Abstract
The underground buds of herbaceous peony (Paeonia lactiflora Pall.) ‘Zhong Sheng Fen’ were used to investigate the effects of indole-3-acetic acid (IAA) on culture initiation, benzyladenine (BA) on axillary shoot induction, and three auxins (IAA, indole-3-butyric acid [IBA] and 1-naphthyleneacetic acid [NAA]) on axillary shoot proliferation. In addition, methods to rejuvenate hyperhydric microshoots were established. Our results showed that the best initiation medium for ‘Zhong Sheng Fen’ was half-strength Murashige and Skoog (MS) medium supplemented with double the concentration of Ca2 + (880 mg L−1), 1 mg L−1 BA, 0.5 mg L−1 gibberellic acid (GA3) and 0.1 mg L−1 IAA. Axillary shoots were successfully induced by 2.0 mg L−1 BA. Several methods allowed the successful rejuvenation of hyperhydric microshoots, allowing them to develop normally. These included the addition of 3 g L−1 activated charcoal, the removal of ammonium nitrate from the medium, doubling the concentration of Ca2 + or eliminating BA from the medium.
Introduction
Herbaceous peony (Paeonia lactiflora Pall.) is a herbaceous perennial in the genus Paeonia, family Paenoiaceae. It has been cultivated in China for more than 3900 years (Wang & Zhang Citation2005). It is a famous garden plant and cut flower, and can also be used as a pot plant or for dried flowers. Herbaceous peony is usually propagated by dividing tuberous root clumps containing three to five dormant vegetative buds (Shannon & Kamp Citation1959; Qin Citation2004). However, the multiplication rate by this method is too low to meet the needs of modern mass production. Thus, direct or indirect shoot induction have been the major objectives of several studies (Hosoki et al. Citation1989; Orlikowska et al. Citation1998; Guo Citation2001; Tian et al. Tian et al. Citation2010; Wang et al. Citation2010). Tissue culture and rapid propagation of herbaceous peony can augment the propagation rate and shorten the breeding period, which is important for production. Although many advances have been made in the micropropagation of peony before, several serious limitations still remain unsolved, including hyperhydricity, which can affect shoot multiplication and culture vigour and can negatively influence rooting and transplanting capacity (Chu & Li Citation1993; Guo Citation2001; Zhang et al. Citation2006; Tian et al. Citation2010).
The performance and survival of in vitro cultures of many plant species are often hampered by hyperhydricity (Debergh et al. Citation1992), a phenomenon of deformity in which the shoots become translucent during tissue culture. Several previous studies indicated that once the cultured microshoots become hyperhydric, it is usually difficult to recover their original form and physiology (An Citation2005). Hyperhydricity can lead to an irreversible loss of regenerative ability of the tissue, other detrimental changes, and even ultimately death. These losses, together with the poor survival rate of hyperhydric shoots when transferred to ex vitro conditions, limit the potential of in vitro techniques for mass propagation (Ivanova & van Staden Citation2008). Hyperhydricity is a complex phenomenon that is affected by several factors such as: medium (An Citation2005); explant age (Bouza et al. Citation1994); temperature (Chu & Li Citation1993); light (Guo Citation2001); subculture age (Guo Citation2001); the type of gelling agent and cytokinins (Ivanova & van Staden Citation2011); nitrogen source, concentration and the NH4 +: NO3-ratio (Ivanova & van Staden Citation2009).
Several researchers have attempted to overcome this problem by using containers with good gaseous exchange (Purohit et al. CitationIn press), different agar concentrations (Kevers & Gasper Citation1986; Miller et al. Citation1991) or changing the levels of potassium nitrate, ammonium nitrate and calcium chloride (Choudhary et al. Citation1993). The accumulation of gases such as ethylene and CO2 has also been found to be responsible for hyperhydricity (De Proft et al. Citation1985). Yadav et al. (Citation2003) reported that an increasing concentration of iron and/or magnesium reduced hyperhydricity during the micropropagation of carnation (Dianthus caryophyllus L.). Many methods have been adopted to control hyperhydricity in Aloe polyphylla such as the combination of low concentrations of cytokinins and NH4NO3 (Ivanova & van Staden Citation2008) and improving the natural ventilation of culture vessels (Ivanova & van Staden Citation2010).
