Abstract
In north-east China, a major region of soybean production in China, soybean yield has increased mainly from long-term application of genetic selection procedures for high yield. Most previous investigations into soybean yield improvement have focused on above-ground morphological and physiological characteristics. Root morphology, in contrast, is much less documented. In this study, eight soybean genotypes are assigned to two pools, based on yield potential performance. The root morphologies of plants from these high- and low-yielding pools are compared temporally and spatially. The high-yielding soybean pool exhibits significantly higher root growth rates from the R1 to R4 stages and lower root senescence rates after the R5 stage compared with the low-yielding pool. Plants from the high-yielding pool not only accumulate more shoot biomass, but also allocate greater biomass to the roots, leading to a 14.5% rise in root-to-shoot ratio. Root length in the high-yielding soybean pool after R5 is 24.2% greater than that in the low-yielding pool. Greater root length duration contributes to the longer leaf life span. These findings indicate that more biomass used in root production and slower root senescence during the reproductive stage play important roles in supporting higher yields. Spatially, the densities of biomass and length of roots from 0 to 45 cm of soil depth at the plant position and halfway between plants from the high-yielding soybean pool are significantly greater than the low-yielding. Thicker root diameters in deeper soil depths are observed in the high-yielding soybean pool. The characteristics of root morphology for high-yielding soybean in north-east China are summarized diagrammatically.
Introduction
The root is a crucial plant organ that contributes to yield because root morphology determines the ability of the plant to acquire soil resources (Costa et al. Citation2000). Many of the root characteristics, such as length, diameter, surface area and biomass, have been used to assess the quantity of roots and the functional size of the root system (Vamerali et al. Citation2003). The size and activity of the root system determine the shoot growth rate and this, in turn, affects the subsequent root growth rate itself (Nemoto et al. Citation1995; Hébert et al. Citation2001; Ingram & Leers Citation2001). Costa et al. (Citation2002) reported that genotypes of corn bearing the leafy trait had greater root lengths and surface areas than the conventional hybrid. The root functions of mechanical and physiological support for the shoot are determined by root architecture (Ennos & Fitter Citation1992). The strong rooting ability of some corn hybrids enables the root system to better withstand the mechanical effects of wind on the shoot (Hébert et al. Citation2001). Deeper root systems may be less susceptible to yield loss in drought-stressed environments (Clarke & Townley-Smith Citation1984). Plant root systems thus have multiple functions in regulating overall plant growth and health (Thaler & Pages Citation1998; Ingram & Leers Citation2001). Utilizing genotypic diversity in the root characteristics has been a widely accepted approach to improving agricultural production (O'Toole & Bland Citation1987).
In soybean, nearly 79% of the yield increase is attributed to genetic improvement (Williams & Specht Citation1979). Genetically, soybean yield both in the US and Canada has been increased by 0.5 to 1% annually (Luedders Citation1977; Wilcox et al. Citation1979; Voldeng et al. Citation1997). A number of researchers have attempted to identify the morphological and physiological characteristics that determine soybean productivity, in order to select potentially better-yielding genotypes (Metz et al. Citation1982; Board et al. Citation1996; Ustun et al. Citation2001). Increasing plant dry matter was a major pathway to achieving higher seed yield (Specht et al. Citation1999; Liu et al. Citation2005). In addition, leaf area, a determinant of photosynthetic ability, was positively related to soybean yield at the R5 stage (Wells et al. Citation1982; Board & Tan Citation1995). Significantly longer leaf duration in new genotypes after pod filling was also reported by Kumudini et al. (Citation2001). Soybean, grown as a main crop in north-east China with sown acreage accounting for 33% of the nation's total (Liu & Herbert Citation2002), has not achieved productivity as high as that achieved in the US, even though the north-east China yield has increased in the past several decades with planting of new cultivars (Liu et al. Citation2008). Further improvement of soybean yield remains a vital task in north-east China (Man et al. Citation2001). However, compared with shoot-dominated phenomena, far less is known about root system growth dynamics and spatial distribution in soil profiles (Del et al. Citation1989; Demotes & Pellerin Citation1992). These are closely associated with the development of the photosynthetic organ, i.e., leaf, and consequently important for identifying more effective markers for use in breeding programmes and modifying farming practices to achieve higher yields in soybean.
In this study, eight soybean genotypes with similar maturation times were used and grouped into high- and low-yielding pools of four genotypes each, based on yield estimations from several previous field experiments, which showed yield differences of approximately 20% between the two pools. Most of these experiments have documented the above-ground characteristics of high-yielding genotypes, such as higher leaf area duration (LAD), photosynthetic rate and dry weight during reproductive stages (Liu et al. Citation2005; Jin et al., Citation2010), while underground characteristics have been largely ignored. Therefore, attention here focused on comparing the two soybean pools to identify differences associated with increased yield on: first, root morphology including root development and spatial distribution along soil profiles; second, root dynamics and leaf area duration; and third, the critical period of root growth in north-east China.
