ABSTRACT
Case history
A 4-year-old, male neutered Borzoi presented for unlocalised pain and frequent episodes of vocalisation.
Clinical findings
Pain was localised to the lumbar spine and radiographs revealed a L3–L4 lesion consistent with discospondylitis. The dog was treated for presumptive bacterial discospondylitis with surgical debridement, spinal stabilisation, and cephalexin. Samples collected from the affected intervertebral disc at the time of surgery revealed lymphoplasmacytic inflammation with no causative agent identified on histopathology or bacterial culture. After an initial period of improvement, signs recurred despite an 8-week antibiotic course, with the development of inappetence, weight loss, polydipsia, and polyuria. Repeat radiographs revealed a new cervical intervertebral lesion, and concurrent pyelonephritis was diagnosed based on blood and urine results. Fungal culture of urine resulted in growth of Rasamsonia argillacea species complex and disseminated fungal disease was clinically diagnosed. Antifungal treatment was commenced, however the dog deteriorated, and euthanasia was performed.
Pathological findings
Multifocal white plaques were grossly visualised in the spleen, mesenteric lymph nodes, cervical vertebrae, and kidneys. Periodic acid-Schiff-positive, fine, parallel-walled, occasionally branching, septate hyphae 5–10 μm in diameter, and conidia 5–7 μm in diameter were found on sectioning all organs. R. argillacea species complex was identified by fungal culture of urine and was considered the species of fungal organism seen histologically. The isolate was subsequently confirmed as R. argillacea by DNA sequencing.
Diagnosis
Disseminated Rasamsonia argillacea infection.
Clinical relevance
Rasamsonia argillacea species complex is a recognised invasive mycosis in veterinary medicine, with disseminated disease causing significant clinical complications and death. This is believed to be the first report of infection caused by R. argillacea in a dog in Australasia and highlights the importance of awareness of a potential fungal aetiology in dogs with discospondylitis.
Abbreviations: CLSI: Clinical and Laboratory Standards Institute; CRI: Constant rate infusion; MEC: Minimum effective concentration; MIC: Minimum inhibitory concentration; PAS: Periodic acid-Schiff
Introduction
Disseminated fungal infections are uncommon in dogs. Aspergillus spp. are the most commonly identified causative agent (Kelly et al. Citation1995; Schultz et al. Citation2008) and although such infections have been reported in a variety of breeds, dolichocephalic breeds, particularly German Shepherds, are over-represented (Schultz et al. Citation2008). Other opportunistic fungi such as Penicillium spp., Paecilomyces spp., and Talaromyces spp. have also been reported to cause disseminated disease in dogs (Zanatta et al. Citation2006; Tappin et al. Citation2012; Whipple et al. Citation2019). Identification of these more unusual opportunistic species has been facilitated by wider availability of molecular identification techniques. Manifestations of disseminated mycoses in dogs depend on the sites that are infected and includes discospondylitis, uveitis, pneumonia, meningitis/meningoencephalitis, kidney disease and cystitis (Grant et al. Citation2009; Elad Citation2019).
Rasamsonia is a genus of non-pigmented, filamentous fungi in the family Trichocomaceae. Within this genus R. argillacea is a species complex consisting of at least four phenotypically similar species: R. argillacea, R. eburnea, R. piperina and R. aegroticola (Houbraken et al. Citation2012). Microbiological laboratories refer to a fungal complex rather than a species when no molecular-based identification is performed. The genus has a wide geographic distribution, including New Zealand (Morris et al. Citation2021), and is highly thermotolerant with an optimal growth temperature of 37–40°C (Houbraken et al. Citation2012). Rasamsonia spp. have been found in hot environments, such as soil with high surface-temperatures, and indoor air. The genus Rasamsonia was previously classified as Talaromyces and Geosmithia but was reclassified in 2012 based on DNA sequencing data (Houbraken et al. Citation2012, Citation2013). R. argillacea is considered an emerging clinical pathogen in humans with invasive and disseminated infections reported (Stemler et al. Citation2020). A small number of case reports describe infections of dogs with Rasamsonia spp. globally including in the USA (Grant et al. Citation2009; Kawalilak et al. Citation2015), the UK (Salgüero et al. Citation2013; Lodzinska et al. Citation2017) and Germany (Lütje et al. Citation2021). A recent case series of eight dogs with disseminated mycosis caused by Rasamsonia spp. in the USA emphasised the infection as a disease of clinical significance and poor prognosis (Dear et al. Citation2021).
