Abstract
During the early period of establishment, productivity of newly planted stool beds is often limited because a low proportion of axillary buds break along the rootstock stem, despite these buds being anatomically well developed. Using newly established stool beds of the apple rootstock ‘M.9’, we applied thidiazuron (TDZ; 1000 mg L−1) on to the rootstock stem at budbreak (on 15 September 2005) or 2 weeks later to ascertain whether axillary budbreak along the rootstock stem could be enhanced. Subsequently, foliar sprays of gibberellins (GA4+7; 400 mg L−1) were applied to determine whether elongation of the resultant axillary shoots could be increased. TDZ applied at budbreak or 2 weeks later increased the percentage of axillary buds that broke to form shoots, with more than 80% of buds breaking on TDZ-treated rootstocks compared with 41% for the untreated control. The elongation growth of newly produced shoots in response to foliar application of GA4+7 was pronounced for the TDZ-treated rootstock stems. TDZ painted on to the rootstock stems at budbreak or 2 weeks later increased the yield of stool shoots per metre length of new stool bed from approximately 18 in the control to c.30 with GA4+7. Paint application of TDZ (1000 mg L−1) followed by foliar application of GA4+7 (400 mg L−1) can increase stool shoot yield in newly planted stool beds.
Introduction
Since the mid 1990s, the New Zealand pipfruit industry has utilized high-density planting systems on dwarfing apple rootstocks for both economic and agronomic advantages. Increased international market demand and export returns for new apple cultivars such as ‘Scifresh’, ‘Tentation™’ and ‘Pink Lady®’ have also been an important catalyst for adoption of intensive planting systems in New Zealand. To supply the market with these new cultivars, growers and marketers must speed up the establishment of new tree plantings, and these plantings must be highly precocious, productive and produce high-quality fruit (Tustin & Palmer Citation2008). Substantial improvement of scion precocity, fruit quality and marketable yield were obtained using dwarfing and semi-dwarfing apple rootstocks (Tustin & Cashmore Citation1994). Presently, approximately 15% of New Zealand apple plantings are intensive systems with ‘M.9’ being the most preferred rootstock. However, inadequate supply of dwarfing rootstocks has been identified as one of the major impediments to the widespread adoption of high-density planting systems (Tustin & Palmer Citation2008). A difficulty in increasing the supply of ‘M.9’ clones is that its stool-bed productivity is typically lower than for other rootstocks, especially during the early years of establishment (Tobakov & Yordanov Citation2006).
Application of 6-benzylaminopurine (BA) alone or with gibberellins (GA4+7) to orchardand nursery apple trees has been reported to significantly increase feathering in some apple cultivars (Elfving Citation1985). Thidiazuron (TDZ) a substituted phenyl urea (N-phenyl-N′-1,2,3,-thiadiazol-5-ylurea; Dropp) mimics cytokinin-like activity (Wang et al. Citation1986) and was shown to promote the breaking of dormant buds in apple (Wang & Faust Citation1988) and a range of other horticultural crops (Alvarado-Raya et al. Citation2000; Arora et al. Citation2003). We have also confirmed the effect of TDZ on stimulating budbreak in blind wood of young ‘Scifresh’ apple trees in the field (Palmer et al. Citation2005). Usually in the first year of stool-bed establishment, only a few strong shoots are produced from each newly planted rootstock stem because of limited and uneven budbreak, even though each rootstock plant has many well-formed axillary buds along its entire length. The possibility of using TDZ to stimulate a high level of budbreak on rootstock stems in newly established stool beds has not been investigated. Induction of budbreak in rootstock stems by using plant growth regulators may provide a higher number of strong shoots in the first year, thereby enhancing nursery stool-bed productivity from the outset. Therefore this study was conducted to investigate the effect of TDZ on enhancing the axillary budbreak of newly planted rootstock stems in stool beds. Gibberellin is a promoter of plant growth that stimulates node neoformation and shoot elongation (Little & McDonald Citation2003) and when applied to apple scions stimulated shoot extension growth (Robitaille & Carlson Citation1976). Consequently, the possibility of elongating new axillary shoots was examined with application of GA4+7 (400 mg L−1) to further enhance the production of horticulturally useful stool shoots.