Materials and methods
Plant material
Eight soybean genotypes with similar maturity (MG00 and MG0) were used in this study. Four genotypes released from 1995 to 2004 (Suinong15, Suinong21, Heinong45 and Beifeng11) were classified as high-yielding, whereas the other four released from 1966 to 1973 (Suinong3, Heinong16, Fengshou10 and Hai9731), as low-yielding. The yield potential difference between the two pools was verified by previous multi-year field experiments (Jin et al. Citation2004; Liu et al. Citation2005; Jin et al. Citation2010).
Pot experiments
Plants were grown in a container (29 cm in height and 25.5 cm in diameter) filled with a mixture of sand and silty-clay loam soil (1:1 v/v). Soil was collected from the tillage layer of 0.1 m depth from farming land located on Songneng plain in north-east China (47°48′ N, 126°47′ E). The soil and sand were air-dried and sieved through a 4 mm sieve before mixing. Basal nutrients were added at the following rates (mg/kg):
* 217 urea
* 219 KH2PO4
* 167 CaCl2·2H2O
* 43 MgSO4·7H2O
* 9 Fe-EDTA
* 6 ZnSO4
* 5 CuSO4
* 0.7 H3BO3
* 6.7 MnSO4·H2O
* 10 ZnSO4·7H2O
* 2 CuSO4·5H2O
* 0.3 CoSO4·7H2O
* 0.2 Na2MoO4·2H2O
Nutrients were thoroughly mixed with the soil and sand. A randomized complete block design was used in which treatments consisted of the two pools, and each genotype received 12 replicates of pots.
Six seeds of uniform size were sown per container on 15 May 2006, and the resulting seedlings were thinned to three per container 6 days after sowing. The plants were grown in a glasshouse with a night temperature range of 16–20°C and a day temperature range of 24–28°C. Soil water content was controlled at 70±5% of field capacity by weighing and watering whenever needed.
Plant samples were taken at the V5 (fifth node), R1 (initial flowering), R4 (full pod), R5 (initial pod filling), R6 (full seed) and R8 (full maturity) stages. For each stage, six plants per genotype (two pots) were removed from the containers by carefully sliding out the entire root mass. The stem was cut off and the root system was completely immersed in a water-filled container and then washed with tap water until free of soil and sand.
Measurement of the root samples was performed with Win-RHIZO version 2004a (Regent Instruments, Quebec, Canada) to obtain root length (RL), root surface area (RSA), average root diameter (RD) and diameter classes of the roots.
Leaf area (LA) was estimated by CI-203 portable laser leaf area analyser (CID, USA). The biomass samples of stem, leaf and root were oven-dried (75°C) for at least 48 hours, and then weighed.
Field experiments
The field experiment was conducted at Hailun Agroecological Experimental Station (47°26′ N, 126°38′ E), Chinese Academy of Sciences. Annual sunshine at the research site was around 2600–2800 h, and growing degree days (GDD) (Dwyer et al. Citation1999) was 1362. The soil is the typical mollisol (black soil), with silty clay loam texture. Chemical characteristics were:
* soil organic matter 50.8 g kg−1
* total nitrogen 2.14 g kg−1
* total phosphorus 0.98 g kg−1
* total potassium 22.0 g kg−1
* available N 168 mg kg−1
* available P 40.2 mg kg−1
* available K 140 mg kg−1
* pH 7.31 (1:5 v/v).
The eight soybean genotypes were grown in a randomized complete block design replicated three times. The seeds were sown on 6 May 2006 and harvested on 2 October. Each plot was 5 m long and 3.5 m wide (five rows, 0.7 m apart). The distance between plants in a row was 12 cm. Di-ammonium phosphate of 50 kg ha−1 (N 18%, P2O5 46%) and composite fertilizer of 150 kg ha−1 (N 18%, P2O5 16%, K2O 16%) were applied before sowing.
The root systems of the eight genotypes were investigated during the R5 to R6 stage using the soil core method (Vamerali et al. Citation2003). Six replicates were considered for each of the following positions: on plant, halfway between two plants in the row; and halfway between two rows. An auger (bi-partite root auger, 8 cm diameter × 15 cm length, The Netherlands) was used to take core samples along soil profiles from 0 cm to 75 cm of soil depth. Each sample was 15 cm long and washed to separate roots from soil. Root length, root volume and average root diameter were measured with the Win-RHIZO system. The root samples were then oven-dried at 75°C for at least 48 hours and weighed.
