ABSTRACT
Aims
To evaluate and compare the pharmacokinetics of IM and oral firocoxib, and IM meloxicam, and detect their effect on renal function and average daily gain (ADG) in lambs undergoing tail docking and castration.
Methods
Seventy-five male Romney lambs, aged 3–6 weeks, were randomised into five treatment groups (n = 15 per group): IM firocoxib (1 mg/kg); oral firocoxib (1 mg/kg); IM meloxicam (1 mg/kg); normal saline (approximately 2 mL, oral); or sham. Following the treatment administration, hot-iron tail docking and rubber ring castration were performed in all groups except the sham group, which did not undergo the procedures, but the animals were handled in the same manner as castrated and tail docked lambs. Blood samples were collected before and 1, 2, 4, 6, 8, 24, 48, 72, 96 and 120 hours after treatment administration, and drug concentrations in plasma were quantified by liquid chromatography and mass spectrometry. Plasma urea and creatinine concentrations were determined at a commercial laboratory. Lamb body weights were recorded before and 2, 4 and 8 weeks after tail docking and castration. The pharmacokinetic analysis was carried out using a non-compartmental approach. Between-group and between-time-point differences were compared using mixed model analyses.
Results
There was no evidence for a difference in plasma elimination half-life between firocoxib given IM (LSM 18.6 (SE 1.4) hours), firocoxib given orally (LSM 18.2 (SE 1.4) hours), and meloxicam given IM (LSM 17. 0 (SE 1.4) hours). Firocoxib (IM) had a significantly greater volume of distribution (LSM 3.7 (SE 0.2) L/kg) than IM meloxicam (LSM 0.2 (SE 0.2) L/kg). Lambs in the meloxicam group had higher (p < 0.05) plasma urea and creatinine concentrations than those in the firocoxib, saline and sham groups. Lambs’ ADG was decreased (p < 0.01) compared to the other treatment groups in the 0–2 week period following meloxicam administration.
Conclusions and clinical relevance
Both formulations of firocoxib had a long plasma elimination half-life and large volume of distribution. There was a transient reduction in ADG in the meloxicam group, possibly due to mild renal toxicity. Comparative studies on dose–response effects of firocoxib and meloxicam in lambs following the procedures are required.
Abbreviations: ADG: Average daily gain; Cmax: Maximum concentration; COX: Cyclooxygenase; LOD: Limit of detection; NSAID: Non-steroidal anti-inflammatory drugs; CL: Plasma clearance; T1/2el: Plasma elimination half-life; Tmax: Time to achieve Cmax; Vd: Volume of distribution
Introduction
Routine on-farm husbandry procedures such as tail docking and castration are performed to, respectively, improve lamb health, and for easy management of the flock by stock people (Sutherland Citation2011). In New Zealand, both procedures are generally carried out at the same time in young lambs of a few weeks of age (Sutherland Citation2011). Several different methods are available to castrate and tail-dock lambs. A combination of rubber ring castration and hot-iron tail docking has been the commonly used method by New Zealand farmers (Kongara et al. Citation2023). Other procedural methods include castration and tail docking using a knife and crushing of the spermatic cords using a Burdizzo clamp for castration. All these procedural methods are associated with a degree of pain and distress to the animal (Stafford Citation2017) and could have a negative impact on animal welfare as they are performed without the use of analgesia.
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID), which has been licensed in New Zealand for the control of pain and inflammation in sheep and lambs from 14 days of age or older. Studies in young farm animals, such as calves, reported both the pharmacokinetics and efficacy of meloxicam after castration and dehorning (Coetzee et al. Citation2012; Melendez et al. Citation2019). Pharmacokinetic and efficacy data for meloxicam are only available in mature sheep (Woodland et al. Citation2019). To date, no reports are available on the pharmacokinetics of meloxicam in lambs despite many studies reporting its efficacy in reducing behaviours associated with acute pain following tail docking and castration (Small et al. Citation2014, Citation2018, Citation2021).
Firocoxib is a NSAID shown to provide long-acting, selective inhibition of cyclooxygenase (COX)-2 in the horse (Barton et al. Citation2014). No reports are available on its COX-2 selectivity in the sheep. It is approved by the US Food and Drug Administration for the control of post-operative pain and inflammation due to soft-tissue and orthopaedic surgery in dogs, and osteoarthritis in the horse (FDA Citation2004, Citation2005, Citation2016). Maternal delivery of firocoxib to nursing piglets prior to tail docking, teeth clipping, and castration significantly reduced the procedural stress for 3 days and increased the average daily gain (ADG). Mean plasma elimination half-life (T1/2el) was 30–48 hours in piglets (Coetzee et al. Citation2019). In other farm animals such as un-weaned calves and adult goats, the plasma T1/2el of a single oral dose (0.5 mg/kg) of firocoxib has been shown to be 18 and 21.5 hours, respectively (Stock et al. Citation2014; Stuart et al. Citation2019). No studies are available on the use of firocoxib in lambs.
Studies investigating the production benefits, such as increased ADG, in lambs due to provision of analgesia at the time of castration and tail docking are very limited. A few studies have shown a non-significant effect of meloxicam treatment on growth rate of lambs following the procedures (Small et al. Citation2014, Citation2018).