To the authors’ knowledge, this report describes the first case of disseminated infection with Rasamsonia spp. in a dog in New Zealand.
Case history
A 4-year-old, male neutered Borzoi presented to the Massey University Veterinary Teaching Hospital (Palmerston North, NZ) for acute agitation and frequent episodes of vocalisation that appeared to be associated with jumping and rising from rest. No traumatic events were reported by the owner; however, they noted that the dog had recently been vocalising when patted over the lumbar vertebrae. The dog’s appetite, water intake, and urination were reported to be unchanged. Prior to this presentation, the dog had been treated at another veterinary clinic for two episodes of unlocalised pain (with similar vocalisation) and transient pyrexia that was partially responsive to 5 days of treatment with meloxicam and enrofloxacin, completed 12 days prior to presentation.
The dog was purchased as a puppy from a local breeder. It had no history of travel outside of New Zealand but did have free-range access to a farm property intermittently throughout the year.
Clinical findings
The dog was bright but agitated and fear aggressive, making thorough physical examination difficult. The dog presented with tachypnoea (panting), normal bronchovesicular sounds and tachycardia (200 beats per minute), but no audible murmur or arrhythmia were detected on thoracic auscultation. Rectal temperature was 39.4°C. The dog was ambulatory on all four limbs with kyphosis of the thoracolumbar spinal region. No overt pain was elicited with lumbar palpation; however the dog was very reactive to palpation of the craniodorsal abdomen. The dog was underweight at 27 kg with a body condition score of 3/9.
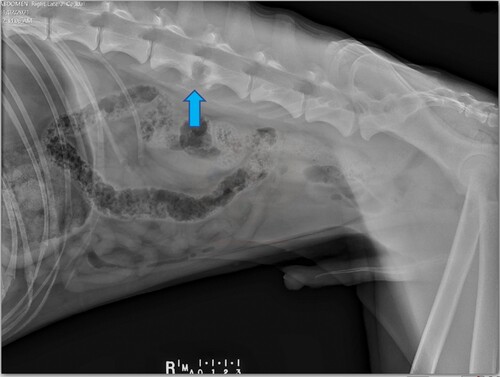
The dog was hospitalised and sedated with 0.3 mg/kg methadone IM (Phebra; Lane Coast West, NSW, Australia) and 0.01 mg/kg medetomidine IM (Mededate; Jurox, Auckland, NZ) to facilitate IV catheter placement, blood sampling and radiography. In-house haematology, serum biochemistry, and venous blood gas analysis (ICatalyst One, ProCyte Dx and VetStat respectively; Iddexx, Palmerston North, NZ) showed no abnormalities. Survey abdominal radiography () revealed collapse of the dorsal aspect of the L3–L4 intervertebral disc space (the L7 vertebrae was sacralised) with well-defined areas of endplate lysis centred over the disc space and extending into the vertebral bodies. There was marked sclerosis in the vertebral bodies, peripheral to the lysis. Ventral to L3–L4 there was smoothly margined, non-bridging, new bone. Sedation and analgesia were provided with a constant rate infusion of dexmedetomidine (Dexdomitor; Jurox) at 0.05–0.1 µg/kg/hour, fentanyl (Mercury Pharma, Auckland, NZ) at 2–5 µg/kg/hour and ketamine (Ceva, Glenorie, NSW, Australia) at 2–5 µg/kg/minute overnight, and the dog was referred to the surgical department the following morning. Reassessment revealed ongoing episodes of vocalisation and pain when moving in the cage despite the analgesia. There was a consistent pain reaction to lumbar spinal palpation and repeatable crepitus when manipulating the lumbar area but no neurological deficits. A presumptive diagnosis of chronic discospondylitis was made based on clinical presentation and radiographic lesions. Though considered less likely, neoplasia was not excluded. Due to the dog’s refractory pain (modified Glasgow pain score 15/20) and the possibility of vertebral instability, it was placed under general anaesthesia so that the spinal lesion could be biopsied surgically and the need for spinal stabilisation could be assessed.
Figure 1. Right lateral radiographic view of the spine and abdomen of a dog that presented with pyrexia, non-localised pain and kyphosis, showing a vertebral lesion at L3–L4 with endplate lysis and adjacent sclerosis (arrow).