Materials and methods
The study was conducted in Havelock North, Hawke's Bay, New Zealand during the 2005–06 growing season using newly established stool beds of ‘M.9’ (sub clone EMLA). Three treatments were applied in early spring: 1) untreated control; 2) 1000 mg L−1 TDZ (BUDCYTTM, Gro-Chem NZ Ltd, Porirua, NZ) + 3% mineral oil (Excel Oil®, Gro-Chem NZ Ltd, Porirua, NZ) at the expected date of budbreak (15 September); and 3) 1000 mg L−1 TDZ + 3% mineral oil 2 weeks later (29 September). Six weeks after the date of the second TDZ treatment, half of each experimental plot received two applications of 400 mg L−1 GA4+7 (GIB-47®, Gro-Chem-NZ Ltd, Porirua, NZ) applied 2 weeks apart. Treatments were randomly allocated within four replicate plots each consisting of a 2 m length of stool bed, using a split plot design with GA treatment as the sub plot. As temperature is a decisive climatic factor on budbreak, the accumulation of growing degree days (GDD10) from planting to the last TDZ application was calculated (Stanley et al. Citation2000) to examine the effectiveness of the timing of TDZ treatments in the context of heat unit accumulation requirement for active budbreak.
The total number of dormant buds was recorded before treatments and the number of breaking buds per rootstock stem was recorded 6 weeks after the ‘late’ TDZ application. The number and the length of extending shoots (>5cm in length) were recorded 8 weeks after the second GA4+7 application in five randomly selected rootstock stems per 1 m of stool bed length (sub plot). New shoots arising from axillary buds were grouped into four length classes according to individual shoot length as ‘<10 cm’, ‘10–20 cm’, ‘20–30 cm’, ‘>30 cm’ and the frequency distribution of shoot length in each category within each treatment was calculated. The number of stool shoots harvested in the following winter was recorded in September 2006 and evaluated against with respect to the induction treatments. Statistical analysis of data was carried out using ANOVA procedure on Genstat Release 8 ([PC/Windows XP] Copyright 2006, Lawes Agricultural Trust [Rothamsted Experimental Station]). Fisher's Protected Least Significant Difference Test wasused for mean separation using the 5% significance level.
Results and discussion
Effect of TDZ on budbreak induction of apple rootstock cuttings
Treatment of rootstock stems with TDZ at the expected date of budbreak (15 September) resulted in an 80% budbreak response compared with 42% budbreak for the untreated control. When TDZ was applied 2 weeks later (29 September), budbreak was further enhanced to 88% (). The increase in budbreak response to TDZ treatments was highly significant (P< 0.001) compared with the control. When the timing of TDZ application is considered, late application could increase budbreak further, by a small but significant margin. In newly planted rootstock stems, only a few buds towards the apical end break at first and grow strongly into sizable shoots that can be harvested as new stool shoots at the end of the growing season. Although the entire length of rootstock stem consists of well-formed axillary buds, on untreated rootstocks most of them fail to develop in the first year probably because of the apical dominance effect established by newly growing shoots that break first in the apical region (, left). Apical dominance is a well-established phenomenon in plant growth. When an apical bud is removed, all the buds below swell for at least 48 hours, but only the top two or three buds will continue development (Faust & Wang Citation1993). Typically, the uppermost bud grows strongly and resumes the role of removed terminal bud (, centre), inhibiting lateral bud growth below it (Faust et al. Citation1995).
Figure 1 Effect of thidiazuron (TDZ, 1000 mg L−1) and application time (at budbreak: TDZ at BB [15 September], and 2 weeks after budbreak: 2WABB [29 September]) on budbreak of newly planted ‘M.9’ (EMLA) apple rootstock stems. Bars with different letters are significantly different (P < 0.001, LSD (5%) = 6.83).