Analysis of data
In the pot experiments with repeated harvests, root length duration (RLD) and leaf area duration (LAD) were calculated as follows (Zhao et al. Citation2002; Liu et al. Citation2005):
where RL1 and RL2 are the root length, and LA1 and LA2 are the leaf area between two growth stages, and T is the day corresponding to RL and LA determination. Root-to-soil volume ratio was the ratio of root volume to soil volume where the root grew in:
Root-to-soil volume ratio (‰) = Root volume×1000/Soil volume
Statistical analyses were performed on the shoot and root morphological characteristics using the Statistical Analysis System (SAS Citation1997) software. Protected ANOVA tests of LSD (Steel & Torrie Citation1980) were used to assess the differences between the two pools. Regression analysis was carried out with SAS procedure REG (SAS Citation1997).
Results
A significant difference in yield between the two pools was observed in the field experiment, with an average yield of 323 g/m2 from the high-yielding pool, which was 22.8% higher than that in the low-yielding pool. The pot experiment showed the same (data not shown).
Root growth dynamics in pots
The use of pot experiments facilitated the characterization of root morphology of whole plants and precise assessment of the relation of root growth to shoot development.
Change of root growth rate
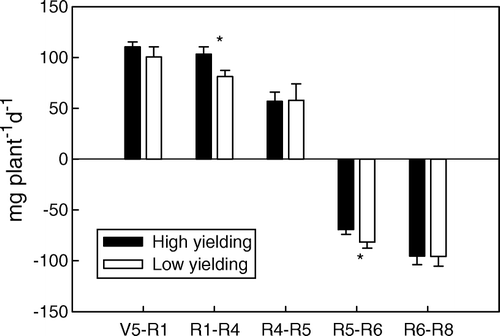
In general, the root growth rate decreased from more than 100 mg/plant/d at the V5 stage to 0 mg/plant/d at the R5 stage. After R5, the root began to senesce, which drove the growth rate to negative (). Compared with the low-yielding soybean pool, the high-yielding soybean pool had a significantly higher root growth rate during the R1 to R4 stage and a lower senescence rate from the R5 to R6 stage ().
Change of root biomass and root-to-shoot ratio
Root biomass was similar in both pools prior to the R4 stage. From R4 to R8, the root biomass in the high-yielding soybean pool exceeded significantly that of the low-yielding, attaining maximum root biomass at R5 (A). The subsequent decline in root biomass was slower for the high-yielding soybean pool so that by maturity (the R8 stage), the significance of the difference in root biomass had increased (P < 0.01).
Fig. 2 Root biomass (A) and root-to-shoot ratio (B) in high- and low-yielding soybean pools across all growth stages. Note: * and ** indicate growth stages at which root biomasses or root-to-shoot ratios of the two yielding pools were significantly different at P < 0.05 and P < 0.01, respectively; bars represent standard error of the mean (n = 4)
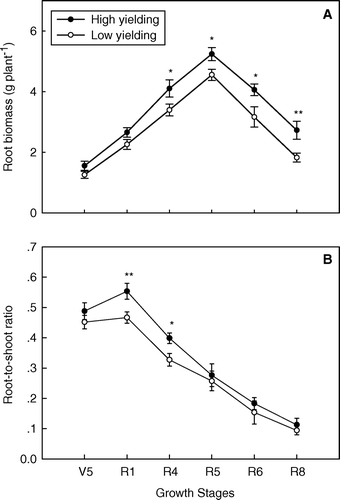
The root-to-shoot ratio reached a maximum at R1 and then declined in both the high- and low-yielding soybean pools. High-yielding soybean had a higher root-to-shoot ratio than low-yielding soybean at R1 and R4 (B).
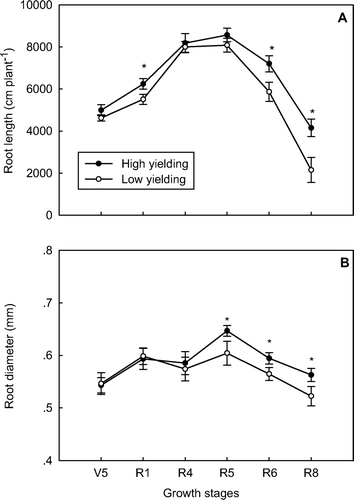
Change of root length and average root diameter
The change in root length during growth was similar to root biomass. The root length in the high-yielding soybean pool was significantly higher than the low-yielding pool at R1 (P < 0.05), R6 (P < 0.05) and R8 (P < 0.01) (A). A similar trend was found in root surface area (data not shown). No significant difference in average root diameters between the high- and low-yielding soybean pools was observed prior to the R5 stage, whereas during the R5 to R8 stage, the root diameter in the high-yielding soybean pool was 6.7%, on average, larger than the low-yielding pool (P < 0.05) (B).