Renal papillary necrosis has been demonstrated in horses following administration of NSAID such as phenylbutazone (Black Citation1986). Elevation of blood urea nitrogen and serum creatinine concentrations was recorded within 24 hours of death in the study animals. Conversely, in a study of firocoxib in pigs (Coetzee et al. Citation2019), examination of liver, kidney and gastrointestinal tract of sows and piglets showed no signs of NSAID-specific toxicity. Given this variability, organ toxicity associated with NSAID use should be considered in susceptible sheep (Small et al. Citation2021).
The aims of the current study were to describe the pharmacokinetics of firocoxib after oral and IM administration, to compare the pharmacokinetics of firocoxib with meloxicam, to evaluate the effects of both firocoxib and meloxicam on plasma urea and creatinine concentrations, and to detect the effects of the treatments on ADG, in lambs undergoing tail docking and castration.
Materials and methods
Animals
The study was approved by Massey University Animal Ethics Committee (protocol number 20/18). Seventy-five entire male, singleton, Romney lambs (mean bodyweight 17.4 (SD 0.6) kg), aged 3–6 weeks, were sourced from the Keebles Farm (Massey University, Palmerston North, NZ). The lambs were randomly selected from a mob of 150 single bearing-ewes, and the selected lamb-ewe mob was left in separate paddocks adjacent to the yards during the study period. The study was conducted in five batches (15 lambs per batch) over a 19-day period. On each study day, a batch of 15 lambs were randomly drafted off the mob, weighed and serially numbered (1–15 in each batch) on the head and rump with coloured stock marker. They were randomly assigned to one of five treatment groups (three lambs per group in each batch), using a random number generator (https://www.graphpad.com/quickcalcs/randomize1/). The lambs were then allocated to one of three pens (n = 5 per pen) in the open yards, so that each pen contained one lamb from each treatment group. Thus, all treatments were represented in each pen. After allocation of lambs to the pens, their dams were left in a long paddock immediately adjacent to the pens; thus, the lambs and their dams were in direct visual contact and close proximity during the study period. Lambs were then left undisturbed for a minimum of 30 minutes to settle in the pens. Video recording of the baseline behaviours was commenced after the settling period.
Treatment administration and recording of body weight
After a 45-minute behaviour recording, a baseline jugular venous blood sample (5 mL) was collected, and the lambs received one of the following five treatments (n = 15 per group, over five batches): firocoxib (1 mg/kg, Equioxx injection; Merial Ltd., Duluth, GA, USA) given IM; firocoxib (1 mg/kg, Equioxx, 0.82% oral paste, Merial Ltd.) given orally; meloxicam (1 mg/kg, Metacam 20; Boehringer Ingelheim, Auckland, NZ) given IM; normal saline (approximately 2 mL) given orally; and sham control. IM treatments were administered into lateral neck muscles using a 20 gauge, 1-inch needle. Firocoxib oral paste was administered through the interdental space onto the back of the tongue, and the lamb’s head was held in an elevated position for 1 minute after administration to ensure the drug was ingested (Kvaternick et al. Citation2007). Lambs in the sham group were handled and restrained in the same manner and for the same duration as castrated and tail docked lambs but did not undergo the procedures.
Following treatment administration, a combined ring castration and hot-iron tail docking was performed by an experienced farm staff member. Castration was performed by the application of a latex rubber ring, proximal to the testes using an elastrator device (Heiniger elastrator ring applicator; Heiniger New Zealand Ltd., Christchurch, NZ) Tail docking was then carried out using a portable gas-heated docking iron (lamb tail docking iron-standard model, Te Pari Livestock Equipment & Handling Solutions, Oamaru, NZ) to remove approximately the last 4/5 of the tail. Castration and tail docking of the 15 lambs in each batch was completed within 15–20 minutes. A fly repellent (Vetrazin Spray-On; Elanco Animal Health, Auckland, NZ) was then applied, and the lambs were returned to their pens and behaviours recorded in the immediate post-procedure period. The behavioural data are presented in a separate article.
Within each batch, the randomly allocated treatments were administered to the lambs in the order of their numerical sequence allocated at random drafting of lambs, and docking and castration were performed in this same order. Body weight of lambs of all groups was recorded at 2, 4 and 8 weeks following tail docking and castration. Ewes and lambs were maintained on pasture containing a mix of ryegrass (Lolium perenne), browntop grass (Agrostis capillaris) and white clover (Trifolium repens), with adequate cover (approximately 1250 kg DM/ha), under routine farm management conditions in New Zealand. Pastures were rotationally grazed.
Blood sampling for pharmacokinetic analysis and assay of serum urea and creatinine
Blood samples were collected 1, 2, 4, 6, 8, 24, 48, 72, 96 and 120 hours after drug administration. Bleeding at all time-points was done as per the numerical sequence of lambs in each batch, allocated at drafting. At each time point, 5 mL blood was collected into a lithium heparin vacutainer via jugular venepuncture. The vacutainer tubes were immediately transferred to a insulated box containing ice and stored for up to 1 hour before transportation to the laboratory at School of Agriculture and Environment, Massey University.
Blood samples were then centrifuged at 1500g for 10 minutes. Plasma was collected into cryovials and stored at −80°C until analysis. Frozen plasma samples were sent on dry ice to a commercial laboratory (Analytica Laboratories, Hamilton, NZ) for measurement of firocoxib and meloxicam concentrations. Fresh plasma samples were submitted to a commercial biochemistry laboratory (IDEXX Laboratories Pty. Ltd., Palmerston North, NZ) for analysis of urea and creatinine concentrations.
