found to be associated with hypercoagulability in one retrospective canine study and multiple human studies (Abdullah et al. Citation2010; Mina et al. Citation2010, Citation2012; Song et al. Citation2016). Further work is needed to determine whether shortened ACT results are similarly associated with hypercoagulability.
There was a moderate positive correlation between aPTT and ACT test results. Modest correlation between the two tests has also been reported in human medicine (Smythe et al. Citation2002; Haas et al. Citation2013). This finding is unsurprising, as both tests assess coagulation factor activity within the intrinsic and common coagulation pathways, although ACT is less sensitive in detecting deficiencies. Activated clotting time prolongation can be seen with over 95% reduction in single factor activity, while prolonged aPTT occurs with single factor activity reduction of over 70% (Topper and Welles Citation2003). The absolute sensitivity and specificity of ACT is not reported, as it is a measure of global haemostasis.
There were too few observations per breed to assess the effect of breed on ACT test results using the C-ACT tubes. Previous investigations into breed-specific clinical pathological indices have shown significant differences between Greyhounds, Dachshunds, and others (Shiel et al. Citation2007; Zaldívar-López et al. Citation2011). In the future, further investigation may result in the formation of breed specific reference intervals.
All subjects included in our study had a Hct within the reference interval (0.35–0.58 L/L) and Hct within this range was not associated with changes in ACT test results. Haemodilution has previously been shown to cause significant prolongation in ACT (Huyzen et al. Citation1996). We are unable to comment on whether dogs with decreased Hct below the reference interval would be expected to have changes in ACT test results using the methods described in this study. Unlike the aPTT test, ACT results have also been demonstrated to be mildly prolonged in the presence of severe thrombocytopaenia, as the test relies on adequate platelet phospholipid for effective clot formation (Topper and Welles Citation2003). All subjects included in our study had either a platelet count within the reference interval (200–520 × 109 cells/L), or evidence of significant clumping on smear review. It is possible that this led to the inclusion of dogs with mild thrombocytopaenia, but the effect of severe thrombocytopaenia on results could also not be assessed.
Thirty-nine dogs in our study had mild bloodwork abnormalities that were unlikely to invalidate the reference range generated in this instance. Mild to moderate lymphocytosis was common and may have represented a physiologic sympathetic nervous system response or, in younger dogs, be physiologically appropriate. Antigenic stimulation, cortisol deficiency or other pathological causes were not excluded (Schultze Citation2010). Lipaemia and hypertriglyceridaemia were also common and were attributed to the non-fasting state of participants. Other findings included marginal hypokalaemia, mild neutropaenia, and decreased creatine kinase, all of which were expected to be clinically benign in this otherwise healthy population and may have been partially due to inappropriately narrow reference limits. These and all other abnormalities reported were not expected to be secondary to disease or anticipated to affect ACT results and are listed in Supplementary Table 1.
Based on our results, ACT test results greater than 80 seconds are suggestive of a marked reduction in intrinsic and/or common coagulation factor pathway activity and should prompt further investigation and, in the setting of frank haemorrhage, aggressive treatment. While a prolonged result is suggestive of a severe clotting factor deficiency, it does not accurately indicate in vivo haemostatic capability and should not be relied upon as a sole indicator of bleeding risk in the absence of clinical bleeding (Segal and Dzik Citation2005; Larsen and Hvas Citation2017; Casado-Méndez et al. Citation2019).
Conclusion
The ACT remains a convenient and inexpensive test to screen for severe intrinsic and common pathway clotting factor abnormalities. The results of this study suggest that the normal reference interval for ACT in dogs using C-ACT tubes in a 37°C water bath is 50–80 seconds. Care should be taken extrapolating the results of this study to the general population, as the smaller study design had less control for confounders than a larger study. That said, test results greater than 80 seconds should be considered prolonged and prompt further investigation. The clinical significance of ACT test results <50 seconds is unknown but warrants further research.