Fig. 3 Root length (A) and average root diameter (B) in high- and low-yielding soybean pools across all growth stages. Note: * and ** indicate growth stages at which root lengths or root diameters of the two yielding pools were significantly different at P < 0.05 and P < 0.01, respectively; bars represent standard error of the mean (n = 4)
Root distribution as classified by root diameter at the R6 stage
Since significant variation of root length between two pools was found during the late reproductive stage, the distribution of root lengths among a series of root-diameter classes at R6 were determined (). Seven root-diameter classes from 0.0 to > 3.0 mm with 0.5 mm increments were classified automatically by the image analysis system. The root length abruptly decreased with increasing root diameter (). The major proportion of root length was in the smallest root-diameter class from 0.0 to 0.5 mm. Roots having a diameter < 0.5 mm comprised 72–73.9% of total measured roots for high- and low-yielding soybean pool, respectively. Furthermore, the differences in root length between the high- and low-yielding soybean pools can be mostly attributed to the roots of the smallest diameter class. The percentage distribution of root length across root-diameter classes, however, did not show any significant difference between the two soybean pools.
Table 1 Root length among seven root-diameter classes in real value and percentage distribution for two yielding soybean pools at the R6 stage
Relationship of root length duration to leaf area duration
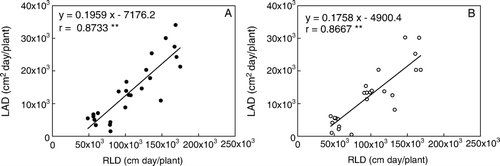
Root length duration (RLD) and leaf area duration (LAD) in the high-yielding soybean pool from R5 to R8 were both significantly greater than those in the low-yielding soybean pool (data not shown). Furthermore, the RLD was linearly correlated to the LAD (P < 0.01), but there was no significant difference between the slopes of the linear regressions for the two pools ().
Distribution of root down the soil profile in the field
The spatial root distribution of the two soybean pools down the soil profile to 75 cm was sampled by a soil coring method when plants were in the R5 to R6 growth stage.
Root biomass and length down the soil profile
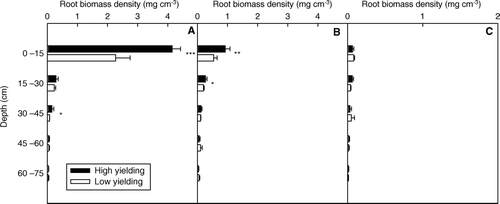
Most root biomass was located at the positions of plant and halfway between plants, whereas less than 4.5% of total sampled root was found at the point halfway between two rows (). Compared with the low-yielding soybean pool, high-yielding soybean had almost double the biomass density in the 0–15 cm soil depth at the plant position and halfway between plants. The high-yielding plants also had significantly higher root biomass densities across 30–45 cm soil depth at the plant position and across 15–30 cm at halfway between plants (A, B). However, at the point halfway between rows, no significant difference between the high- and low-yielding pools was observed (C).
Fig. 5 Root biomass density in high- and low-yielding soybean pools along soil profiles, for each of following positions: on plant (A); at halfway between plants (B); at halfway between rows (C). Note: *, ** and *** indicate depths at which root biomass densities of the two yielding pools were significantly different at P < 0.05, P < 0.01 and P < 0.001, respectively; bars represent standard error of the mean (n = 4)
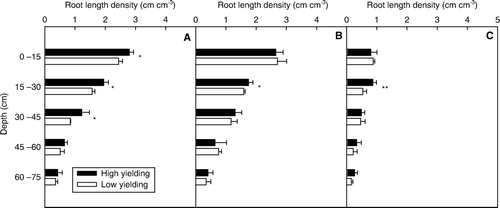
The RLD of the high-yielding soybean pool was significantly higher than that of low-yielding soybean in the 0–45 cm soil depth at the plant position (A) and in the 15–30 cm soil depth at the points half way between plants and rows (B, C).