![Figure 1 Effect of thidiazuron (TDZ, 1000 mg L−1) and application time (at budbreak: TDZ at BB [15 September], and 2 weeks after budbreak: 2WABB [29 September]) on budbreak of newly planted ‘M.9’ (EMLA) apple rootstock stems. Bars with different letters are significantly different (P < 0.001, LSD (5%) = 6.83).](/cms/asset/c60b47c5-6139-410c-be91-de64a0855e5a/tnzc_a_594067_o_f0001g.gif)
Figure 2 The initial breaking of terminal buds in newly planted rootstock stems (left) that later develop into dominant shoots establishing apical dominance. Lower axillary buds either remain dormant or produce a few weak axillary shoots when budbreak is not induced (centre). TDZ (1000 mg L−1) application strongly induced the breaking of axillary buds (right).

Growth regulators that act as cytokinins or that interfere with auxin metabolism have been shown to stimulate breaking of lateral buds and subsequent shoot development when applied to apple trees (Elfving Citation1985). TDZ has strong cytokinin-like growth-promoting activity and is reported to have the ability to break para-dormancy in apple flower buds (Wang & Faust Citation1994) and in a range of other temperate fruit crops (Arora et al. Citation2003). Induction of budbreak and reinvigoration of buds in extinct spur sites along the bare wood in the basal part of 2- and 3-year-old lateral branches of young trees in response to paint application of TDZ was reported by Palmer et al. (Citation2005) with ‘Scifresh’ apple. Insufficient budbreak in rootstock stems is a major setback to the fast increase in productivity of new stool beds. TDZ-induced budbreak achieved in this study may indicate that early spring application of TDZ (1000 mg L−1) acts as a strong budbreaking agent in newly planted apple rootstock stems (, right).
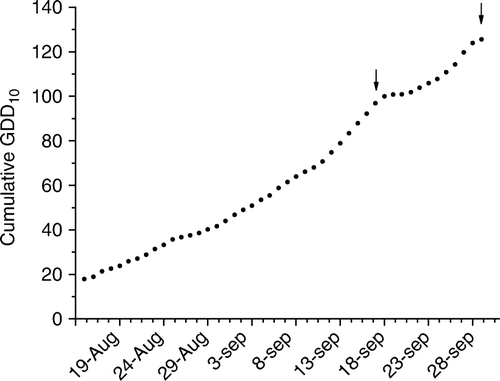
Temperature is one of the main climatic factors that influence budbreak. During winter, deciduous trees first experience a dormant period where a cool period is needed to satisfy the chilling requirement. Following that, a warmer period is needed to start the physiological processes finally resulting in growth of the buds and budbreak (Müller and Braun Citation2008). Anderson et al. (Citation1975) identified a distinct physiological period during budbreak where buds require heat before spring growth begins,which is actually the eco-dormant period (Lang et al. Citation1987). Heat accumulation units are expressed as GDD10. In New Zealand, there is a considerable seasonal and regional variability in heat accumulation among apple growing regions that influence early-season fruit growth significantly. Total accumulation of GDD10 from pollination to harvest for ‘Royal Gala’ in Hawke's Bay, Nelson and Canterbury varied from 652 to 872 (Stanley et al. Citation2000). It was reported that the accumulation of 100 GDD10 was sufficient for inducing apple budbreak in the north Indiana region in the US (Bordelon et al. Citation2007), but there is no information about the GDD10 requirement for apple budbreak in New Zealand. In this study, the GDD10 accumulations from the planting date (15 August) up until the first (15 September) and the second (29 September) TDZ applications were 88 and 126, respectively (). Thus, by the time of the second TDZ application, buds could have been physiologically more responsive to TDZ because of higher heat accumulation. Consequently, budbreak response was increased from 80% to 88% (a 9% rise) when TDZ was applied 2 weeks later (). Probably, this higher GDD10 accumulation may have contributed to the increased budbreak response for late TDZ application (). According to Faust et al. (Citation1995), in applevarieties, TDZ had a considerable effect on budbreak when part of the chilling requirement was satisfied. However, in phenological development processes such as budbreak, a considerable variation is reported in the reception and translation of influencing factors by plants (Müller & Braun Citation2008).These results may indicate that the efficacy of TDZ on budbreak on rootstock stems can be improved by more precisely timing the TDZ application to match the fulfilment of the GDD10 requirement for budbreak.
