ABSTRACT
Aims
To evaluate the effect of IM administration of three sedative drugs, acepromazine, alfaxalone and dexmedetomidine, in combination with morphine, on the size of the feline spleen using ultrasonography.
Methods
Twenty-four client-owned cats undergoing elective de-sexing or minor procedures were recruited for a focused ultrasonographic examination of the spleen prior to and at 10, 20 and 30 minutes following administration of one of three randomly assigned IM sedation protocols: 0.05 mg/kg acepromazine (ACE group), 3 mg/kg alfaxalone (ALF group), or 10 μg/kg dexmedetomidine (DEX group), in combination with 0.5 mg/kg morphine. B-mode images of the spleen were collected and measured following a standardised protocol. Cardiorespiratory parameters and sedation score were also recorded. Mean thickness of the head, body and tail of the spleen for each group at 10, 20 and 30 minutes after drug administration was compared to baseline.
Results
Mean splenic thickness increased over time in the ACE group (thickness of body at T0 = 8.9 (SE 2.1) mm and at T30 = 10.5 (SE 2.0) mm; p = 0.001) and the ALF group (thickness of body at T0 = 8.8 (SE 1.0) mm and at T30 = 10.3 (SE 1.7) mm; p = 0.022) but not in the DEX group (thickness of body at T0 = 8.6 mm (1.2) and at T30 = 8.9 mm (0.6); p = 0.67). Mean arterial blood pressure in the DEX group was significantly higher than in the other groups (p = 0.002). Sedation scores in the DEX group were consistently high for the entire period. However, the sedation score in the ACE group increased over 30 minutes (p = 0.007). Sedation score in the ALF group was highest at 10 minutes but gradually decreased over the following 20 minutes (p = 0.003).
Conclusions
Sedation with IM dexmedetomidine and morphine did not change splenic size, whereas acepromazine or alfaxalone and morphine increased it regardless of the degree of sedation.
Clinical relevance
Where splenomegaly is identified in a cat sedated with acepromazine or alfaxalone, the effects of the sedation protocol could be considered as a possible cause.
Introduction
Abdominal ultrasound is often performed under sedation in clinical practice. The possible effect of different sedation or anaesthetic agents on the size of the feline spleen is an important consideration when splenomegaly is identified, although there is currently limited literature on this topic in cats.
The spleen is known to be able to change in size due to physiological and pathophysiological mechanisms. Normal physiological processes include splenic contraction, which results in the contraction of capsular and trabecular smooth muscle and a reduction in splenic volume. Pathophysiological mechanisms that result in diffuse splenomegaly are broadly categorised into hyperplasia, inflammation, infiltration and congestion (Hanson et al. Citation2001). However, the mechanism of splenic enlargement induced by sedation is not well understood. It is thought to be in part due to the relaxation of capsular smooth muscle allowing accumulation of blood cells in the red pulp (venules and sinusoids) of the spleen (O’Brien et al. Citation2004; Baldo et al. Citation2012). With a lack of sinusoids, it has been postulated that the feline spleen would be less capable of allowing accumulation of blood in the red pulp and therefore less able to increase in size (Auger et al. Citation2019). When feline splenomegaly is identified, it is useful for the clinician to know whether this is likely to be caused by disease or by the drugs administered for sedation.
There are a limited number of studies investigating the effect of different sedation agents on the size of the feline spleen. A recent study of 15 healthy adult research cats showed that acepromazine increased splenic size, while there was no change in splenic size with butorphanol (Auger et al. Citation2019). Another study found that the feline spleen increased in thickness following administration of alfaxalone with butorphanol, but not following administration of dexmedetomidine with butorphanol (Finck et al. Citation2021). A third study of 60 healthy cats showed that there was an increase in splenic size during anaesthesia with sevoflurane (Reese et al. Citation2013).