A left-sided lateral approach was made to the vertebral bodies. The L3–L4 lateral disc annulus was highly vascularised and had a subjectively abnormal consistency. The lateral annulus was fenestrated but purulent material consistent with abscessation was not observed. There was a moderate amount of fibrous nuclear disc material present within the L3–L4 intervertebral disc space. The intervertebral disc material was curetted, and the lytic zones within the articular end plates were located by probing and then debrided with a high-speed pneumatic burr. Samples of the disc material and annulus were retained for aerobic and anaerobic bacterial culture and histopathology. Following sample collection, 22 mg/kg IV cephazolin (Cefazolin-AFT; AFT Pharmaceuticals, Auckland, NZ) was administered. Gentle manipulation demonstrated intervertebral instability and a 4-hole, 3.5-mm locking plate (SOP; Orthomed, Huddersfield, UK) was secured to the vertebral bodies at the junction of the left transverse processes of L3 and L4 using two screws to stabilise the intervertebral disc space. The surgical site was flushed with sterile saline and routinely closed.
The dog responded well to surgical curettage and stabilisation and was discharged 2 days after surgery once it no longer required parenteral analgesia. Ongoing oral medication included 2 mg/kg carprofen (Carprieve; Norbrook, Newry, NI, UK) twice daily, 10 mg/kg paracetamol (Pharmacare; Indoco Remedies, Goa, India) twice daily, 10 mg/kg gabapentin (Neurontin; Pfizer, Freiburg, Germany) twice daily, and 22 mg/kg cephalexin (Rilexine; Virbac, Fort Worth, TX, USA; empirically selected) twice daily.
Histology of the surgical biopsy of the L3–L4 annulus fibrosis revealed inter-fibre haemorrhage with foci of golden-brown pigment (consistent with haemosiderin) and mild infiltrates of inflammatory cells including lymphocytes, plasma cells and scattered neutrophils. There were areas of basophilic degeneration of collagen tissue with plump fibroblastic cells with increased nucleus-to-cytoplasmic ratio. These changes indicated multifocal collagen degeneration of the annulus fibrosis with a mixed, mild inflammatory response. The lymphoplasmacytic infiltrates were interpreted to be in response to exposed tissue antigens. No infectious agents or neoplastic cells were observed histologically, and bacterial culture did not grow any bacteria. However, a decision was made to continue antibiotic treatment for a total of 8 weeks.
When the dog re-presented 2 weeks after surgery for suture removal, the owner reported significant improvement, with only occasional vocalising that resolved with rest. A further 2 weeks later, the dog’s owner was contacted by phone and reported no episodes of pain/vocalisation, and the dog was receiving only paracetamol for analgesia.
Approximately 8 weeks after surgery, the dog re-presented for recurrence of painful episodes. The dog was still being treated with paracetamol and cephalexin orally. At that time, the owner had also noted increased frequency of urination at night. On examination, there was no appreciable pain response on lumbar spinal palpation; however, there appeared to be a consistent reaction to cervical manipulation. The remaining, limited examination did not reveal any further abnormalities. The dog was sedated for orthogonal radiography of the entire spinal column. Lysis of the endplate and widening of the C4–5 disc space was identified, consistent with multifocal discospondylitis. An aseptically collected cystocentesis sample was submitted for laboratory analysis and bacterial culture. Urinalysis revealed a urine specific gravity of 1.008 with mild haematuria and pyuria. An occasional fungal element was identified on cytology of the sediment. Aerobic and anaerobic bacterial culture of the urine was negative. Therefore intervertebral disc aspiration was elected as a more direct means of sample collection to maximise diagnostic sensitivity for either an unidentified but persistent bacterial agent, or a fungal agent, consistent with the fungi seen in the urine.
The dog was subsequently re-sedated for aspiration of the L3–L4 disc space, which was performed aseptically under ultrasonographic and fluoroscopic guidance. Samples were placed into culture vials (BD BACTEC Peds Plus/F; Benex Ltd., Shannon, Ireland) and submitted for aerobic and anaerobic bacterial, fungal culture and cytology (IDEXX Laboratories and New Zealand Veterinary Pathology, Palmerston North, NZ). Carprofen and gabapentin at the doses described previously were re-prescribed for improved analgesia.
While awaiting culture results in the following weeks, the dog started to exhibit signs of inappetence, significant polydipsia/polyuria, weight loss and increasing incidence of painful episodes. The owner was also having difficulty medicating the dog orally. Both bacterial and fungal culture of the disc space aspirates were negative, and cytological examination did not reveal any infectious organisms; however, systemic infectious disease remained high on the list of differential diagnoses.