Effect of GA4+7 treatments on shoot elongation
Another important factor, from the viewpoint of stool-bed productivity, is to achieve good growth from new axillary shoots once inducted. The numbers of extending shoots after TDZ and GA4+7 treatments per rootstock stem are presented in . The average number of axillary buds per rootstock stem was initially uniform (14–16 axillary buds per rootstock stem). When rootstock stems were treated with TDZ, a substantially larger number of axillary buds were activated to break compared with the control, but only a limited number of those activated buds elongated into axillary shoots and the rest remained very short (<5 cm) shoots. Probably the large number of growing axillary shoots per rootstock (14–16 growing shoots per rootstock stem in TDZ treatments compared with seven in the control) may compete with one another for photosynthetic and mineral resources, limiting the extent of growth achieved by individual shoots.
Table 1 Extending shoot number per rootstock stem in response to thidiazuron (TDZ, 1000 mg L−1) treatments applied at budbreak (BB) or 2 weeks later (2WABB) and subsequent gibberellin (GA4+7, 400 mg L−1) treatment.
However, within this limited number of extending shoots, TDZ-induced budbreak with subsequent GA4+7 applications could significantly increase the number of extending shoots per rootstock stem (). The extending axillary shoot number per rootstock stem was increased up to 4.5 (+31%) and 4.9 (+37%), respectively, by early and late TDZ treatments compared with 3.1 in the untreated control. GA4+7 applications increased the extending shoot number from 3.9 to 4.4 (+11%) shoots per rootstock (). Late TDZ treatment followed by GA4+7 applications had the highest extending axillary shoot number (5.4 shoots per rootstock stem), but the interaction between TDZ and GA4+7 treatments was not significant.
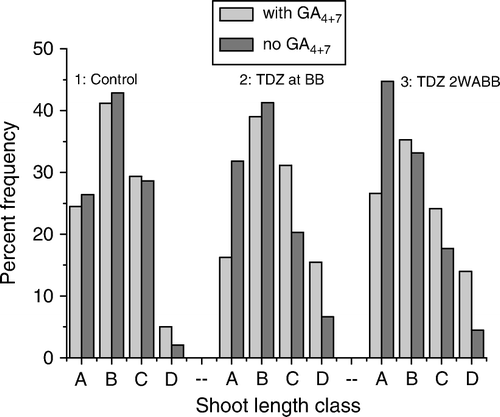
The axillary shoot length distribution in response to GA4+7 applications is presented in . The shoot length distributions were skewed towards shoots less than 20 cm in length in all treatments. The proportion ofshoots within each length class was more or less the same with or without GA4+7 when there was no TDZ-induced budbreak early in the season ([1]). A gradual transition in axillary shoot length from shorter (<10 cm) to longer shoots in response to GA4+7 treatment was observed in rootstock stems treated with TDZ at budbreak ([2]) or later ([3]). When TDZ was applied at budbreak, 32% of newly formed shoots remained as small shoots (<10 cm length class) on rootstock stems without GA4+7, but it was decreased to 16% when treated with GA4+7 ([2]). Furthermore, GA4+7 treatments resulted in more shoot extension growth, producing 46% of shoots >20 cm in length including 15% of >30 cm length. In comparison, only 27% of shoots reached more than 20 cm length and only 7% of shoots reached >30 cm length without GA4+7 ([2]). Following the late TDZ treatment supplemented by GA4+7 applications, only 27% of new axillary shoots were smaller than 10 cm in length, whereas 38% reached >20 cm in length, including 14% in the >30 cm length class. Without GA4+7 treatment, 45% of shoots remained below 10 cm in length and only 22% reached more than 20 cm length ([3]). Lateral buds being released from dormancy and activated to grow into small shoots as a result of TDZ application may have provided a favourable physiological status for GA-induced shoot extension growth. Both neoformed and preformed shoot elongation can be promoted by exogenous GA1, GA3, GA4 or GA4+7 (Little & Pharis Citation1995). It is reported that GA promotes shoot elongation primarily by stimulating the activity of the sub-apical meristem of growing shoots (Hansen et al. Citation1999). In this study, the effect of GA4+7 was found to be greater in rootstock stems following TDZ-induced budbreak, but a substantial number of shorter shoots remained unextended despite two applications of GA4+7 indicating that there may be further potential to manipulate shoot extension by increasing the number of gibberellin applications.