Common sedative or pre-anaesthetic protocols in cats include dexmedetomidine, alfaxalone or acepromazine in combination with an opioid (e.g. morphine). Dexmedetomidine is an alpha-2 agonist, which causes systemic vasoconstriction via alpha-adrenergic receptors (Jaeger et al. Citation2019). Alfaxalone is a neuroactive steroid that produces strong sedation via GABAA receptors and dose-dependent cardiovascular effects such as decreasing systemic vascular resistance (Muir et al. Citation2009). Acepromazine is a phenothiazine derivative that affects vasodilation via alpha-2 receptor blockade (Clarke et al. Citation2014). Morphine is a mu-opioid and is commonly administered for analgesia when performing unpleasant procedures. Evaluation of the effect of different drugs on the size of the feline spleen, with concurrent measurement of cardiovascular parameters and level of sedation, is needed to better understand sedation-associated splenomegaly in cats.
The objective of this study was to determine the effects of three commonly used sedative protocols on the splenic size of cats, determined via ultrasonography using a standardised protocol for measurement. We hypothesised that the spleen would increase in size following the administration of acepromazine with morphine and alfaxalone with morphine, and that there would be no evidence of difference in size following the administration of dexmedetomidine with morphine. Secondarily, we hypothesised that the change in splenic size would be associated with the degree of sedation of each protocol.
Methods and materials
This study was approved by the Animal Ethics Committee of Massey University (Palmerston North, NZ; Protocol ID: 18/85).
Cats undergoing elective desexing surgery (n = 22) or a minor procedure (i.e. grooming) (n = 4) were included in this study. Informed consent was obtained from all owners. The cats were all reported by their owners to be in good health with no existing medical conditions. All cats were fasted overnight prior to the examination as per routine pre-anaesthetic procedure. A descriptive temperament score was assigned on their arrival (1: quiet, 2: anxious, 3: nervous, 4: aggressive). Patients were randomly assigned to one of three sedation protocols using Excel software (Microsoft, Redmond, WA, USA). The IM sedation protocols were 0.05 mg/kg acepromazine (ACE; Acezine 2; Ethical Agents Ltd., Auckland, NZ), 3 mg/kg alfaxalone (ALF; Alfaxan Anaesthetic Injection; Jurox New Zealand Ltd., Auckland, NZ), or 10 μg/kg dexmedetomidine (DEX; Dexdomitor; Zoetis New Zealand Ltd., Auckland, NZ), in combination with 0.5 mg/kg morphine (DBL Morphine Sulfate Injection BP; Pfizer New Zealand Ltd., Auckland, NZ).
The fur overlying the spleen on the left abdominal wall was clipped. Patients were manually restrained in right lateral recumbency and underwent the first focused ultrasonographic examination of the entire spleen to establish baseline data before sedation. Patients were excluded from the study if they were fractious or had any sonographic splenic abnormalities. The assigned protocol (either ACE, ALF or DEX) was then administered by a single author (HS) into the epaxial muscles of the patient. Cats then underwent repeat focused ultrasonographic examinations of the spleen 10, 20 and 30 minutes after administration of the sedative combination. Following the final ultrasonographic examination, the cat’s involvement in the study was completed, and it was anaesthetised for the required procedure.
All evaluations were performed using the same ultrasound machine (Xario 200, model TUS-X200; Canon Medical Systems USA Inc., Tustin, CA, USA) with a high frequency (12.0 MHz) linear array transducer (PLU-1204BT 18L7; Canon Medical Systems).