In this experiment, underground buds of herbaceous peony (P. lactiflora Pall.) ‘Zhong Sheng Fen’, which has a high ornamental value and vigorous shoot-forming capacity, were chosen as the experimental material to induce axillary shoots and to explore methods to optimize the rejuvenation of hyperhydric microshoots.
Materials and methods
Materials
The well-developed underground buds of P. lactiflora ‘Zhong Sheng Fen’ were collected in winter from the Society of Forestry Experimental Base and used as the explants.
Explant sterilization and culture conditions
Before inoculation, the underground buds were washed in tap water for 30 min. The outer scales were peeled off and buds were soaked in 75% ethanol for 30 s, immediately followed by 10 min sterilization with a dilute solution of HgCl2 (0.1% w/v). Plant material was rinsed five times (5 min/rinse) in autoclaved distilled water.
All sterilized explants (i.e. underground buds) were placed on agar (0.7%) solidified medium. The base medium was half-strength (macro- and micronutrients) MS medium (Murashige and Skoog Citation1962) with double-strength calcium chloride (Ca2 +) supplemented with 30 g L−1 sugar. Buds were cultured in 100 ml Erlenmeyer flasks containing 30 ml agar solidified medium with one bud per flask.
Treatments
Four experiments were conducted. In the first experiment, the effects of indole-3-acetic acid (IAA) (0.0, 0.1, 0.3 and 0.5 mg L−1) combined with 1 mg L−1 6-benzyladenine (BA) and 0.5 mg L−1 gibberellic acid (GA3) on culture initiation of ‘Zhong Sheng Fen’ were tested. In the second experiment, the effects of BA (0.5, 1.0, 1.5 and 2.0 mg L−1) combined with 0.5 mg L−1 kinetin (KT) on axillary shoot induction were tested. In the third experiment, the effects of different auxins (IAA, indole-3-butyric acid [IBA], 1-naphthyleneacetic acid [NAA]) on axillary shoot proliferation were tested. In the fourth experiment, four protocols were tested to explore the optimum method for rejuvenation of hyperhydric microshoots: 1) BA at 0 or 0.2 mg L−1; 2) activated charcoal (AC) at 0, 1 or 2 g L−1; 3) ammonium nitrate at 0, 206.25, 412.5 or 825 mg L−1 (control); 4) calcium at 440 (control), 880 or 1320 mg L−1.
The pH of all media was adjusted to 5.8 prior to autoclaving at 118 °C for 18 min. Culture vessels were placed in a growth chamber at 25±2 °C in a 14-h photoperiod with 50 µmol m−2 s−1 photosynthetic photon flux density (PPFD) using cool white fluorescent tubes. Data were collected after 30 days of culture.
Experimental design and statistical analyses
Experiments were laid out in a completely randomized design. Means were separated by one-way analysis of variance and significant differences were assessed using Duncan's multiple range test at P = 0.05 using SPSS software version 13.0. Each experiment was replicated three times with 10 explants per treatment.
Results
Culture initiation
Different concentrations of IAA had a significant effect on the number of induced lateral shoots, but did not affect the initiation rate, leaf expansion rate, the rate of yellowing shoot tips or height (). However, the addition of IAA favoured the growth of shoots with an earlier emergence time and increased the number of axillary shoots and shoot length in response to IAA. After inoculation, these buds grew very rapidly and the main and lateral shoots elongated (A). Leaves expanded gradually. The highest lateral shoot initiation (92.8%), number of lateral shoots (2.13) and shoot length (4.2 cm) and the lowest rate of yellowing shoot tips (17.43%) were observed in medium containing 0.1 mg L−1 IAA + 1 mg L−1 BA + 0.5 mg L−1 GA3 (). A higher concentration of IAA (0.3–0.5 mg L−1) did not favour leaf expansion and lateral shoot development.
Figure 1 Culture of underground buds of Paeonia lactiflora Pall. A, Induction of underground buds. B, Proliferation of axillary shoots. C, Serious hyperhydricity. D, Mild hyperhydricity. E, Rejuvenation of hyperhydric microshoots with activated charcoal. F, Rejuvenation of hyperhydric microshoots with ammonium nitrate.