Fig. 6 Root length density in high- and low-yielding soybean pools along soil profiles, for each of following positions: on plant (A); at halfway between plants (B); at halfway between rows (C). Note: * and ** indicate depths at which root densities of the two yielding pools were significantly different at P < 0.05 and P < 0.01, respectively; bars represent standard error of the mean (n = 4)
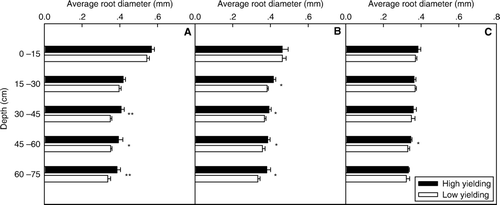
Average root diameter distribution down the soil profile
The average root diameter decreased with increase of soil depth. A greater average root diameter in the high-yielding soybean pool was found below 30 cm at the plant position and half way between plants, and in 45–60 cm soil depth half way between rows (). No great difference of average root diameter between the high- and low-yielding soybean pools was found in the 0–15 cm soil depth at any position ().
Fig. 7 Average root diameter in high- and low-yielding soybean pools along soil profiles, for each of following positions: on plant (A); at halfway between plants (B); at halfway between rows (C). Note: * and ** indicate depths at which average root diameters of the two yielding pools were significantly different at P < 0.05 and P < 0.01, respectively; bars represent standard error of the mean (n = 4)
Distribution of root-to-soil volume ratio down the soil profile
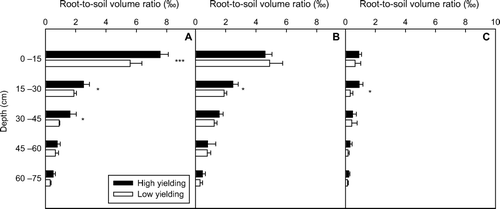
The space occupied by roots in the soil can be presented as root-to-soil volume ratio. This ratio was significantly greater for the high-yielding soybean pool at 0–45 cm soil depth at the plant position and at 15–30 cm soil depth for the points halfway between plants and between rows, compared with the low-yielding soybean pool ().
Fig. 8 Root-to-soil volume ratio in high- and low-yielding soybean pools along soil profiles, for each of following positions: on plant (A); at halfway between plants (B); at halfway between rows (C). Note: * and *** indicates depths at which root-to-soil volume ratios of the two yielding pools were significantly different at P < 0.05 and P < 0.001, respectively; bars represent standard error of the mean (n = 4)
Discussion
The development and function of the root system controls the growth and yield of a crop by the delivery of water and nutrients to the shoot, although the relationship between shoot development and root growth is not always synchronized (Yamaguchi & Tanaka Citation1990). Identifying those features of root morphology that distinguish high-yielding from low-yielding soybean across all growth stages is crucial for understanding how these plants achieve higher yields. The comparatively higher root growth rate from R1 to R4 () and root-to-shoot ratio (B) of the high-yielding soybean pool suggest that allocation of more photosynthate to the root has enhanced root growth, with consequent accumulation of more root biomass at the R4 stage (A). Moreover, this additional biomass persisted after the root growth peak at R5 (A), with a lower rate of loss in the R5-R6 stage (). High root biomass is of benefit to strong shoot growth (Oliveira et al. Citation2000; Qu et al. Citation2003), which is necessary for high yield production. An increase of aerial biomass has been found in genetic improvement of soybean for high yield (Koutroubas et al. Citation1998; Specht et al. Citation1999; Liu et al. Citation2005). Additionally, the root-to-shoot ratio is likely to influence the lodging behaviour. Those genotypes with high root-to-shoot ratios are likely to be better equipped to resist lodging, thus increasing productivity (Hébert et al. Citation2001).
Root length and surface area are thought to be better indicators of root uptake ability than root biomass (Van Noordwijk & de Willigen; Citation1991 Costa et al. Citation2000). There is ample evidence to indicate a linear relationship between yield and total root length in rice and soybean (Silberbush & Barber Citation1985; Yamaguchi & Tanaka Citation1990; Board & Tan Citation1995; Kumudini Citation2002). In the present study, compared with the low-yielding soybean pool, the significantly higher root length and root surface area of high-yielding soybean at the R1, R6 and R8 stages indicate that rapid root elongation at the early reproductive stage and slow root senescence at the late seed filling stage may play a much more important role in promoting yield than any other stage.