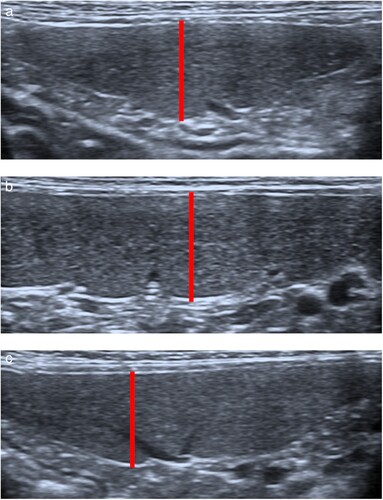
Ultrasound examinations of all study subjects were performed by one sonographer (ES). The sonographer was blinded as to which sedation protocol was administered. The entire spleen was scanned in both sagittal and transverse planes to identify any splenic abnormalities and cats with such abnormalities were subsequently excluded from the study. Splenic size was assessed using a previously reported ultrasonographic protocol (Sayre and Spaulding Citation2014). This protocol included measuring the thickness of the splenic head and tail in a transverse plane and the thickness of the splenic body in a sagittal plane. For each of the focused examinations, the head, tail and body of the spleen were each imaged three times in sequence. B-mode images were collected, and then measurements were made later by the sonographer who remained blinded to treatment groups. The thickness was measured in each location using the imaging measurement function within the ultrasound machine. Callipers were placed on the near edge of the parietal capsule margin and the far edge of the visceral capsule margin, accounting for capsule thickness with each calliper placement, as shown in . The mean thickness at each site was used for statistical analysis.
Figure 1. Ultrasound images demonstrating position of cursors (connected by solid line) for measurement of splenic thickness. For the head (a) and tail (c) of the spleen, which were imaged in a transverse plane, measurement cursors were placed on the near edge of the parietal capsule margin and the far edge of the visceral capsule margin, adjacent to the indentation from the splenic vein radicle. For the body of the spleen (b), which was imaged in a sagittal plane, the measurement was similarly made from a clearly defined splenic radicle to the opposite surface perpendicular to the long axis of the spleen.
Immediately before, and 10, 20 and 30 minutes after drug administration, the following parameters were recorded: heart rate (HR), respiratory rate (RR), rectal temperature, mean arterial blood pressure (MAP), and sedation score. HR and MAP were measured using an oscillometric blood pressure device (Pettrust; BioCare, Taoyuan City, Taiwan) with an appropriately sized cuff attached to the tail. RR was manually counted based on the movement of the chest, and temperature was measured using a rectal thermometer (Digital thermometer, Henry Schein Inc, Melville, NY, USA). Sedation was evaluated by a single author (YI) who was blinded to the sedation protocol administered, using a modified multidimensional composite scale previously described by Bhalla et al. (Citation2018). The total sedation score (0 (no sedation) to 10 (heavy sedation)) consisted of the evaluation of posture (0–4), response to clipper sounds (0–2), response to clipping (0–2), and response to restraint (0–2). Posture was evaluated from inside the cage, then the clippers were turned on in front of the door to judge the response to clipper sounds. Next, the door was opened and the response to clipping was assessed by clipping some fur over the abdominal area. An attempt to place the cat in lateral recumbency outside the cage was made to assess response to restraint.
Statistical analysis
Statistical analysis was performed using the lme4 package in R (R version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria). A power calculation was conducted prior to the start of the study based on a previous study that showed that the mean proximal height (i.e. thickness) of the spleen was 7.1 (SD 1.2) mm (Sayre and Spaulding Citation2014). It was estimated that a sample size of eight cats per group was required in order to detect a 3-mm difference of splenic thickness before and after premedication (α = 0.05, β = 0.8, SD = 1.2 mm and mean splenic thickness of 7 and 10 mm before and after premedication).
A Shapiro–Wilk test was used to check data distribution for normality.
Pre-anaesthetic parameters such as body weight, age, temperament score, HR, RR, temperature, and MAP were compared with the Kruskal–Wallis H test, and sex (female/male ratio) was compared with the χ2 test. Splenic thickness, measured 10, 20 and 30 minutes after drug administration in each splenic location, was compared to that prior to drug administration using a repeated one-way ANOVA. Sedation scores after each premedication were evaluated by the Friedman test.