Supplemental Material
Download MS Word (32.6 KB)Acknowledgements
Funding was provided by the University of Queensland School of Veterinary Science John and Mary Kibble Trust Fund. This study was presented in part as a research abstract at the 2022 Australian and New Zealand College of Veterinary Scientists College Science Week conference. The authors thank Dr Phil Thomas for his ongoing mentorship and support, Claire Lambe and Emily Ngo for their assistance with sample collection and analysis, and Dr Deborah Keys for statistical analyses.
Notes
1 G. Rallings, Helena Laboratories (Australia) Pty Ltd., Mount Waverley, VIC, Australia.
References
- Abdullah WZ, Moufak SK, Yusof Z, Mohamad MS, Kamarul IM. Shortened activated partial thromboplastin time, a hemostatic marker for hypercoagulable state during acute coronary event. Translational Research 155, 315–9, 2010. https://doi.org/10.1016/j.trsl.2010.02.001
- *Atkins LB. Blood sample collection and handling. In: Burkitt Creedon JM, Davis H (eds). Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care. 1st Edtn. Pp 601–10. Wiley Blackwell, Ames, IA, USA, 2012
- Bonett DG, Wright TA. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika 65, 23–8, 2000
- Byars TD, Ling G, Ferris NA, Keeton KS. Activated coagulation time (ACT) of whole blood in normal dogs. American Journal of Veterinary Research 37, 1359–61, 1976
- Casado-Méndez M, Fernandez-Pacheco J, Arellano-Orden V, Rodríguez-Martorell FJ, Díaz-Martín A, Pastor de las Heras Á, Dusseck-Brutus R, Pérez-Torres I, Leal-Noval SR. Relationship of thromboelastography and conventional clotting test values with severe bleeding in critically ill patients with coagulopathy: a prospective study. International Journal of Laboratory Hematology 41, 671–8, 2019. https://doi.org/10.1111/ijlh.13086
- Dirkmann D, Nagy E, Britten MW, Peters J. Point-of-care measurement of activated clotting time for cardiac surgery as measured by the Hemochron signature elite and the Abbott i-STAT: agreement, concordance, and clinical reliability. BMC Anesthesiology 19, 174, 2019. https://doi.org/10.1186/s12871-019-0846-z
- FitzGerald D, Patel A, Body S, Garvin S. The relationship between heparin level and activated clotting time in the adult cardiac surgery population. Perfusion 24, 93–6, 2009. https://doi.org/10.1177/0267659109106729
- Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Veterinary Clinical Pathology 41, 441–53, 2012. https://doi.org/10.1111/vcp.12006
- Glaus T, Hudak-Glaus D, Hoeptner C, Arnold P, Lutz H. The activated coagulation time (ACT): two simple screening tests for evaluating coagulation disorders in dogs. Schweizer Archiv Fur Tierheilkunde 138, 532–6, 1996
- Haas T, Spielmann N, Mauch J, Schmugge M, Weiss M. Correlation of activated clotting times and standard laboratory coagulation tests in paediatric non-cardiac surgery. Scandinavian Journal of Clinical and Laboratory Investigation 73, 29–33, 2013. https://doi.org/10.3109/00365513.2012.732239
- Hattersley PG. Activated coagulation time of whole blood. Journal of the American Medical Association 196, 436–40, 1966. https://doi.org/10.1001/jama.1966.03100180108036
- Huyzen RJ, van Oeveren W, Wei F, Stellingwerf P, Boonstra PW, Gu YJ. In vitro effect of hemodilution on activated clotting time and high-dose thrombin time during cardiopulmonary bypass. The Annals of Thoracic Surgery 62, 533–7, 1996. https://doi.org/10.1016/0003-4975(96)00324-4