To investigate the cause of the emergent urinary tract disease (suspected occult pyelonephritis) and progressive discospondylitis, sterile blood and urine samples were collected 3 weeks after the disc space aspirates and submitted for bacterial and fungal culture. Complete blood count and serum biochemistry revealed leucocytosis (19.5 × 109/L; reference range 6.0–17.0 × 109/L), azotaemia (creatinine 195 μmol/L; reference range 53–123 μmol/L) and hyperglobulinaemia (55 g/L; reference range 17–39 g/L). Urine culture results revealed significant fungal growth of mat-forming, fluffy, grey-white colonies. Blood culture was negative for bacteria or fungi. A presumptive diagnosis of systemically disseminated fungal disease was made, and treatment with 4 mg/kg itraconazole (Itrazole; Mylan, Auckland, NZ) twice daily was commenced. A review of the original surgical biopsy was requested to include staining for fungi. Periodic acid-Schiff (PAS) staining revealed variable fungal forms including budding forms and septate (nonbranching) hyphae within degenerative tissue debris (). The morphology was described as not unlike Candida, but also similar to Aspergillus spp. In the ensuing weeks, fungal culture and phenotypic identification from the urine sample was performed at LabPLUS (Mycology & Anaerobic Reference Laboratory, Auckland City Hospital, Auckland, NZ). Based on morphology and using various agars and temperatures (see Supplementary Material 1), Rasamsonia argillacea species complex was identified as previously described in the literature (Houbraken et al. Citation2012). Minimum inhibitory (MIC) and effective (MEC) concentrations for antifungals were determined using the Sensititre YeastOne Y10 panel (TREK Diagnostic Systems, East Grinstead, UK), which confirmed sensitivity for itraconazole (). Despite antifungal treatment and analgesia, the dog continued to progressively deteriorate with ongoing polyuria, reduced appetite, weight loss and pain. Five months from initial presentation, the dog presented collapsed to the emergency centre following an episode of acute vomiting. The owner elected euthanasia due to the rapid deterioration and the dog was submitted to Massey University School of Veterinary Science Pathobiology Service for necropsy.
Figure 2. Photomicrograph of a section from a biopsy of the L3/L4 intervertebral disc of a dog, demonstrating branching fungal hyphae within the centre (arrows) (periodic acid-Schiff; bar = 100 µm).
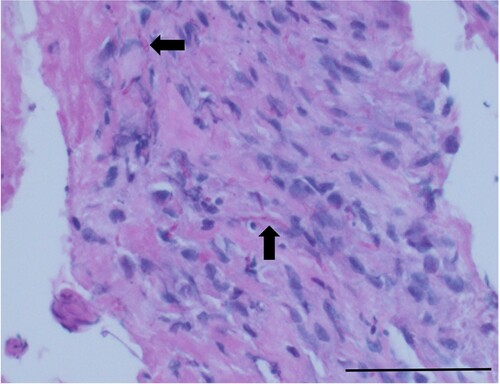
Table 1. Antifungal susceptibility test results for the Rasamsonia spp. isolate cultured from urine of a dog with pyelonephritis and discospondylitis.
Necropsy findings
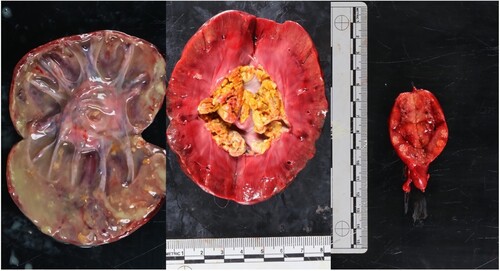
Gross abnormalities were present in multiple organs. The right kidney was enlarged and had mixed multifocal, 4–6-mm white plaques and dark haemorrhagic areas over the serosal surface. The renal pelvis and cortex had been replaced completely by copious purulent fluid with large yellow plaques floating within, which extended down the dilated ureter (). The left kidney was firm to palpate, with a nodular appearance. Within the pelvis, a yellow, florid, dry, caseous material was present (). The spleen contained a focal 10 × 10-mm white raised nodule on the serosal surface. Two mesenteric lymph nodes near the duodenum/pancreas were pale with multifocal to coalescing, irregular, dark areas over the surface, and contained multifocal white areas within the cortex (). The lumbar spine at the L3–L4 region contained the implant on the lateral surface of the vertebrae. Ventral spondylosis was present in the region. The cervical spine at the C4–5 region, contained rough, irregular, lytic lesions on both vertebral bodies near the intervertebral disc space.