A mixed-effects model was fitted to the data to account for the lack of independence between repeated measurements taken on the same cat over time. The mixed model included the fixed effects of drug (DEX, ALF or ACE) and sedation period, and their interaction, plus cat as a random effect. The explanatory variable sedation period was coded as an ordered factor with T0 (pre-administration), T10 (10 minutes after administration), T20 (20 minutes after administration) and T30 (30 minutes after administration). The model was fitted to the data separately for each of the three spleen locations. Diagnostic residual and random-effects plots were examined to assess adherence to model assumptions of within-group errors that are normally distributed, were centred at zero and had constant variance, and random effects that were normally distributed, with mean zero, and were independent for different groups. In addition, an analysis of deviance with its accompanying Wald test was carried out to indicate the quality of each model fit to the data. The data in the model were expressed as mean (SE). Significance was set at p < 0.05.
Results
Twenty-six cats were included in the study. Two cats were fractious during the initial physical examination and were excluded. A total of 24 cats completed the study. All cats were domestic shorthairs and included 11 males (nine intact, two neutered), and 13 females (11 intact, two neutered). No evidence for differences in body weight, age, temperament, HR, RR or MAP was detected between groups ().
Table 1. Characteristics of cats (n = 24) included in a study comparing the effect of acepromazine (ACE), alfaxalone (ALF) and dexmedetomidine (DEX), all in combination with morphine, on the size of the spleen measured ultrasonographically.
There were eight cats in each of the three study groups (ACE, ALF and DEX). The mean pre-sedation baseline ultrasonographic measurement of splenic thickness of the head, body and tail were 7.75 (SD 1.38) mm, 8.74 (SD 1.46) mm and 8.23 (SD 1.69) mm, respectively. Increases in mean splenic thickness over time were identified in the ACE group (thickness of body at T0 = 8.9 (2.1) mm vs. T30 = 10.5 (2.0) mm; p = 0.001) and ALF group (thickness of body at T0 = 8.8 (1.0) mm vs. T30 = 10.3 (1.7) mm; p = 0.022) but not in the DEX group (thickness of body at T0 = 8.6 (1.2) mm vs. T30 = 8.9 (0.6) mm; p = 0.67) ( and ). In the ALF group, there was a trend for the change in splenic size to be greater in the head and body than the change in size in the tail.
Figure 2. Mean (SE) thickness of the splenic head (a, transverse plane), body (b, sagittal plane) and tail (c, transverse plane) prior to (T0) and 10 (T10), 20 (T20) and 30 (T30) minutes following IM administration of 0.05 mg/kg acepromazine (ACE), 3 mg/kg alfaxalone (ALF) or 10 μg/kg dexmedetomidine (DEX), each in combination with 0.5 mg/kg morphine in cats (n = 8 per group). Asterisks indicate statistical significance compared to T0: *p < 0.05; **p < 0.01; ***p < 0.001.
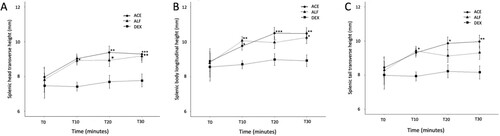
Table 2. Mean (SE) thickness (mm) of the splenic head (transverse plane), body (sagittal plane) and tail (transverse plane) prior to (0 minutes) and 10, 20 and 30 minutes following IM administration of 0.05 mg/kg acepromazine (ACE), 3 mg/kg alfaxalone (ALF) or 10 μg/kg dexmedetomidine (DEX), in combination with 0.5 mg/kg morphine in cats (n = 8 per group).
Mean arterial pressure in the DEX group was higher than that in the ALF and ACE groups (p = 0.002; ). Sedation scores in the DEX group were consistently high for the entire period. However, the sedation score in the ACE group increased over 30 minutes (p = 0.007). Sedation in the ALF group was highest at 10 minutes but the score gradually decreased over the following 20 minutes (p = 0.003; ). HR reduced from baseline after each drug administration. No evidence of a significant change in RR or temperature was detected between groups over the study period.
Figure 3. Mean (± SE) arterial pressure prior to (T0) and 10 (T10), 20 (T20) and 30 (T30) minutes following IM administration of 0.05 mg/kg acepromazine (ACE), 3 mg/kg alfaxalone (ALF) or 10 μg/kg dexmedetomidine (DEX), each in combination with 0.5 mg/kg morphine in cats (n = 8 per group). Statistically significant compared to DEX: *p < 0.05; **p < 0.01; ***p < 0.001.