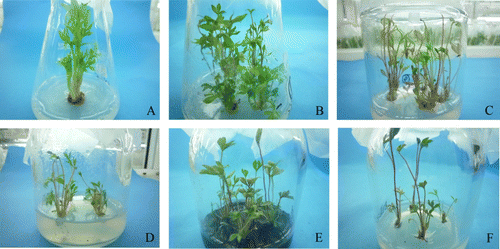
Table 1 The effects of various concentrations of indole-3-acetic acid (IAA) on initiation culture (n = 10). Underground buds were inoculated on half-strength MS medium (double-strength Ca2 +) supplemented with 1.0 mg L−1 benzylaminopurine (BA) plus 0.5 mg L−1 gibberellic acid (GA3) and different concentrations of IAA, respectively. Different letters within a column indicate significant differences according to Duncan's multiple range test (P = 0.05).
Axillary shoot induction
After initiation of culture, elongated shoots were cut into nodal sections and transferred to fresh medium for axillary shoot induction. Thirty days later, one to several new shoots developed from each bud eye region. Different concentrations of BA had significant effects on induction rate, number of lateral shoots and percentage of death (). The highest induction rate (79.83%) was obtained on medium with 0.5 mg L−1 BA; the highest number of axillary shoots and the lowest percentage of death were observed on medium with 2.0 mg L−1 BA. The medium with 1.5 mg L−1 BA showed the worst effect by producing the lowest induction rate (62.03%), number of axillary shoots (3.89) and the highest percentage of death (20.37). Axillary shoots induced by a lower concentration of BA (0.5–1.0 mg L−1) were weaker, shorter and leaves did not extend. Thus, the induction of axillary shoots was favoured by a higher concentration of BA (2.0 mg L−1).
Table 2 The effects of various concentrations of benzylaminopurine (BA) on axillary shoot induction (n = 10). Shoots were inoculated on half-strength MS medium (double-strength Ca2+) supplemented with 0.5 mg L−1 kinetin (KT) plus different concentrations of BA, respectively. Different letters within a column indicate significant differences according to Duncan's multiple range test (P=0.05).
Shoot proliferation
Different auxins had significant effects on the height of microshoots and on the incidence of hyperhydricity (, B). The addition of an auxin to the medium caused a significant increase in the incidence of hyperhydricity compared with the control. The highest multiplication rate (2.40) and the lowest incidence of hyperhydricity (7.01%) were observed on auxin-free medium. Although the addition of IBA or IAA increased shoot length, hyperhydricity was also aggravated. The lowest rate of multiplication and shoot length were observed in the treatment with NAA, which caused less shoot hyperhydricity than when microshoots were exposed to the other two auxins (i.e. IAA and IBA). However, the treatment with NAA resulted in considerable callus at the base of the shoots and leaf deformity, which did not favour shoot development. In conclusion, auxin did not favour shoot proliferation.
Table 3 The effects of different auxins on axillary shoot proliferation (n = 10). Shoots were inoculated on half-strength MS medium (double-strength Ca2+) supplemented with 0.2 mg L−1 benzylaminopurine (BA) plus 0.1 mg L−1 gibberellic acid (GA3) and different auxins, indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) and 1-naphthyleneacetic acid (NAA), respectively. Different letters within a column indicate significant differences according to Duncan's multiple range test (P=0.05).
Rejuvenation of hyperhydric microshoots
Effect of BA on growth of hyperhydric microshoots
Even a low concentration of BA (0.2 mg L−1) aggravated hyperhydricity (, C). In this state, no hyperhydric microshoots (D) reversed to normal shoots and the proliferation rate of hyperhydric microshoots also decreased: only 20.26% normal shoots were obtained in the treatment without BA. In conclusion, hyperhydricity was strongly linked to BA, and even a low concentration of BA (0.2 mg L−1) did not benefit the growth of hyperhydric microshoots. Our experiments indicate that it was better to not use BA or to add other plant growth regulators (PGRs) or additives to the medium to reduce hyperhydricity.
Table 4 The effects of benzylaminopurine (BA) on the growth of hyperhydric microshoots (n = 10). Shoots were inoculated on half-strength MS medium (double-strength Ca2+) supplemented with 0.2 mg L−1 or 0 mg L−1 BA.