Quantification of firocoxib and meloxicam
Sample preparation
Calibration stock solutions of firocoxib and meloxicam were prepared in acetonitrile at 1, 10, 100, and 1000 ng/mL and kept at −20°C. Working standards were prepared across the range of 0.05–100 ng/mL (meloxicam) and 0.02–100 ng/mL (firocoxib) by adding respective drug stock standard solution in acetonitrile to 200 µL of extractant containing 50 ng/mL meloxicam-d3 (HPC Standards GmbH, Leipzig, Germany) or firocoxib-d6 (HPC Standards GmbH, Leipzig, Germany) in an amber glass vials. Mobile phase A was added to make a total volume of 1 mL. A 100-µL aliquot of spiked quality control plasma (2, 20 and 200 ng/mL) or sample plasma was added to 400 µL of 0.1% formic acid in acetonitrile containing 50 ng/mL meloxicam-d3 or firocoxib-d6 in a 96-well plate. The plate was shaken on a vortex mixer, sonicated for 10 minutes, and centrifuged for 5 minutes at 2,500g. Supernatant (100 µL) was transferred to a 96-well microtitre plate containing 300 µL of mobile phase A. This was shaken on a vortex mixer and used for liquid chromatography mass spectrometry/mass spectrometry analysis.
Liquid chromatography
An ultra-performance liquid chromatography system (ExionLC AD Series; Sciex, Framingham, MA, USA) was used, and the separation was made with a column (XSELECT HSS T3 Column XP, 2.1 × 50 mm, 2.5 µm; Waters Corp., Drinagh, Ireland) fitted with an in-line filter (KrudKatcher Ultra; Phenomenex, Torrance, CA, USA). Gradient chromatography was used to separate analytes from other sample components by changing the ratio of mobile phase A and B. For firocoxib, the mobile phase was composed of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B), and for meloxicam it was 10 mM ammonium formate containing 0.1% formic acid in water (A) and methanol (B).
For firocoxib, composition started at 80% mobile phase A:20% mobile phase B, and changed to 5% mobile phase A:95% mobile phase B over a period of 0.8 minutes; mobile phase composition was maintained at this ratio for a further 0.5 minutes, before returning to the starting conditions (total run time 2.2 minutes). For meloxicam, composition started at 80% mobile phase A:20% mobile phase B, and was held at that ratio for 0.8 minutes, then changed to 30% mobile phase A:70% mobile phase B over a period of 2.2 minutes, before returning to the starting conditions (total run time 5 minutes). The flow rate was set at 0.6 mL/minute for firocoxib and 0.8 mL/minute for meloxicam. The injection volume was 2 μL and the auto-sampler temperature was maintained at 15°C for both drugs. The column temperature was maintained at 40°C and 60°C for firocoxib and meloxicam, respectively.
Mass spectrometry
Detection was performed using a quadrupole mass spectrometer (Triple Quad 6500 MS/MS; Sciex, Framingham, MA, USA) equipped with an electrospray ionisation source operated in positive ion mode. The optimum mass spectrometry conditions included the following parameters: desolvation temperature 350°C (firocoxib) or 250°C (meloxicam), and capillary voltage 3 kV. Nitrogen was used as the desolvation gas, with declustering potential 100 V. Quantification was performed using multiple reaction monitoring for the transitions with mass-to-charge ratio (m/z) 337 > 283 (quantifier, firocoxib) or 352 > 115 (quantifier, meloxicam) and 337 > 237 (qualifier, firocoxib) or 352 > 141 (qualifier, meloxicam). The transition m/z 341 > 289 or 355 > 115 was used to quantify firocoxib-d6 (internal standard) or meloxicam-d3 (internal standard).
Pharmacokinetic analysis
The plasma drug concentration-time data obtained after oral and IM administration of firocoxib, and IM meloxicam were analysed using a non-compartmental method for extravascular administration (PCModfit software, version 7.4, UK; https://www.pcmodfit.co.uk). The maximum plasma concentration (Cmax) and time to achieve Cmax (Tmax) were determined as direct observations from plasma drug concentration-time data. The other pharmacokinetic parameters such T1/2el, area under the concentration–time curve, mean residence time, apparent volume of distribution (Vd) and clearance (CL) were calculated by the linear trapezoidal rule in the PCmodfit programme.
Statistical analyses
The dependent outcome variables of the study were the plasma drug concentrations, pharmacokinetic parameters, plasma urea and creatinine concentrations, and ADG. Using body weight recorded at docking and castration (time-point 0, T0), and 2, 4 and 8 weeks after these procedures, ADG (grams) during three periods (0–2, 2–4, and 4–8 weeks), and overall (0–8 weeks) were estimated. The predictor variables for ADG, and concentrations of drugs, urea and creatinine in plasma were treatment, time, and their interaction. The predictor variable for pharmacokinetic parameters was treatment. Comparisons were made using generalised linear mixed model in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The model used included the fixed effects of the predictor variables for each of the variables specified above, and the random effects of lambs. Since there were significant between-group differences in body weight at T0, body weight at docking was fitted as a co-variate in the model for ADG. In case of variables with repeated measures (plasma drug, urea and creatinine concentrations, and ADG), the covariance error structure for repeated measures over time-points, within animals and within each group was determined using Akaike’s information criterion. A heterogeneous first-order autoregressive model was found to be the most appropriate error structure. To normalise residuals of data, data on all dependent variables were Blom-transformed (Blom 1958) prior to mixed model analysis. To facilitate multiple comparisons of LSM, Bonferroni adjustment of p-values was made. The significance level was set at Bonferroni-corrected p < 0.05. For all variables, data is presented in graphs and tables as LSM (SE) in the original scale.