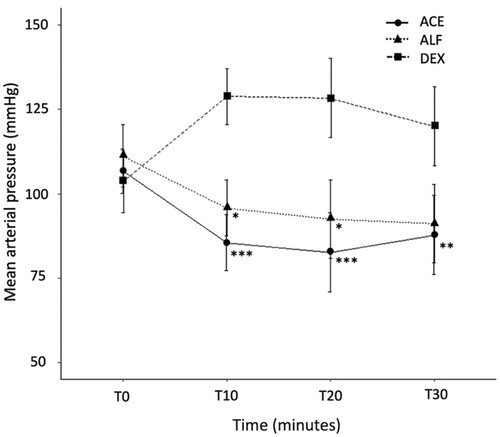
Table 3. Mean (SE) heart rate (HR), respiratory rate (RR) and temperature, and median (IQR) sedation scores prior to (0 minutes) and 10, 20 and 30 minutes following IM administration of 0.05 mg/kg acepromazine (ACE), 3 mg/kg alfaxalone (ALF) or 10 μg/kg dexmedetomidine (DEX), each in combination with 0.5 mg/kg morphine, in cats (n = 8 per group).
Discussion
Findings from the current study show that certain common sedative protocols can result in drug-induced splenomegaly in cats. Splenic enlargement was identified following administration of alfaxalone and acepromazine in combination with morphine. However, there was no evidence for splenic enlargement following administration of dexmedetomidine with morphine. These findings supported our hypothesis that the feline spleen would increase in size following administration of acepromazine and alfaxalone, and would not change significantly in size following administration of dexmedetomidine.
It is thought that drug-induced splenic enlargement is, in part, due to relaxation of capsular smooth muscle allowing passive accumulation of blood cells in the red pulp of the spleen, although this process is poorly understood (Baldo et al. Citation2012; Hasuik et al. Citation2018). Other possible mechanisms discussed in the literature include blood flow redistribution secondary to dose-dependent decrease in arterial blood pressure (Muir et al. Citation2009) and red blood cell sequestration (Hasuik et al. Citation2018).
Our study found an increase in splenic size following administration of alfaxalone and morphine, which is comparable to findings in dogs and, more recently, in cats. A study in dogs showed increased splenic volume 15 and 30 minutes following a bolus of alfaxalone (Hasiuk et al. Citation2018). A recent study in cats found the spleen increased in size following administration of alfaxalone with butorphanol (Finck et al. Citation2021).
We found no evidence of a change in splenic size with administration of dexmedetomidine and morphine, which is comparable to a recent study in cats where the spleen did not increase in size following administration of dexmedetomidine with butorphanol (Finck et al. Citation2021). Additionally, in a study by Auger et al. (Citation2019), which investigated the effect of specific sedation agents on the size of the feline spleen determined via radiographs and ultrasonography, it was found that there was a trend of increased splenic size 15–30 minutes after administration of dexmedetomidine, but this did not reach statistical significance on the ultrasonographic measurements. The findings are similar to those in dogs, where Baldo et al. (Citation2012) used computed tomography to determine canine splenic volume and found that following administration of dexmedetomidine, the splenic size did not change significantly. As postulated by others, it is possible that redistribution of blood flow to vital organs and/or a lack of functional post-synaptic α2-adrenoreceptor in the splenic vasculature may explain the lack of splenomegaly following sedation with dexmedetomidine (Finck et al. Citation2021).
Our study showed a statistically significant increase in feline splenic size with administration of acepromazine in combination with morphine. This result is comparable with other studies identifying splenic enlargement in cats following acepromazine administration. Auger et al. (Citation2019) investigated the effect of specific sedation agents on the size of the feline spleen determined via radiographs and ultrasonography and found a significant increase in splenic size after administration of acepromazine. This is also comparable to dogs, in which splenic size is reported to increase following administration of acepromazine (O'Brien et al. Citation2004; Mosallanejad et al. Citation2006; Baldo et al. Citation2012).