Effect of activated charcoal on rejuvenation of hyperhydric microshoots
Activated charcoal (AC) had a significant effect on the rejuvenation of hyperhydric microshoots (E). The rate of normal shoots increased significantly from 4.41% (control) to 34.37% or 34.22%. There was no difference between 1 and 2 g L−1 of AC on the reversion rate of hyperhydric microshoots. However, the highest rate of normal shoots (34.37%) was observed when 2 g L−1 of AC was used (). Furthermore, the addition of 2 g L−1 AC significantly resulted in the highest number of multiple shoots (1.71) and shoot length (4.02 cm). In addition, shoots strengthened and leaves turned green. However, shoots became slightly withered and leaves turned yellow after long exposure (30 days) to AC, possibly due to the strong adsorbability of AC (Gao Citation2008). AC is likely to have adsorbed harmful substances in the medium but may have also adsorbed vital nutrients that are needed for shoot development, resulting in shoot wilting. It was concluded that a short period of inoculation (15–20 days) in AC-supplemented medium favoured the rejuvenation of hyperhydric microshoots.
Table 5 The effect of active charcoal (AC) on rejuvenation of hyperhydric microshoots (n = 10). Shoots were inoculated on PGR-free half-strength MS medium (double-concentration Ca2+) containing different concentrations of activated charcoal. Different letters within a column indicate significant differences according to Duncan's multiple range test (P=0.05).
Effect of ammonium nitrate on rejuvenation of hyperhydric microshoots
Ammonium nitrate had a significant effect on the rejuvenation and height of hyperhydric microshoots, but did not affect multiple shoot formation (). The best treatment was when the medium contained no ammonium nitrate. The highest percentage of normal shoots (28.43%) (F) was observed in this treatment. Reducing ammonium nitrate to 412.5 or 206.25 mg L−1 in the medium aggravated hyperhydricity compared with the control (825 mg L−1). However, reducing the concentration of ammonium nitrate significantly inhibited spindling of the shoots. Height decreased 0.55–1.13 cm compared with the control (4.70 cm). In conclusion, removing all the ammonium nitrate from the medium was the best means to overcome hyperhydricity.
Table 6 The effect of ammonium nitrate on rejuvenation of hyperhydric microshoots (n = 10). Shoots were inoculated on PGR-free half-strength MS medium (double-strength Ca2+) containing different strength of ammonium nitrate. The normal strength of ammonium nitrate in 1/2 MS medium was 825 mg L−1. Different letters within a column indicate significant differences according to Duncan's multiple range test (P=0.05).
Effect of calcium on rejuvenation of hyperhydric microshoots
The highest percentage of normal shoots (26.18%) was obtained in the treatment in which 880 mg L−1 calcium (double strength) was applied. However, this also significantly inhibited overgrowth of the shoots more than other treatments (). The treatment with 1320 mg L−1 calcium (three-fold strength) caused spindling and produced maximum height (4.81 cm), although shoots were more deformed. The level of calcium in the control, i.e. 440 mg L−1, had the worst effect: the lowest percentage of normal shoots (10.74%). The shoot stems became more transparent with curled leaves. Thus, double-strength calcium (880 mg L−1) was better for rejuvenation of hyperhydric microshoots.
Table 7 The effect of calcium on rejuvenation of hyperhydric microshoots (n = 10). Shoots were inoculated on PGR-free half-strength MS medium containing different concentration of calcium. The normal strength of calcium in 1/2 MS medium was 440 mg L−1. Different letters within a column indicate significant differences according to Duncan's multiple range test (P = 0.05).
Discussion
Several previous studies have reported on shoot proliferation stages of P. lactiflora Pall. (Hosoki et al. Citation1989; Gabryszewska Citation1998; Guo Citation2001; Ding et al. Citation2010). BA is the most commonly used cytokinin for peony shoot proliferation (Hosoki et al. Citation1989; Bouza et al. Citation1994; Gabryszewska Citation1998). In the study of Paeonia suffruticosa, 6-benzyladenine (BAP) was the only cytokinin effective for leaf and axillary bud development and, when combined with another cytokinin, reduced its efficiency (Bouza et al. Citation1994). The addition of a low concentration of auxin to the medium containing BA increased multiple shoots but stimulated callus formation which was unfavourable for shoot growth of tree peony (Kong & Zhang Citation1998; Wang et al. Citation2010). Our study showed that auxin did not favour shoot proliferation and growth although, ironically, IAA stimulated more shoots than the control treatment. Those shoots became easily hyperhydric. An examination of other peony studies indicated that most authors have not mentioned this problem, although it was commonly found in tree peony in vitro culture.