Results
Pharmacokinetics
Firocoxib (IM and oral) and meloxicam (IM) could be detected at the first sampling point after the procedures (1 hour) in the plasma of all animals in the respective groups.
For both IM and oral formulations, concentrations of firocoxib in plasma were above the limit of detection (LOD 0.6 ng/mL; limit of quantification: 1 ng/mL) in all lambs until 96 hours after treatment. Plasma concentrations of meloxicam were above LOD (1.7 ng/mL; limit of quantification: 2.5 ng/mL) until 120 hours in all lambs. At the 120-hour sampling point, three lambs in the IM firocoxib group and four lambs in the oral firocoxib group had plasma concentrations below the LOD. and depict concentration-time curves with LSM ± SE concentrations for the two firocoxib formulations, and IM meloxicam, respectively. shows the pharmacokinetic data for the three drugs. LSM T1/2el for IM and oral firocoxib were 18.6 (SE 1.4) and 18.2 (SE 1.4) hours, respectively, and for IM meloxicam 17.0 (SE 1.4) hours. There was no evidence for a difference between the Blom-transformed mean estimates. LSM CL of IM meloxicam (8.3 (SE 10.0) mL/hour/kg) was lower than IM firocoxib (144.2 (SE 10.0) mL/hour/kg).
Figure 1. Least square mean (±SE) concentration of firocoxib in plasma after IM (dotted line; 1 mg/kg) and oral (solid line; 1 mg/kg) administration to unweaned lambs (n = 15 lambs per group) undergoing hot-iron tail docking and rubber ring castration. Asterisks indicate that the mean values for the two routes of administration differed (p < 0.05) at 1, 6 and 8 hours after administration.
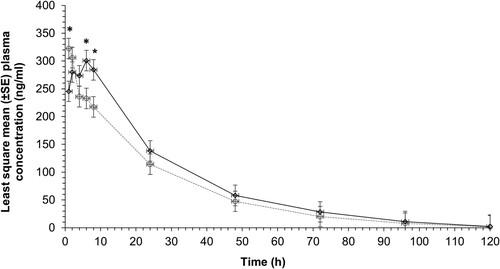
Figure 2. Least square mean (±SE) plasma concentrations of meloxicam after IM (1 mg/kg) administration to unweaned lambs (n = 15 lambs) undergoing hot-iron tail docking and rubber ring castration.
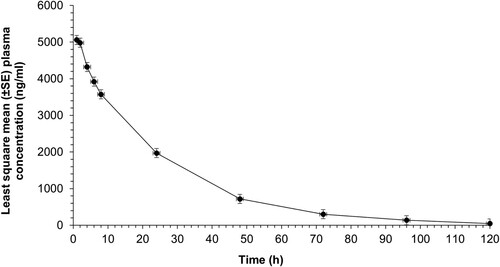
Table 1. Least squares mean (±SE)Table Footnotea pharmacokinetic parameters of firocoxib and meloxicam following oral or IM administration to lambs (n = 15, 3–6 weeks old) at a dose of 1 mg/kg.
Plasma urea and creatinine
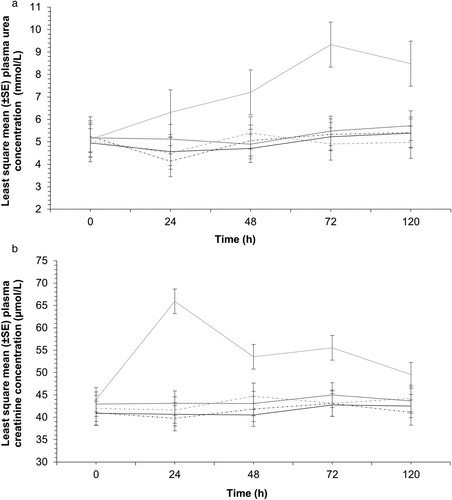
There was no evidence for a difference in plasma urea ((A)) or creatinine ((B)) concentrations between baseline and defined time points in all groups except the meloxicam group. The mean concentrations of urea and creatinine in the meloxicam group increased (Bonferroni-corrected p < 0.05) at all defined time points compared to their corresponding baseline values. Overall, the meloxicam group had higher (Bonferroni-corrected p < 0.05) plasma urea and creatinine concentrations compared to both groups that received firocoxib, and the saline and sham control groups. Overall plasma urea concentrations in the meloxicam group were 2.3 (95% CI = 1.0–3.6) mmol/L, 2.0 (95% CI = 0.7–3.3) mmol/L, 2.3 (95% CI = 0.9–3.6) mmol/L, and 2.3 (95% CI = 0.9–3.7) mmol/L higher (Bonferroni-corrected p < 0.05) than in the IM firocoxib, oral firocoxib, saline and sham groups, respectively. Similarly, the overall plasma creatinine concentrations in the meloxicam group were 12.2 (95% CI = 6.4–18.1) µmol/L, 10.1 (95% CI = 4.1–16.2) µmol/L, 12.3 (95% CI = 6.3–18.5) µmol/L, and 10.6 (95% CI = 4.3–16.8) µmol/L higher (Bonferroni-corrected p < 0.05) than in the IM firocoxib, oral firocoxib, saline and sham groups, respectively. However, the elevated urea and creatinine concentrations measured after treatment in the meloxicam group were within the reference ranges (plasma urea 5.1–15.6 mmol/L; creatinine 73–150 µmol/L) supplied by the laboratory.