Figure 3. Photographs of organs of a dog with disseminated fungal infection with Rasamsonia argillacea species complex, showing (left) copious purulent material occupying the entire right kidney, (middle) the left kidney with dry, caseous material within the renal pelvis, and (right) a mesenteric lymph node with multiple white nodules on the cut surface.
Representative samples of the lesions were embedded in paraffin wax and 5-μm sections were cut and stained with H&E.
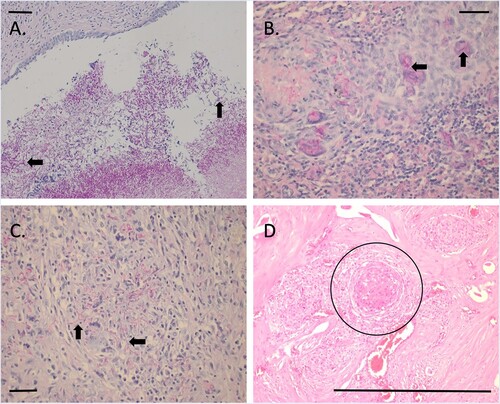
Multifocally within the kidneys, surrounding the arcuate arteries and veins, were large regions of granulomatous inflammation, admixed with fibrin, and cellular debris (necrosis). The adventitia, tunica media and endothelium were eroded, and numerous PAS-positive, 5–10-μm diameter, fine, parallel-walled, occasionally branching, septate hyphae were present, along with 5–7-μm diameter conidia (angioinvasion). Within the renal pelvis, there was a large area of amphophilic necrotic debris, mixed with abundant PAS-positive fungal organisms ((A)) Similar lesions were also noted in mesenteric lymph node, spleen, and vertebral sections with numerous PAS-positive fungal organisms present throughout ((B–D)).
Figure 4. Photomicrographs of sections from tissues from a dog with disseminated Rasamsonia argillacea species complex infection: (A) renal pelvis with numerous fungal organisms (arrows) (periodic acid-Schiff; bar = 100 µm); (B) splenic parenchyma showing multinucleate giant cells with fungal organisms within the cytoplasm (arrows) (periodic acid-Schiff; bar = 100 µm); (C) lymph node cortex with numerous extracellular fungal organisms (arrows) (periodic acid-Schiff; bar = 100 µm); and (D) vertebrae, showing large regions of granulomatous inflammation (circle) surrounding larger blood vessels (H&E; bar = 100 μm).
The histological lesions observed in this case were consistent with a diagnosis of disseminated fungal infection with localisation in the spleen, mesenteric lymph nodes, spinal column, and kidneys. Rasamsonia argillacea species complex was cultured from the urine and was considered the species of fungal organism seen histologically.
Molecular identification
The fungal isolate from the urine was retrospectively identified (LabPLUS) post-mortem to the species level using PCR amplification of large ribosomal subunit rDNA sequences with primers LR0R and LR16 (see Supplementary Material 2) followed by sequencing of the resulting amplicon. The DNA sequence from the cultured fungus showed 99.8% identity with a sequence from R. argillacea strain CBS 408.73 (accession number MH872429.1). Together with the phenotypic features of the fungus, these results confirmed the isolated organism was Rasamsonia argillacea. The sequence was submitted to GenBank as accession number OQ730211.
Discussion
To our knowledge this is the first report in Australasia of Rasamsonia argillacea causing disseminated disease in a dog. The first case of disseminated Rasamsonia (reported as Geosmithia argillacea) spp. infection in humans or animals was reported by Grant et al. in 2009. Due to morphological similarities to other fungal species (e.g. Paecilomyces spp., Talaromyces spp.), Rasamsonia spp. have been misidentified and their true prevalence underestimated (Stemler et al. Citation2020). Human Rasamsonia spp. infections show a spectrum of disease from simple colonisation to invasion, with disseminated disease occasionally reported in severely ill or immunocompromised patients (Abdolrasouli et al. Citation2018; Stemler et al. Citation2020; Eshaghi et al. Citation2021). Whether immunosuppression is similarly related to invasiveness of Rasamsonia spp. infections in dogs is unknown.