BA was considered to be the major factor causing hyperhydricity. Reducing the concentration of BA or adopting other cytokinins such as kinetin (KT) instead of BA can overcome this problem (Liu et al. Citation2003; Li et al. Citation2004). The highest recovery of hyperhydricity in tree peony was only obtained in medium without PGRs or with PGRs at low concentrations (An Citation2005). Our study suggested that even a low concentration of BA (0.2 mg L−1) aggravated hyperhydricity. Besides, the addition of some adsorbant substances can reduce hyperhydricity such as polyvinyl alcohol, AC and penicillin G potassium (Liu et al. Citation2003). In the current study, AC favoured the rejuvenation of hyperhydric microshoots. A short period of AC treatment (approximately 20 days) would be better considering its strong adsorbability and eventual long-term negative effects by adsorbing important nutrients from the medium. Reducing or removing ammonium nitrate in the medium or using other media containing a low concentration of ammonium nitrate could reduce the occurrence of hyperhydricity (Liu et al. Citation2003). The removal of ammonium nitrate in the medium reduced the percentage of hyperhydric shoots greatly in the study of Daphne odora Thunb (Zhou et al. Citation1990). Decreasing the ratio of NH4 +: NO3 − in MS medium reduced hyperhydricity in Aloe polyphylla (Ivanova & van Staden Citation2009). In that study, the medium contained no ammonium nitrate, significantly reducing hyperhydricity. Other media and PGRs need to be tested to overcome hyperhydricity.
Acknowledgements
This work was supported by the National Science and Technology Program in the 12th Five-year Plan of China (20113AD12B02).
References
- An BY 2005 . Studies on the establishment of in vitro regeneration system of Paeonia suffruticosa Andr . MS thesis, Northeast Forestry University . Pp. 48 – 50 .
- Bouza , L , Jacques , M and Miginiac , E . 1994 . In vitro propagation of Paeonia suffruticosa Andr. cv. ‘Mme de Vatry’: developmental effects of exogenous hormones during the multiplication phase . Scientia Horticulturae , 57 : 241 – 251 .
- Choudhary , ML , Prakash , ML and Prakash , P . 1993 . Effect of different levels of agar and MS macro salts on the production of hardened carnation in vitro . Advances in Horticulture and Forestry , 3 : 165 – 169 .
- Chu , CC and Li , DW . 1993 . The vitrification of tree peony . Journal of Henan Normal University (Natural Science) , 20 : 70 – 73 .
- De Proft , M , Maene , J and Debergh , P . 1985 . Carbon dioxide and ethylene evolution in the culture atmosphere of magnolia cultured in vitro . Physiologia Plantarum , 65 : 375 – 379 .
- Debergh , P , Aitken-Christie , J , Cohen , D , Grout , B , von Arnold , S , Zimmerman , R and Ziv , M . 1992 . Reconsideration of the term ‘vitrification’ as used in micropropagation . Plant Cell, Tissue and Organ Culture , 30 : 135 – 140 .
- Ding , Y , He , S-L , Teixeira da Silva , JA , Li , G-Y and Tanaka , M . 2010 . Effects of a new light source (cold cathode fluorescent lamps) on germination and growth of peony plantlets in vitro . Scientia Horticulturae , 125 : 167 – 169 .
- Gabryszewska , E . 1998 . The influence of cytokinins, thidiazuron, paclobutrazol and red light on shoot proliferation of herbaceous peony cv. Jadwiga in vitro . Journal of Fruit and Ornamental Plant Research , 6 : 157 – 169 .
- Gao , CY . 2008 . Research progress of browning in tissue culture of tree Paeony . Northern Horticulture , 3 : 79 – 81 .