In the field, high-yielding soybean spatially had greater root biomass and root length not only in shallow soil, but also in deeper soil at the plant position and halfway between plants ( and ). This kind of distribution of root in high-yielding soybean could be the result of genetic improvement associated with adaptation to the regional climatic environment. Seasonal drought stress in north-east China occurs frequently and causes abnormal senescence in soybean plants with weakening photosynthetic activity, shortening leaf life span and consequently decreasing yield (Meng Citation2007; Yu et al. Citation2009). Since rooting depth determines the plant potential to capture water and solutes in soil (Himmelbauer et al. Citation2005), the deeper distribution of root in soil profiles could alleviate the adverse effects of water deficit. As the drought lowers the availability of nutrients in soil, especially in the shallow soil layers which roots populate densely, deeper roots should minimize the depletion of available resources. Sponchiado et al. (Citation1980) found that the response of soybean roots to water deficit stress was genotype dependent. Carter (Citation1989) suggested that enhanced rooting, associated with increased ability to extract soil water, was perhaps the most promising genetic mechanism for improving soybean drought tolerance. Better genotype selection of soybean, with particular attention given to root response to dry conditions, was needed for soybean grown in the semi-arid Great Plains of the United States (Benjamin & Nielsen Citation2006). Therefore, it would seem likely that the breeding pressure in the changing climatic environment of north-east China would result in the large size and deeper root genotypes ( and ). The production and maintenance of extra roots (high root-to-shoot ratio) requires the consumption of additional carbon resources, but is probably compensated for by the additional water and nutrient uptake achieved.
Roots of larger average diameter could contribute to deeper rooting and slower senescence (B, ). Many researchers have found that large-diameter roots have a longer life span (Costa et al. Citation2002) and, furthermore, thicker roots tend to elongate vertically and deeply (Yamazaki & Tanaka Citation1983), thus improving root functionality.
The life spans of roots and leaves are significantly associated. Given the linear relationship between root length duration and leaf area duration (), high-yielding soybean could have the ability to enhance leaf development and delay leaf senescence through the increased uptake of nutrients and water, and decrease root senescence during the late reproductive stage. In pots, the majority of the total root length was in roots of less than 0.5 mm diameter and these were significantly increased in the high-yielding soybean pool (). Merrill et al. (Citation2002) showed that the most fine roots belong to laterals that influence leaf senescence (Hsia & Kao Citation1978). Therefore, the increased number of fine roots in high-yielding soybean may promote a more leafy population during later stages in the field, which corroborates with research findings in maize (Costa et al. Citation2002). One possible mechanism for this may be related to a molecular signal—cytokinin—because cytokinins in lateral roots play a predominant role in leaf senescence and the normal supply of root cytokinins is important in leaf metabolism (Hsia & Kao Citation1978; Yang et al. Citation1997). However, direct evidence for this is lacking and the relation between lateral root abundance and cytokinin synthesis and its subsequent role in leaf function maintenance need further research.
Conclusions
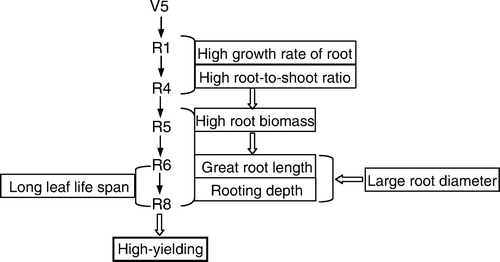
In north-east China, the temporal and spatial characteristics of soybean root morphology have changed as a result of the breeding process aimed at increasing yield. High-yielding soybean, in comparison with low-yielding, had faster root growth rate and partitioned more carbohydrate to the root at the R1 and R4 stage, as summarized in . The additional roots with large biomass and length after the R5 stage contributed to a longer leaf life span. The longer-lasting and greater length density of roots down soil profiles to more than 45 cm soil depth during the late reproductive period could be crucial for improvement of soybean yield by overcoming conditions of fluctuating nutrient and water contents in the soil, which are strongly influenced by the erratic climatic environment of agricultural ecosystems in north-east China.
Acknowledgements
The authors are grateful to Dr Ross McC Lilley, University of Sydney, Australia, for his comments and critical review of this manuscript. This research was funded by the National Natural Science Foundation of China (40701084), the National Science & Technology Pillar Program (2009BADB3B06-2) and the Innovation Project, the Chinese Academy of Sciences (KZCX3-SW-NA3-23; KSCX1-YW-09-09).
References
- Benjamin , JG and Nielsen , DC . 2006 . Water deficit effects on root distribution of soybean, field pea and chickpea . Field Crop Research , 97 : 248 – 253 .
- Board , JE and Tan , Q . 1995 . Assimilatory capacity effects on soybean yield components and pod number . Crop Science , 35 : 846 – 851 .
- Board , JE , Zhang , W and Harville , BG . 1996 . Yield ranking for soybean cultivars grown in narrow and wide rows with late planting dates . Agronomy Journal , 88 : 240 – 245 .
- Carter TE Jr , 1989 . Breeding for drought tolerance in soybean: Where do we stand? In : Pascale AJ Proceeding World Soybean Conference IV , Buenos Aires , Argentina . 1001 1008 .
- Clarke , JM and Townley-Smith , TF . 1984 . Drying rates of spring triticale compared to wheat . Agronomy Journal , 76 : 454 – 456 .