Rasamsonia spp. infection is an oligosymptomatic disease and, as such, clinicopathological presentation varies depending on the site of infection. In this case, the initial clinical signs were attributable to discospondylitis, which is commonly reported in Rasamsonia infections in dogs (Dear et al. Citation2021). Other commonly reported presenting signs are poor appetite, back pain, polydipsia, and polyuria (Elad Citation2019; Dear et al. Citation2021). The hyperglobulinaemia and leucocytosis observed in this case were compatible with chronic inflammation and are consistent with previous reports of disseminated disease in dogs (Schultz et al. Citation2008; Dear et al. Citation2021). Discospondylitis is more frequently reported to be caused by bacteria than fungi in veterinary literature: in the largest report of discospondylitis in dogs, most cases had a bacterial cause while only 2/57 had a fungal cause (Burkert et al. Citation2005). The clinicopathological picture for mycotic and bacterial discospondylitis are also similar and cannot be differentiated without microscopic or microbiological examination.
Determining the causative agent of discospondylitis can be challenging as failure to culture the organisms is common, and a negative microbial culture does not rule out the presence of infection. Burkert et al. (Citation2005) reported that combined blood and urine culture identified a causative agent in 40% of cases. Percutaneous intervertebral disc aspiration may be more sensitive, disclosing a causative agent in 60–75% of cases (Fischer et al. Citation1997; Wirtz et al. Citation2000; Burkert et al. Citation2005). Surgical biopsy, while more invasive, is considered the most sensitive method for identifying microbes in cases of discospondylitis (Kornegay and Barber Citation1980; Fischer et al. Citation1997). Surgical curettage provided a direct means of sample collection in this case, and once PAS staining was performed on stored tissue, it readily identified fungal elements. The opportunity to diagnose fungal discospondylitis was missed initially due to a low index of suspicion for a fungal cause, based on its rarity, especially in breeds other than German Shepherd dogs. Confounding the diagnostic process was the dog’s positive response to surgery and antibiotic treatment. In retrospect, clinical improvement was likely due to successful stabilisation of the collapsed disc resulting in improved pain control (McKee et al. Citation1990). Had PAS staining and fungal culture been performed on the surgical biopsy, it would have yielded an earlier diagnosis.
Due to the small number of reported cases of disseminated R. argillacea infection in dogs, there is little information to guide treatment recommendations. Early diagnosis and prompt initiation of an antifungal is key in treating disseminated fungal disease (Elad Citation2019). However, choosing an appropriate antifungal is difficult because there are no established susceptibility patterns, epidemiological cut-off values or clinical breakpoints in veterinary medicine and thus predicting efficacy in clinical cases is problematic. Results of antifungal susceptibility tests are influenced by various methodological factors, which has led to the development of standards by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antifungal testing performed under CLSI or EUCAST standards can yield different MIC due to methodological differences. Consequently, results generated by one method must not be interpreted with criteria used by the other (Kidd et al. Citation2018). For this case, the Sensititre broth microdilution technique was used by the reference laboratory and was interpreted using CLSI criteria. Susceptibility based on CLSI-compatible Sensititre may be inaccurate due to falsely low MIC for azoles, as seen with other filamentous fungal species (Lyskova et al. Citation2018). Whether this also occurs with Rasamsonia spp. is unknown. Regardless of the methodology, the absence of interpretive breakpoints warrants caution when using MIC to guide treatment in clinical cases (Hoenigl et al. Citation2021).
Successful treatment of mycoses in animals is challenging due to widespread antifungal resistance and the high costs of newer drugs (Elad Citation2019; Stemler et al. Citation2020). The MIC/MEC results for this isolate were consistent with those reported in veterinary literature as well as those recently reported in a review of antifungal susceptibility of moulds in New Zealand (Dear et al. Citation2021; Morris et al. Citation2021). Itraconazole was empirically chosen in this case, and this was appropriate according to the MIC results, however despite 8 weeks of treatment, the dog continued to deteriorate. Alternate antifungals that could have been used for this case included posaconazole or an echinocandin. Unfortunately, echinocandins such as caspofungin require daily IV injections, making them cost-prohibitive in New Zealand. Compared to itraconazole, newer antifungals have unknown optimum doses and adverse effects in animals. A global consensus on treatment of moulds in humans suggested avoiding azoles for Rasamsonia spp. infections and recommended first-line therapy with echinocandins (Hoenigl et al. Citation2021). Whether the recommendation should be the same in dogs has not been established.