Figure 3. Least square mean (±SE) plasma concentrations of urea (a) and creatinine (b) after administration of IM firocoxib (solid black line; 1 mg/kg), oral firocoxib (dotted black line; 1 mg/kg), IM meloxicam (solid grey line; 1 mg/kg), saline (black dashdot line) to lambs (n = 15 lambs per group) that were castrated and docked on Day 0 or that were neither castrated nor docked (sham, n = 15; grey dashdot line). Mean urea and creatinine concentrations in plasma after tail docking and castration differed (Bonferroni-corrected p < 0.05) from baseline values in the meloxicam group.
Average daily gain
There was no evidence for a difference in ADG in body weight between the oral firocoxib, IM firocoxib, saline, and sham groups during the 2-week period following tail docking and castration (). However, ADG for lambs in the meloxicam group was 110.9 (95% CI = 16.1–205.7) g, 95.6 (95% CI = −0.7–191.9) g, 104.8 (95% CI = 6.8–202.8) g, and 105.2 (95% CI = 5.2–205.1) g less (Bonferroni-corrected p < 0.05) during this time period, compared to the lambs in IM firocoxib, oral firocoxib, saline and sham groups, respectively. There was no evidence for a difference in ADG between the treatment groups in the 2–4 and 4–8 week periods. The point estimate for the LSM for overall ADG (0–8 weeks after procedures) was 261.4 (SE 22.7) g and 261.7 (SE 23.5) g in the IM and oral firocoxib groups, respectively, with 240.1 (SE 23.5) g in the meloxicam group, 253.2 (SE 24.4) g in the saline group and 252.8 (SE 25.3) g in the sham group. However, there was no evidence for a difference between these values.
Figure 4. Least square mean (±SE) average daily gain (ADG) in body weight during different time-periods in groups of lambs (n = 15 per group) that were treated with an analgesic drug (firocoxib IM: 
 , meloxicam IM:
, meloxicam IM:  ) or and saline (oral:
) or and saline (oral:  ) and then docked and castrated, or not (sham:
) and then docked and castrated, or not (sham:  ). ADG in the meloxicam IM group during 0–2 weeks following castration and docking was (Bonferroni-corrected p < 0.05) lower than the other groups. There were no between-group differences in ADG during 2–4 weeks or 4–8 weeks or overall period (0–8 weeks).
). ADG in the meloxicam IM group during 0–2 weeks following castration and docking was (Bonferroni-corrected p < 0.05) lower than the other groups. There were no between-group differences in ADG during 2–4 weeks or 4–8 weeks or overall period (0–8 weeks).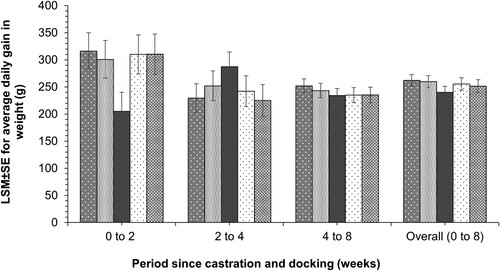
Discussion
This is the first study evaluating the pharmacokinetics of firocoxib in unweaned lambs undergoing tail docking and castration. Pharmacokinetic parameters such as Cmax, CL and T1/2el of firocoxib did not differ significantly between the oral and IM routes. As expected, firocoxib given IM attained peak plasma concentration significantly earlier (LSM Tmax = 1.4 (SE 0.4) hours) than when administered orally (LSM Tmax = 3.7 (SE 0.4) hours). This could be due to the slower absorption of the drug after oral administration.
The dose of firocoxib (1 mg/kg) used for both IM and oral administration was chosen from previous studies in un-weaned calves where oral doses of 0.5 mg/kg (Stock et al. Citation2014) and 4.2 mg/kg (Wagner et al. Citation2021) were reported. Since this is the first study in unweaned lambs, a dose (1 mg/kg) that is within the range of these previous studies was selected. The dose of meloxicam (1 mg/kg) was selected according to the manufacturer’s recommendations in sheep and lambs.
In the current study, firocoxib was detected in the plasma 1 hour after administration by both IM and oral routes. Rapid onset of effect is required after analgesic administration for on-farm procedures such as tail docking and castration. Although the oral route of analgesic administration is preferred in field situations (Ingvast-Larsson et al. Citation2011), the slower rate of oral drug absorption compared to systemic routes may delay the onset of drug effects. One limitation of the current study is the lack of data for plasma drug concentrations earlier than 1 hour after administration. It was not possible to collect blood samples immediately after drug administration due to the need to dock and castrate the lambs and record pain behaviours. More studies are required to detect the actual (earliest) time at which firocoxib can be found in plasma after administration by systemic and oral routes, and its minimum effective plasma concentration in lambs.
In the current study, IM and oral firocoxib had long T1/2el (median 18.6 and 17.5 hours, respectively) and large Vd (median; 3.5 and 3 L/kg, respectively) which were comparable with those reported in unweaned calves (T1/2el, median 18.9 hours; Vd: 3.27 L/kg) by Stock et al. (Citation2014). A long T1/2el, which was detected for both firocoxib and meloxicam in this study following a single dose, potentially may result in long lasting analgesia, which is desirable in farm animals undergoing painful husbandry procedures (Coetzee et al. Citation2019). This reduces the labour costs, the need for repeated dosing, and improves animal wellbeing. It may also have the potential for increased uptake by farmers.