- Costa , C , Dwyer , LM , Hamilton , RI , Hamel , C , Nantais , L and Smith , DL . 2000 . A sampling method for measurement of large root systems with scanner-based image analysis . Agronomy Journal , 92 : 621 – 627 .
- Costa , C , Dwyer , LM , Zhou , XM , Dutileul , P , Hamel , C , Reid , LM and Smith , KDL . 2002 . Root morphology of contrasting maize genotypes . Agronomy Journal , 94 : 96 – 101 .
- Del , CD , Acock , DB , Reddy , V and Acock , M . 1989 . Elongation and branching of roots on soybean plants in a carbon dioxide-enriched aerial environment . Agronomy Journal , 81 : 692 – 695 .
- Demotes , MS and Pellerin , S . 1992 . Effect of mutual shading on the emergence of nodal roots and the root: shoot ratio of maize . Plant and Soil , 147 : 87 – 93 .
- Dwyer , LM , Stewart , DW , Garngan , SL , Ma , BL , Neave , P and Balchin , D . 1999 . Guidelines for comparisons among different maize maturity rating systems . Agronomy Journal , 91 : 946 – 949 .
- Ennos , AR and Fitter , AH . 1992 . Comparative functional morphology of the anchorage systems of annual dicots . Functional Ecology , 6 : 71 – 78 .
- Hébert , Y , Guingo , E and Loudet , O . 2001 . The response of root/shoot portioning and root morphology to light reduction in maize genotypes . Crop Science , 41 : 363 – 371 .
- Himmelbauer , ML , Puschenreiter , M , Schnepf , A , Loiskand , W and Wenzel , W . 2005 . Root morphology of Thlaspi goesingense Hálácsy grown on a serpentine soil . Journal of Plant Nutrition and Soil Science , 168 : 138 – 144 .
- Hsia , CP and Kao , CH . 1978 . The importance of roots in regulating the senescence of soybean primary leaves . Physiologia Plantarum , 43 ( 4 ) : 385 – 389 .
- Ingram , KT and Leers , GA . 2001 . Software for measuring root characters from digital images . Agronomy Journal , 93 : 918 – 922 .
- Jin , J , Liu , XB , Wang , GH and Herbert , SJ . 2004 . Some eco-physiological characteristics at R4-R5 stage in relation to soybean yield differing in maturities . Agricultural Science in China , 3 : 425 – 434 .
- Jin , J , Liu , XB , Wang , GH , Mi , L , Shen , ZB , Chen , XL and Herbert , SJ . 2010 . Agronomic and physiologic contributions to the yield improvement of soybean cultivars released from 1950 to 2006 in Northeast China . Field Crop Research , 115 : 116 – 123 .
- Koutroubas , SD , Papakosta , DK and Gagianas , AA . 1998 . The importance of early dry matter and nitrogen accumulation in soybean yield . European Journal of Agronomy , 9 : 1 – 10 .
- Kumudini , S . 2002 . Trials and tribulations: a review of the role of assimilate supply in soybean genetic yield improvement . Field Crops Research , 75 : 211 – 222 .
- Kumudini , S , Hume , DJ and Chu , G . 2001 . Genetic improvement in short season soybeans. I. Dry matter accumulation, partitioning, and leaf area duration . Crop Science , 41 : 391 – 398 .
- Liu , XB and Herbert , SJ . 2002 . Fifteen years of research examining cultivation of continuous soybean in Northeast China . Field Crops Research , 79 : 1 – 7 .
- Liu , XB , Jin , J , Herbert , SJ , Zhang , QY and Wang , GH . 2005 . Yield components, dry matter, LAI and LAD of soybeans in Northeast China . Field Crop Research , 93 : 85 – 93 .
- Liu , XB , Jin , J , Wang , GH and Herbert , SJ . 2008 . Soybean yield physiology and development of high-yielding practices in Northeast China . Field Crop Research , 105 : 157 – 171 .
- Luedders , VD . 1977 . Genetic improvement in yield of soybeans . Crop Science , 17 : 971 – 972 .
- Man WQ Du WG Zhang GR Luan XY Chen Y Gu XZ 2001 . Study on super-high yield potentiality of soybean . Soybean Science 20 : 94 97 (in Chinese) .
- Meng XC 2007 . The annual change of arid tendency in the past fifty years of Heilongjiang province . Heilongjiang Agricultural Sciences 6 : 37 39 (in Chinese) .
- Merrill , SD , Tanaka , DL and Hanson , JD . 2002 . Root length growth of eight crop species in Haplustoll soils . Soil Science Society of America Journal , 66 : 913 – 923 .