Figure 4 Frequency distribution of axillary shoot length in response to GA4+7 treatments. Rootstock stems were: (1) not treated with TDZ, (2) treated with TDZ (1000 mg l−1) at budbreak or (3) two weeks later. Subsequently, half of each treatment plot was treated with GA4+7 (400 mg l−1) as a split plot treatment. New axillary shoots were grouped in to four length classes as A:<10 cm, B: 10–20 cm, C: 20–30 cm and D:>30 cm.
Effect of treatments on stool shoot yield
The standard growth required for stool shoots may vary but the optimum girth and length is considered as 6–10 mm calliper and 30–40 cm length (Waterman et al. Citation1993). The total number of harvested stool shoots per metre length is presented in . The harvested stool shoot numbers closely reflect the previously observed trends in the number of extending axillary shoots per rootstock stem (). TDZ-induced budbreak increased the stool shoot yield from 18 stool shoots per metre in the untreated control up to 29 (+37%) or 32 (+ 46%), respectively, in early or late TDZ + GA4+7 treatments. When there was no follow-up, GA4+7 application stool shoot yield was increased only up to 25 (+27%) and 29 (+37%) stool shoots per metre, compared with 18 in the control in at budbreak and late TDZ treatments (). TDZ-induced breaking of lateral buds made available higher numbers of growing shoots for GA-mediated shoot elongation in TDZ-treated rootstockstems than found in the control. However, the effect of GA4+7 on stool shoot yield was not statistically significant ().
Table 2 Number of apple stool shoots harvested per metre length of stool bed in response to thidiazuron (TDZ, 1000 mg L−1) treatments applied at budbreak (BB) or 2 weeks later (2WABB) and subsequent gibberellin (GA4+7, 400 mg L−1) treatment.
Conclusions
Application of TDZ (1000 mg L−1) early in the spring enhanced the budbreak of newly planted rootstock stems. Sufficient heat unit accumulation at the time of TDZ application may be a prerequisite for successful TDZ-induced budbreak. The elongation growth of axillary shoots was found to be greater when the TDZ-induced budbreak was followed by GA4+7 (400 mg L−1) applications. However, the majority of axillary shoots developed in response to TDZ and GA4+7 treatments did not reach the required size by the end of the season. The means to develop these axillary shoots into stool shoots of usable length and girth by the end of the season may be possible by further manipulation of exogenous gibberellin applications. That would contribute to increase stool-bed productivity, which is a pressing need for the New Zealand pipfruit industry.
Acknowledgements
The authors are thankful to The Nursery and Garden Industry Association of New Zealand for funding the project, Peak View Nursery in Havelock North for their assistance and Gro-Chem New Zealand Ltd for the supply of growth regulators.
References
- Alvarado-Raya , H , Rodriguez-Alcazar , J , Calderon-Zavala , G and Cardenas-Soriano , E . 2000 . Thidiazuron, flower bud break and ovary dimensions in Japanese plum (Prunus salicina L) ‘Shiro’ . Agrociencia , 34 : 321 – 327 .
- Anderson , JL , Ashcroft , GL , Richardson , EA , Alfaro , JF , Griffin , RE , Hanson , GR and Keller , J . 1975 . Effects of evaporative cooling on temperature and development of apple buds . Journal of the American Society of Horticultural Science , 100 : 229 – 231 .
- Arora , R , Rowland , LJ and Tanino , K . 2003 . Induction and release of bud dormancy in woody perennials: A science comes of age . HortScience , 38 : 911 – 921 .
- Elfving , DC . 1985 . Comparison of cytokinin and apical dominance inhibiting growth regulators for lateral branch induction in nursery and orchard apple trees . Journal of Horticultural Science , 60 : 447 – 454 .