Studies on other NSAID such as flunixin meglumine (1 mg/kg, IV; Welsh et al. Citation1993) and ketoprofen (3 mg/kg, IV; Ali et al. Citation2012) in sheep showed much shorter T1/2el (3.8 and approximately 2 hours, respectively) than for firocoxib in the present study. Although carprofen (4 mg/kg), another NSAID, had longer T1/2el (approximately 46–47 hours) following SC, IM, and oral administration in sheep, its slower rate of absorption and consequently longer Tmax (8, 2.06 and 11.73 hours, respectively) precludes its use for rapid onset of analgesia. Muscle damage at injection site is another drawback of carprofen use via the IM route (Coskun et al. Citation2022).
In the present study, IM firocoxib had a significantly larger Vd than meloxicam. High lipophilicity of firocoxib facilitates its wide distribution throughout the body tissues, synovial fluid (Kvaternick et al. Citation2007), and aqueous humour (Hilton et al. Citation1991) in horses, and milk in pigs (Coetzee et al. Citation2019). Approximately 30% of the plasma drug concentration was found in synovial fluid of the horse (Kvaternick et al. Citation2007). Although increased distribution of firocoxib into the synovial fluid or other body fluids has not been studied in animals with natural or induced inflammation and/or disease, it is expected that firocoxib rapidly distributes into inflammatory tissue based on its pharmacokinetic properties in healthy animals (Fadel and Giorgi Citation2023).
In the present study, the CL and Vd of meloxicam (0.008 (SE 0.001) L/hour/kg and 0.2 (SE 0.2) L/kg, respectively) corroborate the values reported in a previous study in sheep (0.006 (SE 0.002) L/kg/hour and 0.11 (SE 0.05) L/kg; Woodland et al. Citation2019). The combination of a low CL and Vd would have resulted in high plasma concentrations (LSM Cmax = 5362.8 (SE 94.8) ng/mL), and a long T1/2el (LSM = 17.0 (SE 1) hours) in meloxicam-treated lambs of the current study. Coetzee et al. (Citation2009) reported similar findings in calves treated with meloxicam.
Elimination half-life is a function of both CL and Vd. Although IM firocoxib had higher CL than meloxicam, there was no evidence for a difference in the T1/2el (LSM = 18.6 (SE 1.4) hours) for IM firocoxib compared to IM meloxicam (LSM = 17.0 (SE 1) hours). This could be because the Vd calculated from plasma concentrations of firocoxib might be lower than the actual physiologic volume in which it distributes into tissues and other body fluids.
In the present study, high urea and creatinine concentrations in the plasma of lambs treated with meloxicam (1 mg/kg, IM) indicates a possible transient detrimental effect on kidney function. Renal toxicity of meloxicam has been documented in other species such as cats (Robson et al. Citation2006; Wun et al. Citation2003). No reports are available for sheep or other ruminants. NSAID-associated renal adverse effects depend on the degree of COX-1 and -2 inhibition, which in turn depends on the dose administered and the plasma concentrations achieved in vivo with these doses (Lees et al. Citation2004). In vitro studies in horses, dogs, cats and sheep have demonstrated that meloxicam is a preferential COX-2 inhibitor (Engelhardt et al. Citation1996; Brideau et al. Citation2001; Naylor et al. Citation2014). The dose–response curves for the COX selectivity of NSAID show loss of selective inhibition or the preferential COX sparing effect at high doses (Kay-Mugford et al. Citation2000). In the present study, it is possible that the high plasma concentrations found in lambs treated with 1 mg/kg meloxicam IM would have resulted in partial or complete loss of selective COX-2 enzyme inhibition (or COX-1 sparing effect) and consequently reduced renal function, reflected as increased plasma concentrations of urea and creatinine. However, dose–response studies of meloxicam are not available for lambs.
In the current study, lower (p < 0.01) ADG in the immediate 2-week period following the procedures in the meloxicam group could be due to transient renal stress as indicated by elevated concentrations of renal markers. This finding of our study correlates with a recent study (Small et al. Citation2021) reporting reduced ADG during the marking-to-weaning period in Merino lambs treated with meloxicam (1 mg/kg, SC) at ring castration and tail docking. However, as the elevated plasma urea and creatinine concentrations were within the laboratory reference range, the observed effects of meloxicam may not be biologically relevant.
A multitude of factors contribute to differences in drug pharmacokinetics between ruminant and pre-ruminant animals (Hinchcliff et al. Citation1991). No studies are available on meloxicam pharmacokinetics in lambs despite many studies reported in mature sheep. A dose of 1 mg/kg for both mature sheep and young lambs from 14 days of age has been recommended by the manufacturer. Mosher et al. (Citation2012) reported a variation in meloxicam pharmacokinetics between ruminant and pre-ruminant calves and suggested dose adjustment in pre-ruminant calves. Based on the current study findings and those from of Small et al. (Citation2021), it appears that a dose < 1 mg/kg may be more appropriate in young lambs to reduce the transient potential effects on the kidneys and ADG.
In the current study, the modelling of the data suggests that there is no evidence for a difference in ADG over the period 0–8 weeks between the treatment groups. A dose-dependent increase in ADG has been reported 21 days after husbandry procedures in piglets that consumed milk from sows treated with IM firocoxib at four different doses (Coetzee et al. Citation2019). Future studies focussing on dose–response effects of firocoxib on lambs ADG are required.