- Larsen JB, Hvas AM. Predictive value of whole blood and plasma coagulation tests for intra- and postoperative bleeding risk: a systematic review. Seminars in Thrombosis and Hemostasis 43, 772–805, 2017. https://doi.org/10.1055/s-0037-1602665
- *Lubas G, Caldin M, Wiinberg B, Kristensen AT. Laboratory testing of coagulation disorders. In: Weiss DJ, Wardrop KJ, Schalm OW (eds). Schalm‘s Veterinary Hematology. 6th Edtn. Pp 1082–100. Wiley Blackwell, Ames, IA, USA, 2010
- Maslow A, Chambers A, Chevees T, Sweeney J. Assessment of heparin anticoagulation measured using i-STAT and hemochron activated clotting time. Journal of Cardiothoracic and Vascular Anesthesia 32, 1603–8, 2018. https://doi.org/10.1053/j.jvca.2018.01.027
- Middleton DJ, Watson ADJ. Activated coagulation times of whole blood in normal dogs and dogs with coagulopathies. Journal of Small Animal Practice 19, 417–22, 1978. https://doi.org/10.1111/j.1748-5827.1978.tb05516.x
- Mina A, Favaloro EJ, Koutts J. Relationship between short activated partial thromboplastin times, thrombin generation, procoagulant factors and procoagulant phospholipid activity. Blood Coagulation & Fibrinolysis 23, 203–7, 2012. https://doi.org/10.1097/MBC.0b013e32834fa7d6
- Mina A, Favaloro EJ, Mohammed S, Koutts J. A laboratory evaluation into the short activated partial thromboplastin time. Blood Coagulation & Fibrinolysis 21, 152–7, 2010. https://doi.org/10.1097/MBC.0b013e3283365770
- *Schultze AE. Interpretation of canine leukocyte responses. In: Weiss DJ, Wardrop KJ, Schalm OW (eds). Schalm‘s Veterinary Hematology. 6th Edtn. Pp 321–34. Wiley Blackwell, Ames, IA, USA, 2010
- See AM, Swindells KL, Sharman MJ, Haack KL, Goodman D, Delaporta A, Robertson I, Foster SF. Activated coagulation times in normal cats and dogs using MAX-ACT TM tubes. Australian Veterinary Journal 87, 292–5, 2009. https://doi.org/10.1111/j.1751-0813.2009.00450.x
- Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 45, 1413–25, 2005. https://doi.org/10.1111/j.1537-2995.2005.00546.x
- Shiel RE, Brennan SF, O’Rourke LG, McCullough M, Mooney CT. Hematologic values in young pretraining healthy Greyhounds. Veterinary Clinical Pathology 36, 274–7, 2007. https://doi.org/10.1111/j.1939-165X.2007.tb00223.x
- Smythe MA, Koerber JM, Nowak SN, Mattson JC, Begle RL, Westley SJ, Balasubramaniam M. Correlation between activated clotting time and activated partial thromboplastin times. Annals of Pharmacotherapy 36, 7–11, 2002. https://doi.org/10.1345/aph.1A141
- Song J, Drobatz KJ, Silverstein DC. Retrospective evaluation of shortened prothrombin time or activated partial thromboplastin time for the diagnosis of hypercoagulability in dogs: 25 cases (2006–2011). Journal of Veterinary Emergency and Critical Care 26, 398–405, 2016. https://doi.org/10.1111/vec.12478
- Thenappan T, Swamy R, Shah A, Nathan S, Nichols J, Bond L, Jolly N. Interchangeability of activated clotting time values across different point-of-care systems. The American Journal of Cardiology 109, 1379–82, 2012. https://doi.org/10.1016/j.amjcard.2011.12.033
- *Topper MJ, Welles EG. Hemostasis. In: Latimer KS, Mahaffey EA, Prasse KW (eds). Duncan & Prasse’s Veterinary Laboratory Medicine: Clinical Pathology. 4th Edtn. Pp 99–135. Iowa State Press, Ames, IA, USA, 2003
- Zaldívar-López S, Marín LM, Iazbik MC, Westendorf-Stingle N, Hensley S, Couto CG. Clinical pathology of Greyhounds and other sighthounds. Veterinary Clinical Pathology 40, 414–25, 2011. https://doi.org/10.1111/j.1939-165X.2011.00360.x
- *Non-peer-reviewed