The effects of the ALF protocol on the sedation score reached a maximum at 10 minutes after administration and declined at 20 and 30 minutes. However, splenic thickness was increased at 10 minutes and remained increased at 20 and 30 minutes after ALF administration. In contrast, the spleen of cats that received the ACE protocol reached the maximum size after 20 minutes and remained the same at 30 minutes, whereas the peak sedation effects of ACE protocol were observed at 30 minutes. Thus, even though the sedative effect of the ACE protocol did not result in profound sedation, it produced splenomegaly in the cats under study. The onset of sedation after parenteral administration of acepromazine in cats is slow, and the duration of action is several hours (Bortolami et al. Citation2013). The current study showed that the onset of change in the spleen size induced by acepromazine occurred before peak sedative effects. Unfortunately, this study could not evaluate the duration of the splenomegaly effect induced by acepromazine. Further investigation may be required to assess the duration of the splenomegaly effect. The association between change in splenic size and degree of sedation with each protocol was not statistically tested in this study, however further research could investigate a possible relationship.
Drug-related splenomegaly is incompletely understood. It is thought to be at least in part due to the relaxation of capsular smooth muscle allowing accumulation of blood cells in the red pulp (venules and sinusoids) of the spleen (O'Brien et al. Citation2004; Baldo et al. Citation2012). It appears that despite the non-sinusoidal structure of the feline spleen, significant splenomegaly occurs after administration of some sedative agents. In this study, MAP in the DEX group was significantly higher in than the ALF and ACE groups, possibly due to the different vasomotor effects of dexmedetomidine (vasoconstriction) compared to acepromazine and alfaxalone (vasodilation). Interestingly, spleen size in the DEX group did not change, while the ALF and ACE groups had significant changes in spleen size. Therefore, we postulate that the non-sinusoidal structure may limit the relative magnitude of changes in spleen size in cats (and other species with a non-sinusoidal spleen), although more research is required to investigate this. Additionally, further research could be considered to investigate any relationship between MAP and splenic size, independent of sedation agents.
Several limitations should be considered in this study. Assessment of systemic health was confined to a general physical examination, owners reporting patients to be clinically well, and a focused ultrasonographic examination of the spleen. Other studies investigating drug-induced splenic enlargement have included assessments such as haematological and biochemical panels, testing for feline immunodeficiency virus and feline leukaemia virus, complete abdominal ultrasound and cytologic examination of splenic fine needle aspirates. It is possible that cats with occult disease may have been included in this study. The cats included in the study had variable temperament scores, and it is unknown if this may affect splenic size secondary to splenic contraction. Additionally, the sample was relatively small and comprised a large proportion of sexually intact and relatively young cats. A study on dogs has shown that the sonographic appearance of the spleen in young dogs can differ from that of adult dogs (Hwang et al. Citation2020). Moreover, it is unknown if there are age-related variations in the size of the feline spleen. Sonographic splenic measurements were performed by a single observer, which does not enable control for inter-observer variability. Additionally, this study did not evaluate the effect of a single drug or have a control group with no drug administration, as repeat examinations in an un-sedated cat were considered likely to cause undue stress and were not considered to be vital for addressing the study aims. Additive or synergistic effects of each drug in combination with morphine may also need to be considered. However, it is unlikely to bias the result of this study because all cats received morphine.
Furthering our understanding of the effect of sedative agents on the size of the feline spleen is clinically important for the interpretation of splenomegaly. We conclude that where splenomegaly is identified in a cat sedated with acepromazine or alfaxalone in conjunction with morphine, drug-induced splenomegaly could be considered as a possible cause.