In conclusion, findings of the present study show no evidence for a difference in T1/2el and Tmax between lambs treated IM with firocoxib and meloxicam. Both oral and IM formulations of firocoxib had a large Vd and no evidence for an effect on renal function following administration of a single dose (1 mg/kg) prior to tail docking and castration of lambs. Treatment with meloxicam (1 mg/kg, IM) was associated with an increase in plasma urea and creatinine concentrations and a transient decrease in ADG. However, the present study cannot indicate if these are causally related or if there were any adverse consequences from the elevated plasma urea and creatinine concentrations. Since correlation between plasma concentration and efficacy of NSAID has long been debated, studies are required to evaluate the comparative analgesic effect of these NSAID in lambs after castration and tail docking.
Acknowledgements
Dr Mhairi Sutherland, Senior Advisor, research programmes at Beef + Lamb New Zealand is gratefully acknowledged for her valuable advice in conceptualisation of this project. The research was funded through a Sustainable Food and Fibre Futures grant (SFFF-20030) from the Ministry for Primary Industries, Massey University Research Fund and Wairarapa Veterinary Association Research Grant.
References
- Ali A, Afzal S, Ashraf M, Amin S, Javeed A, Usman M. Pharmacokinetic study of ketoprofen in healthy sheep under local conditions of Pakistan. The Journal of Animal & Plant Sciences 22, 588–92, 2012
- Barton MH, Paske E, Norton N, King D, Giguère S, Budsberg S. Efficacy of cyclo-oxygenase inhibition by two commercially available firocoxib products in horses. Equine Veterinary Journal 46, 72–5, 2014. https://doi.org/10.1111/evj.12095
- Black HE. Renal toxicity of non-steroidal anti-inflammatory drugs. Toxicologic Pathology 14, 83–91, 1986. https://doi.org/10.1177/019262338601400110
- Brideau C, Van Staden C, Chung Chan C. In vitro effects of cyclooxygenase inhibitors in whole blood of horses: dogs, and cats. American Journal of Veterinary Research 62, 1755–60, 2001. https://doi.org/10.2460/ajvr.2001.62.1755
- Coetzee JF, KuKanich B, Mosher R, Allen PS. Pharmacokinetics of intravenous and oral meloxicam in ruminant calves. Veterinary Therapeutics 10, E1–E8, 2009
- Coetzee JF, Mosher RA, KuKanich B, Gehring R, Robert B, Reinbold JB, White BJ. Pharmacokinetics and effect of intravenous meloxicam in weaned Holstein calves following scoop dehorning without local anesthesia. BMC Veterinary Research 8, 153, 2012. https://doi.org/10.1186/1746-6148-8-153
- Coetzee JF, Sidhu PK, Seagen J, Schieber T, Kleinhenz KE, Kleinhenz MD, Wulf LW, Cooper VL, Mazloom R. Delivery of firocoxib to piglets reduces stress and improves average daily gain after castration, tail docking, and teeth clipping. Journal of Animal Science 97, 2750–68, 2019. https://doi.org/10.1093/jas/skz143
- Coskun D, Corum O, Corum DD, Uney K, Elmas M. Pharmacokinetics and bioavailability of carprofen in sheep. Journal of Veterinary Pharmacology and Therapeutics 45, 543–9, 2022. https://doi.org/10.1111/jvp.130852
- Engelhardt G, Bögel R, Chr Schnitzer, Utzmann R. Meloxicam: influence on arachidonic acid metabolism: part 1. In vitro findings. Biochemical Pharmacology 51, 21–8, 1996. https://doi.org/10.1016/0006-2952(95)02111-6
- Fadel C, Giorgi M. Synopsis of the pharmacokinetics, pharmacodynamics, applications, and safety of firocoxib in horses. Veterinary and Animal Science 19, 100286, 2023. https://doi.org/10.1016/j.vas.2023.100286
- *FDA. Freedom of information summary NADA 141-230: Previcox Chewable Tablets (Firocoxib). https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/768 (accessed 13 June 2023). Food and Drug Administration, United States Government, Silver Springs, MD, USA, 2004
- *FDA. Freedom of information summary NADA 141-253: Equioxx Oral Paste, 0.82% Firocoxib (w/w). https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/805. (accessed 13 June 2023). Food and Drug Administration, United States Government, Silver Springs, MD, USA, 2005
- *FDA. Freedom of information summary, NADA 141-458: Equioxx Firocoxib Tablets for Horses. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/944. (accessed 13 June 2023). Food and Drug Administration, United States Government, Silver Springs, MD, USA, 2016
- Hinchcliff KW, Jernigan AD, Upson DW, Constable PD. Ruminant pharmacology. Veterinary Clinics of North America: Food Animal Practice 7, 633–49, 1991. https://doi.org/10.1016/S0749-0720(15)31076-8
- Ingvast-larsson C, Högberg M, Mengistu U, Olsén L, Bondesson U, Olsson K. Pharmacokinetics of meloxicam in adult goats and its analgesic effect in disbudded kids. Journal of Veterinary Pharmacology and Therapeutics 34, 64–9, 2011. https://doi.org/10.1111/j.1365-2885.2010.01195.x
- Kay-Mugford P, Benn SJ, LaMarre J, Conlon P. In vitro effects of nonsteroidal anti-inflammatory drugs on cyclooxygenase activity in dogs. American Journal of Veterinary Research 61, 802–9, 2000. https://doi.org/10.2460/ajvr.2000.61.802