- Guo FY 2001 . Study on tissue culture of Paeonia lactiflora . MS thesis, Beijing Forestry University. Pp. 37 – 38 .
- Hosoki , T , Ando , M , Kubara , T , Hamada , M and Itami , M . 1989 . In vitro propagation of herbaceous peony (Paeonia lactiflora Pall.) by a longitudinal shoot-split method . Plant Cell Reports , 8 : 243 – 246 .
- Ivanova , M and van Staden , J . 2008 . Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla . Plant Cell, Tissue and Organ Culture , 92 : 227 – 231 .
- Ivanova , M and van Staden , J . 2009 . Nitrogen source, concentration, and NH4+: NO3− ratio influence shoot regeneration and hyperhydricity in tissue cultured Aloe polyphylla . Plant Cell, Tissue and Organ Culture , 99 : 167 – 174 .
- Ivanova , M and van Staden , J . 2010 . Natural ventilation effectively reduces hyperhydricity in shoot cultures of Aloe polyphylla Schonland ex Pillans . Plant Growth Regulation , 60 : 143 – 150 .
- Ivanova , M and van Staden , J . 2011 . Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla . Plant Cell, Tissue and Organ Culture , 104 : 13 – 21 .
- Kevers , C and Gasper , T . 1986 . Vitrification of carnation in vitro: change in water contents, extracellular space, air volume and ion levels . Physiologie Vegetale , 24 : 647 – 653 .
- Kong , XS and Zhang , MX . 1998 . Fast propagation of tree peony . Northwest Horticulture , 3 : 87 – 89 .
- Li , YL , Zhang , J and Pang , YZ . 2004 . Vitrification of shoots in tissue culture and advances in its prevention and cure . Journal of Sichuan Agricultural University , 22 : 278 – 282 .
- Liu , QL , Ma , H and Zheng , YM . 2003 . Tissue culture of flower , 55 – 56 . Beijing : China Agriculture Press .
- Miller , RM , Kaul , V , Hutchinson , SF and Richards , D . 1991 . Adventitious shoot regeneration in carnation (Dianthus caryophyllus) from axillary bud explants . Annals of Botany , 67 : 35 – 42 .
- Murashige , T and Skoog , F . 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiologia Plantarum , 15 : 473 – 497 .
- Orlikowska , T , Marasek , A and Kucharska , D . 1998 . Regeneration of Paeonia mloksewitschii Lom. and P. tenuifolia L. in vitro from different explants . Acta Societatis Botanicorum Poloniae , 67 : 223 – 227 .
- Purohit SD , Teixeira da Silva JA , Habibi N In press . Current approaches for cheaper and better micropropagation technologies . International Journal of Plant Developmental Biology .
- Qin , KJ . 2004 . Herbaceous peony , 76 Beijing : China Forestry Press .
- Shannon , J and Kamp , JR . 1959 . Trials of various possible propagation methods on herbaceous peonies . Illinois State Florists Association Bulletin , 197 : 4 – 7 .
- Tian , D , Tilt , KM , Dane , F , Woods , FM and Sibley , JL . 2010 . Comparison of shoot induction ability of different explants in herbaceous peony (Paeonia lactiflora Pall.) . Scientia Horticulturae , 123 : 385 – 389 .
- Wang , JF , Li , Q and Meng , H . 2010 . Induction and regeneration of callus tissues in five peony cultivars . Journal of Beijing Forestry University , 32 : 213 – 216 .
- Wang , JG and Zhang , ZS . 2005 . Herbaceous peony of China , 1 Beijing : China Forestry Press .
- Yadav , MK , Gaur , AK and Garg , GK . 2003 . Development of suitable protocol to overcome hyperhydricity in carnation during micropropagation . Plant Cell, Tissue and Organ Culture , 72 : 153 – 156 .
- Zhang , QR , Sun , JZ , Ren , NH , Dong , XY , Liu , ZM and Zhai , M . 2006 . Tissue culture of Paeonia lactiflora Pall . Journal of Henan Agriculture Science , 24 : 88 – 90 .
- Zhou , JH , Lin , ZM and Liang , HM . 1990 . Studies on vitrification of regenerated shoots in Daphne . Acta Horticulturae , 17 : 229 – 232 .