References
- Auger M, Fazio C, de Swarte M, Bussières G, Schaefer D, Springer CM. Administration of certain sedative drugs is associated with variation in sonographic and radiographic splenic size in healthy cats. Veterinary Radiology & Ultrasound 60, 717–28, 2019. https://doi.org/10.1111/vru.12791
- Baldo CF, Garcia-Pereira FL, Nelson NC, Hauptman JG, Shih AC. Effects of anesthetic drugs on canine splenic volume determined via computed tomography. American Journal of Veterinary Research 73, 1715–9, 2012. https://doi.org/10.2460/ajvr.73.11.1715
- Bhalla RJ, Trimble TA, Leece EA, Vettorato E. Comparison of intramuscular butorphanol and buprenorphine combined with dexmedetomidine for sedation in cats. Journal of Feline Medicine and Surgery 20, 325–31, 2018. https://doi.org/10.1177/1098612X17709612
- Bortolami E, Murrell JC, Slingsby LS. Methadone in combination with acepromazine as premedication prior to neutering in the cat. Veterinary Anaesthesia and Analgesia 40, 181–93, 2013. https://doi.org/10.1111/j.1467-2995.2012.00736.x
- Clarke KW, Trim CM, Hall LW. Anaesthesia of the cat. In: Veterinary Anaesthesia. 11th Edtn. Pp 499–534. Elsevier, Oxford, UK, 2014
- Finck C, Steagall P, Beauchamp G. Effects of butorphanol with alfaxalone or dexmedetomidine on feline splenic size and appearance on ultrasound and computed tomography. Frontiers in Veterinary Science 8, 572146, 2021. https://doi.org/10.3389/fvets.2021.572146
- Hanson JA, Papageorges M, Girard E, Menard M, Hebert P. Ultrasonographic appearance of splenic disease in 101 cats. Veterinary Radiology & Ultrasound 42, 441–5, 2001. https://doi.org/10.1111/j.1740-8261.2001.tb00967.x
- Hasiuk MMM, Garcia-Pereira FL, Berry CR, Ellison GW. Effects of a single intravenous bolus injection of alfaxalone on canine splenic volume as determined by computed tomography. Canadian Journal of Veterinary Research 82, 203–7, 2018
- Hwang Y, Noh D, Choi S, Choi H, Lee Y, Lee K. Changes of ultrasonographic pattern of the spleen examined with a high-frequency linear transducer during growth in puppies. Veterinary Radiology & Ultrasound 61, 577–82, 2020. https://doi.org/10.1111/vru.12873
- Jaeger AT, Pypendop BH, Ahokoivu H, Honkavaara J. Cardiopulmonary effects of dexmedetomidine, with and without vatinoxan, in isoflurane-anesthetized cats. Veterinary Anaesthesia and Analgesia 46, 753–64, 2019. https://doi.org/10.1016/j.vaa.2019.05.012
- *Mosallanejad B, Avizeh R, Ghadiri A, Ezzati M. Radiographic features of acepromazine-induced splenic enlargement and its relationship with hematocrit and total protein changes in cats of Iran. World Small Animal Veterinary Association World Congress Proceedings, 2006
- Muir W, Lerche P, Wiese A, Nelson L, Pasloske K, Whittem T. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Veterinary Anaesthesia and Analgesia 36, 42–54, 2009. https://doi.org/10.1111/j.1467-2995.2008.00428.x
- O’Brien RT, Waller KR III, Osgood TL. Sonographic features of drug-induced splenic congestion. Veterinary Radiology & Ultrasound 45, 225–7, 2004. https://doi.org/10.1111/j.1740-8261.2004.04039.x
- Reese SL, Zekas LJ, Iazbik MC, Lehman A, Couto CG. Effect of sevoflurane anesthesia and blood donation on the sonographic appearance of the spleen in 60 healthy cats. Veterinary Radiology & Ultrasound 54, 168–75, 2013. https://doi.org/10.1111/j.1740-8261.2012.01990.x
- Sayre RS, Spaulding KA. Formulation of a standardized protocol and determination of the size and appearance of the spleen in healthy cats. Journal of Feline Medicine and Surgery 16, 326–32, 2014. https://doi.org/10.1177/1098612X13508947
- *Non-peer-reviewed