- Kongara K, Corner-Thomas R, Bruere S, Lawrence K, Gates MC. Practices and opinions of New Zealand sheep farmers towards pain management in lambs during castration and/or tail docking. New Zealand Veterinary Journal 71, 8–17, 2023. https://doi.org/10.1080/00480169.2022.2135626
- Kvaternick VM, Pollmeier J, Fischer AD, Hanson PD. Pharmacokinetics and metabolism of orally administered firocoxib, a novel second generation coxib in horses. Journal of Veterinary Pharmacology and Therapeutics 30, 208–17, 2007. https://doi.org/10.1111/j.1365-2885.2007.00840.x
- Lees P, Landoni MF, Giraudel J, Toutain PL. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. Journal of Veterinary Pharmacology and Therapeutics 27, 479–90, 2004. https://doi.org/10.1111/j.1365-2885.2004.00617.x
- Meléndez DM, Marti S, Pajor EA, Sidhu PK, Gellatly D, Janzen ED, Schwinghamer TD, Coetzee JF, Schwartzkopf-Genswein, KS. Pharmacokinetics of oral and subcutaneous meloxicam: Effect on indicators of pain and inflammation after knife castration in weaned beef calves. PLoS ONE 14, e0217518, 2019. https://doi.org/10.1371/journal.pone.0217518
- Mosher RA, Coetzee JF, Cull CA, Gehring R, KuKanich B. Pharmacokinetics of oral meloxicam in ruminant and preruminant calves. Journal of Veterinary Pharmacology and Therapeutics 35, 373–81, 2012. https://doi.org/10.1111/j.1365-2885.2011.01331.x
- Naylor RF, Taylor AH, Knowles EJ, Wilford S, Linnenkohl W, Mair TS, Johns IC. Comparison of flunixin meglumine and meloxicam for post operative management of strangulating small intestinal lesions. Equine Veterinary Journal 46, 427–34, 2014. https://doi.org/10.1111/evj.12224
- *Robson M, Chew D, Van S Aalst. Intrinsic acute renal failure (ARF) associated with non-steroidal anti-inflammatory drug (NSAID) use in juvenile cats undergoing routine desexing – 16 cases 1998–2005. Research Abstract Program of the 24th Annual ACVIM Forum. Pp 740. American College of Veterinary Internal Medicine, Greenwood Village, CO, USA, 2006
- Small AH, Belson S, Brewer H, Schmoelzl SM. Marking to weaning production aspects of lambs provided with NSAID analgesia compared with lambs receiving no analgesia at the time of elastrator ring marking. Australian Veterinary Journal 99, 40–3, 2021. https://doi.org/10.1111/avj.13037
- Small AH, Belson S, Holm M, Colditz IG. Efficacy of a buccal meloxicam formulation for pain relief in Merino lambs undergoing knife castration and tail docking in a randomised field trial. Australian Veterinary Journal 92, 381–8, 2014. https://doi.org/10.1111/avj.12241
- Small AH, Marini D, Dyall T, Paull D, Lee C. A randomised field study evaluating the effectiveness of buccal meloxicam and topical local anaesthetic formulations administered singly or in combination at improving welfare of female Merino lambs undergoing surgical mulesing and hot knife tail docking. Research in Veterinary Science 118, 305–11, 2018. https://doi.org/10.1016/j.rvsc.2018.03.006
- *Stafford KJ. Husbandry procedures. In: Ferguson DM, Lee C, Fisher A (eds). Advances in Sheep Welfare, Vol. 1. Pp 211–26. Woodhead Publishing. Cambridge, UK, 2017. https://doi.org/10.1016/B978-0-08-100718-1.00011-X
- Stock ML, Gehring R, Barth LA, Wulf LW, Coetzee JF. Pharmacokinetics of firocoxib in preweaned calves after oral and intravenous administration. Journal of Veterinary Pharmacology and Therapeutics 37, 457–63, 2014. https://doi.org/10.1111/jvp.12124
- Stuart AK, KuKanich B, Caixeta LS, Coetzee JF, Barrell EA. Pharmacokinetics and bioavailability of oral firocoxib in adult: mixed-breed goats. Journal of Veterinary Pharmacology and Therapeutics 42, 640–6, 2019. https://doi.org/10.1111/jvp.12795
- Sutherland MA, Tucker C. The long and short of it: a review of tail docking in farm animals. Applied Animal Behaviour Science 135, 179–91, 2011. https://doi.org/10.1016/j.applanim.2011.10.015
- Wagner SA, Fajt VR, Lo CP, Byrd CJ. Pharmacokinetics of oral firocoxib in un-weaned calves. Journal of Veterinary Pharmacology and Therapeutics 44, 793–8, 2021. https://doi.org/10.1111/jvp.12971
- Welsh EM, McKeller QA, Nolan AM. The pharmacokinetics of flunixin meglumine in the sheep. Journal of Veterinary Pharmacology and Therapeutics 16, 181–8, 1993. https://doi.org/10.1111/j.1365-2885.1993.tb00162.x
- Woodland AN, Van der Saag D, Kimble B, White PJ, Govendir M, Lomax S. Plasma pharmacokinetic profile and efficacy of meloxicam administered subcutaneously and intramuscularly to sheep. PLoS One 14, e0215842, 2019. https://doi.org/10.1371/journal.pone.0215842
- Wun MK, Leister E, King T, Korman R, Malik R. Acute kidney injury in 18 cats after subcutaneous meloxicam and an update on non-steroidal anti-inflammatory drug usage in feline patients in Australia. Australian Veterinary Journal 101, 90–8, 2003. https://doi.org/10.1111/avj.13222
- *Non-peer-reviewed