Molecular techniques can be used to accurately identify fungi to help guide appropriate antifungal treatment. DNA sequencing confirmed the species infecting this dog was R. argillacea. However, this test was performed post-mortem and so had no clinical bearing. Molecular testing may prevent misidentification of Rasamsonia spp. in laboratories unfamiliar with the genus, however, it is expensive and not routinely accessible. In one study, five of six dogs diagnosed with Rasamsonia spp. infections cross-reacted with the Aspergillus galactomannan enzyme immunoassay, leading the authors to recommend routine use of PCR sequencing to avoid misdiagnosis of mould species (Dear et al. Citation2021). Interestingly, Stemler et al. (Citation2020) found that misidentification of Rasamsonia as a different genus of fungi was not a significant predictor of mortality in human cases. Despite correct species identification and treatment guided by susceptibility testing, infection with Rasamsonia spp. led to euthanasia in most of the veterinary cases reported due to progressive disease. The median survival time for the eight dogs in the recent case series was 82 days (Dear et al. Citation2021).
This case highlights the importance of increased awareness of the possibility of a fungal aetiology in discospondylitis cases of any breed of dog. Culture of urine, blood or intervertebral disc samples for both bacterial and fungal agents are recommended early in diagnostic investigations, especially in those with multi-systemic signs. PAS staining of a surgical biopsy identified the causative agent in this case and is recommended routinely in all cases of discospondylitis. Finally, clinicians should be aware that, as in this case, a diagnosis of infection with R. argillacea appears to carry a poor prognosis.
Supplemental Material
Download PDF (231.4 KB)Acknowledgements
The authors would like to acknowledge LabPlus Mycology Department for the phenotypic identification and LabPlus Microbiology Molecular Department for the molecular identification of the R. argillacea isolate.
References
- Abdolrasouli A, Bercusson AC, Rhodes JL, Hagen F, Buil JB, Tang AYY, de Boer LL, Shah A, Milburn AJ, Elborn JS, et al. Airway persistence by the emerging multiazole-resistant Rasamsonia argillacea complex in cystic fibrosis. Mycoses 61, 665–73, 2018. https://doi.org/10.1111/myc.12789
- Burkert BA, Kerwin SC, Hosgood GL, Pechman RD, Fontenelle JP. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980–2001). Journal of the American Veterinary Medical Association 227, 268–75, 2005. https://doi.org/10.2460/javma.2005.227.268
- Dear J, Reagan KL, Hulsebosch SE, Chai-Fei L, Munro MJL, Byrne BA, Affolter VK, Wiederhold N, Cañete-Gibas C, Sykes JE. Disseminated Rasamsonia argillacea species complex infections in 8 dogs. Journal of Veterinary Internal Medicine 35, 2232–40, 2021. https://doi.org/10.1111/jvim.16244
- Elad D. Disseminated canine mould infections. The Veterinary Journal 243, 82–90, 2019. https://doi.org/10.1016/j.tvjl.2018.11.016
- Eshaghi H, Moradi L, Adimi P, Gharagozlou M, Movahedi M, Parvaneh N. Invasive Rasamsonia argillacea infection in chronic granulomatous disease: report of a new case and literature review. Journal of Medical Mycology 31, 101–6, 2021. https://doi.org/10.1016/j.mycmed.2020.101106
- Fischer A, Mahaffey MB, Oliver JE. Fluoroscopically guided percutaneous disk aspiration in 10 dogs with diskospondylitis. Journal of Veterinary Internal Medicine 11, 284–7, 1997. https://doi.org/10.1111/j.1939-1676.1997.tb00466.x
- Grant DC, Sutton DA, Sandberg CA, Tyler RD, Thompson EH, Romanelli AM, Wickes BL. Disseminated Geosmithia argillacea infection in a German Shepherd dog. Medical Mycology 47, 221–6, 2009. https://doi.org/10.1080/13693780802559023
- Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, Lackner M, Sprute R, Al-Hatmi AMS, Bassetti M, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. The Lancet Infectious Diseases 21, E246–57, 2021. https://doi.org/10.1016/S1473-3099(20)30784-2
- Houbraken J, Spierenburg H, Frisvad JC. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek 101, 403–21, 2012. https://doi.org/10.1007/s10482-011-9647-1
- Houbraken J, Giraud S, Meijer M, Bertout S, Frisvad JC, Meis JF, Bouchar JP, Samson RA. Taxonomy and antifungal susceptibility of clinically important Rasamsonia species. Journal of Clinical Microbiology 51, 22–30, 2013. https://doi.org/10.1128/JCM.02147-12
- Kawalilak LT, Chen AV, Roberts GR. Imaging characteristics of disseminated Geosmithia argillacea causing severe diskospondylitis and meningoencephalomyelitis in a dog. Clinical Case Reports 3, 901–6, 2015. https://doi.org/10.1002/ccr3.372
- Kelly SE, Shaw SE, Clark WT. Long-term survival of four dogs with disseminated Aspergillus terreus infection treated with itraconazole. Australian Veterinary Journal 72, 311–3, 1995. https://doi.org/10.1111/j.1751-0813.1995.tb03562.x
- Kidd SE, Halliday CL, Morris AJ, Chen SC. Antifungal susceptibility testing in Australasian clinical laboratories: we must improve our performance. Pathology 50, 257–60, 2018. https://doi.org/10.1016/j.pathol.2018.01.001
- Kornegay JN, Barber DL. Diskospondylitis in dogs. Journal of the American Veterinary Medical Association 177, 337–41, 1980.