- Bordelon B , Beckerman J , Hirst P , Dennis J , Foster R 2007 Facts for fancy fruit Technical Bulletin, Department of Horticulture & Landscape Architecture, July 2007, School of Agriculture, Purdue University, Indiana, US. 9 p.
- Faust , M and Wang , SY . 1993 . Biochemical events associated with resumption of growth in temperate zone fruit trees . Acta Horticulturae , 329 : 257 – 264 .
- Faust , M , Dehua Liu , SY , Wang , SY and Stutte , GW . 1995 . Involvement of apical dominance in winter dormancy of apple buds . Acta Horticulturae , 395 : 47 – 56 .
- Hansen , E , Olsen , JE and Junttila , O . 1999 . Gibberellins and sub apical cell division in relation to bud set and bud break in Salix pentandra . Journal of Plant Growth Regulation , 18 : 167 – 170 .
- Lang , GA , Early , JD , Martin , GC and Darnell , RL . 1987 . Endo-, para- and eco-dormancy: physiological terminology and classification for dormancy research . HortScience , 22 : 371 – 377 .
- Little , CHA and McDonald , JE . 2003 . Effect of exogenous gibberellin and auxin on shoot elongation and vegetative bud development in seedlings of Pinus sylvestris and Picea glauca . Tree Physiology , 23 : 73 – 83 .
- Little CHA , Pharis RP 1995 Hormonal control of radial and longitudinal growth in the tree stem In : Gartner BL Plant stems: physiology and functional morphology. Academic Press San Diego 281 319
- Müller M , Braun P 2008 Development of phenological models over time—a review Proceedings of the VIIIth International Symposium on Fruit Research. Ed: Samietz J, International Society of Horticultural Science, ActaHort. Pp. 803.
- Palmer , JW , Diack , R , Seymour , S , Dayatilake , D and Tustin , DS . 2005 . New approaches to the alleviation of bare wood in young apple trees . Journal of Horticultural Science and Biotechnology , 80 : 623 – 627 .
- Robitaille , H and Carlson , R . 1976 . Gibberellic and abscisic acid-like substances and the regulation of apple shoot extension . Journal of American Society of Horticultural Science , 101 : 388 – 392 .
- Stanley , CJ , Tustin , DS , Lupton , GB , McArtney , S , Cashmore , WM and De Silva , HN . 2000 . Towards understanding the role of temperature in apple fruit growth responses in three geographical regions within New Zealand . Journal of Horticultural Science & Biotechnology , 75 : 413 – 422 .
- Tobakov , S and Yordanov , A . 2006 . Performance of some vegetatively propagated apple rootstocks in the nursery . Sordininkyste ir Darzininkyste , 25 : 85 – 89 .
- Tustin , DS and Palmer , JW . 2008 . Adoption and implementation of intensive planting systems for apple—the New Zealand experience . Acta Horticulturae , 772 : 475 – 481 .
- Tustin , DS and Cashmore , WM . 1994 . Rootstock and spacing effect on precocity, yield and fruit quality of ‘Fuji’ apple using slender pyramid tree management . Compact Fruit Tree , 23 : 83 – 87 .
- Wang , SY and Faust , M . 1988 . Metabolic activities during dormancy and blooming of deciduous fruit trees . Israel Journal of Botany , 37 : 227 – 243 .
- Wang , SF and Faust , M . 1994 . Apical dominance in Malus Domestica Borkh: The possible role of indole-3-acetic acid . Journal of the American Society of Horticultural Science , 119 : 1215 – 1221 .
- Wang , SF , Steffins , GL and Faust , M . 1986 . Breaking bud dormancy in apple with a plant growth regulator, thidazuron . Phytochemistry , 25 : 311 – 317 .
- Waterman P , Hogue EJ , Quamme H 1993 In: Tree fruit home nurseries. A growers’ manual Okanagan Valley Tree Fruit Authority, the British Columbia Ministry of Agriculture, Fisheries and Food and Agriculture, Canada. Summerland, British Columbia, Canada.