- Metz , GL , Green , DE and Shibles , RM . 1982 . Relationship between soybean yield in narrow rows and leaflet, canopy, and developmental characters . Crop Science , 24 : 462 – 547 .
- Nemoto , K , Morita , S and Baba , T . 1995 . Shoot and root development in rice related to the phyllochron . Crop Science , 35 : 24 – 29 .
- O'Toole , JC and Bland , WL . 1987 . Genotypic variation in crop plant root systems . Advances in Agronomy , 41 : 91 – 145 .
- Oliveira , MRG , Van Noordwijk , M , Gaze , S , Brouwer , G , Bona , S , Moska , G and Hairiah , K . 2000 . “ Auger sampling, ingrowths cores and pinboard methods ” . In Root methods , Edited by: Smit , A , Bengough , A , Engels , C , Van Noordwijk , M , Pellerin , S and Van DeGeijin , S . 175 – 210 . Berlin, Heidelberg, Springer-Verlag .
- Qu , LY , Ali , MQ and Takayoshi , K . 2003 . Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes . Plant and Soil , 255 : 293 – 302 .
- SAS Institute 1997 . SAS for Windows . Release 6.12 . SAS Institute , Cary, NC .
- Silberbush , M and Barber , SA . 1985 . Root growth, nutrient uptake and yield of soybean cultivars grown in the field . Communications in Soil Science and Plant Analysis , 16 ( 1 ) : 119 – 127 .
- Specht , JE , Hume , DJ and Kumudini , SV . 1999 . Soybean yield potential—a genetic and physiological perspective . Crop Science , 39 : 1560 – 1570 .
- Sponchiado , BN , White , JW , Castillo , JA and Jones , PG . 1980 . Effects of plant-water stress on root distribution of corn, soybeans, and peanuts in sandy soil . Agronomy Journal , 72 : 548 – 550 .
- Steel , RG and Torrie , JH . 1980 . Principles and procedures of statistics: A biometrical approach , 2nd edn , New York, McGraw-Hill .
- Thaler , P and Pages , L . 1998 . Modeling the influence of assimilate availability on root growth and architecture . Plant and Soil , 201 : 307 – 320 .
- Ustun , A , Allen , FL and English , BC . 2001 . Genetic progress in soybean of the U.S. midsouth . Crop Science , 41 : 993 – 998 .
- Vamerali , T , Saccomani , M , Bona , S , Mosca , G , Guarise , M and Ganis , A . 2003 . A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids . Plant and Soil , 255 : 157 – 167 .
- Van Noordwijk , M and de Willigen , P . 1991 . “ Review of quantitative root length data in agriculture ” . In Plant roots and their environment , Edited by: McMichael , BL and Persson , H . 5 Elsevier, New York. Pp .
- Voldeng , HD , Cober , ER , Hume , DJ , Gillard , C and Morrison , MJ . 1997 . Fifty-eight years of genetic improvement of short-season soybean cultivars in Canada . Crop Science , 37 : 428 – 431 .
- Wells , R , Schulze , LL , Ashley , DA , Boerma , HR and Brown , RH . 1982 . Cultivar differences in canopy apparent photosynthesis and their relationship to seed yield in soybeans . Crop Science , 22 : 886 – 890 .
- Wilcox , JR , Schapaugh , WT Jr , Cooper , RL , Fehr , WR and Niehaus , MH . 1979 . Genetic improvement of soybeans in the Midwest . Crop Science , 19 : 803 – 805 .
- Williams JH , Specht JE 1979 . A perspective on yield advances attributable to soybean variety development . In : Agronomy abstracts . ASA, Madison , WI . 81 .
- Yamaguchi , J and Tanaka , A . 1990 . Minimum space for root development without mechanical stress for obtaining a high yield of rice . Soil Science and Plant Nutrition , 36 ( 3 ) : 515 – 518 .
- Yamazaki K Tanaka A 1983 . Primary root formation in the growing shoots of several cereal crops . Japanese Journal of Crop Science 52 : 342 348 (in Japanese) .
- Yang , X , Römheld , V , Marschner , H , Baligar , VC and Martens , DC . 1997 . Shoot photosynthesis and root growth of hybrid and conventional rice cultivars as affected by N and K levels in the root zone . Pedosphere , 7 ( 1 ) : 35 – 42 .
- Yu , M , Xing , JJ and Yu , HM . 2009 . Air temperature change in Heilongjiang province in recent 46 years . Journal of Natural Disasters , 18 ( 3 ) : 158 – 164 .
- Zhao HJ Li Y Zou Q 2002 . A comparative study on characteristics of radiation and photosynthesis in canopy of two different spike-type cultivars of wheat . Acta Agronomica Sinica 28 5 : 654 659 (in Chinese) .