- Lodzinska J, Cazzini P, Taylor CS, Harris J, Kilpatrick S, Liuti T, Paterson GK. Systemic Rasamsonia piperina infection in a German Shepherd cross dog. JMM Case Reports 4, e005125, 2017. https://doi.org/10.1099/jmmcr.0.005125
- Lütje M, Bauer N, Engelen C, Lubke-Becker A, Drumm I, Hapke H. Systemic mycosis in a German Shepherd mixed-breed dog caused by Rasamsonia piperina. Kleintierpraxis 66, 516–23, 2021. [English Abstract]
- Lyskova P, Hubka V, Svobodova L, Barrs V, Dhand NK, Yaguchi T, Matsuzawa T, Horie Y, Kolarik M, Dobias R, et al. Antifungal susceptibility of the Aspergillus viridinutans complex: comparison of two in vitro methods. Antimicrobial Agents and Chemotherapy 62, e01927–17, 2018. https://doi.org/10.1128/AAC.01927-17
- McKee W, Mitten R, Labuc R. Surgical treatment of lumbosacral discospondylitis by a distraction-fusion technique. Journal of Small Animal Practice 31, 15–20, 1990. https://doi.org/10.1111/j.1748-5827.1990.tb00647.x
- Morris AJ, McKinney WP, Rogers K, Freeman JT, Roberts SA. Antifungal susceptibility of clinical mould isolates in New Zealand, 2001–2019. Pathology 53, 639–44, 2021. https://doi.org/10.1016/j.pathol.2020.09.030
- Salgüero R, Borman AM, Herrtage M, Benchekroun G, Abbondati E, Piola V, Vanhaesebrouck A. Rasamsonia argillacea mycosis in a dog: first case in Europe. Veterinary Record 172, 581, 2013. https://doi.org/10.1136/vr.101373
- Schultz RM, Johnson EG, Wisner ER, Brown NA, Byrne BA, Sykes JE. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. Journal of Veterinary Internal Medicine 22, 851–9, 2008. https://doi.org/10.1111/j.1939-1676.2008.0125.x
- Stemler J, Salmanton-García J, Seidel D, Alexander BD, Bertz H, Hoenigl M, Herbrecht R, Meintker L, Meißner A, Mellinghoff SC, et al. Risk factors and mortality in invasive Rasamsonia spp. infection: analysis of cases in the FungiScope registry and from the literature. Mycoses 63, 265–74, 2020. https://doi.org/10.1111/myc.13039
- Tappin SW, Ferrandis I, Jakovljevic S, Villiers E, White RAS. Successful treatment of bilateral Paecilomyces pyelonephritis in a German Shepherd dog. Journal of Small Animal Practice 53, 657–60, 2012. https://doi.org/10.1111/j.1748-5827.2012.01268.x
- Whipple KM, Shmalberg JW, Joyce AC, Beatty SS. Cytologic identification of fungal arthritis in a Labrador Retriever with disseminated Talaromyces helicus infection. Veterinary Clinical Pathology 48, 449–54, 2019. https://doi.org/10.1111/vcp.12777
- Wirtz DC, Genius I, Wildberger JE, Adam G, Zilkens KW, Niethard FU. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis – an evaluation of 59 cases. Archives of Orthopaedic Trauma and Surgery 120, 245–51, 2000. https://doi.org/10.1007/s004020050457
- Zanatta R, Miniscalco B, Guarro J, Gene J, Capucchio MT, Gallo MG, Mikulicich B, Peano A. A case of disseminated mycosis in a German Shepherd dog due to Penicillium purpurogenum. Medical Mycology 44, 93–7, 2006. https://doi.org/10.1080/13693780500302726
