Abstract
Daylily or Hemerocallis spp. (Xanthorrhoeaceae) is one of the most economically important flowering crops in the world. Ploidy level of 149 genotypes both local to China and introduced from the United States and New Zealand were assessed with an outcome of 79 diploids, 22 triploids and 48 tetraploids. A large proportion of introduced cultivars were tetraploid (42%), half were diploid and the rest, 8%, were triploid. Most Chinese cultivars were diploid; only one was tetraploid. Among the 29 wild genotypes collected from the Taihang Mountain range, 13 (45%) were triploid and 16 (55%) were diploid; no tetraploids were identified. For the 31 genotypes with known ploidy, 22 matched previous counts with the rest showing a lower ploidy. As different ploidy is common in daylily, we suggest that ploidy levels should be assessed in breeding programmes for specific breeding purposes. Karyotypes of three diploid, three triploid and two tetraploid genotypes were constructed and they were assigned to ‘3A’, ‘2B’ and ‘3B’ categories based on Stebbins’ karyotype classification.
Introduction
Polyploidization is an important means of plant evolution (Huang & Qin Citation2008). Paleopolyploidy is very common in plants. It has been found that almost all flowering plants have undergone at least one round of genome duplication at some point during their evolutionary history. However, the evolution of most crop plants has undergone a more recent polyploidization, so called neopolyploidy (Wang et al. Citation2011). About 70% of angiosperm species have undergone polyploidization multiple times during their evolutionary history (Masterson Citation1994); the resulting polyploid with multiple genomes shows a greater potential for improvement and a wider ability to adapt to different environments. For example, polyploid kiwifruit exhibit increased fruit size and improved fruit shape (Wu et al. Citation2012). In addition, polyploidy can alter the number of flowers (Balao et al. Citation2011), flowering time (Mayfield et al. Citation2011), stomatal density, chloroplast number and other characteristics (Singsit Citation1991). Therefore, breeding through ploidy manipulation is often used to change agronomic traits of crops.
Daylily (Hemerocallis spp.) is one of the most economically important flowering crops in the world because of its large, showy flowers and its adaptation to a wide range of soils and climates. Genetic resources of Hemerocallis spp. are abundant; there are about 15–18 species in the genus around the world, 11 of which are widely distributed in China (Chen Citation1980). Through extensive breeding, many cultivars have been developed. With the development of chromosome technology in recent years, cytological markers are widely used for plant identification, biodiversity evaluation and the study of plant evolution. Earlier studies indicated that the base chromosome number in Hemerocallis is 11. Most wild daylilies are diploid 2n = 2x = 22, H. fulva var. kwanso is a triploid with chromosome 2n = 3x = 33 and some cultivars are tetraploid with 2n = 4x = 44. All karyotypes studied so far belong to the 2B type based on Stebbins’ genome classification (Matsuoka Citation1971; Kong & Wang Citation1993; Li & Zhang Citation1995; Kong Citation1998; Tomkins et al. Citation2001; Plodeck Citation2002; Hiroyuki et al. Citation2003; Li et al. Citation2009).
In this experiment, we studied the chromosome number, ploidy and karyotypes of selected daylily germplasm from different sources with the aim of providing information for daylily germplasm enhancement and genetic improvement.
Materials and methods
This research was conducted at the Institute of Horticulture, Shanxi Academy of Agricultural Sciences in China. A total of 149 genotypes were studied. All genotypes were kept at the field station of the institute. Among the 149 genotypes, 120 are commercial cultivars: 82 from the United States, 30 from New Zealand, and eight local cultivars from China. The remaining 29 genotypes were collected from natural populations occurring in different localities in the Taihang Mountain range, Shanxi Province, China ().
Table 1 Chromosome number and ploidy level of daylily cultivars (Hemerocallis spp.). Accessions collected from natural populations are in bold.
Chromosome number was counted in root tips using the conventional squash method. Plants were watered in the morning, root tips about 1 cm long were collected at c. 0930 h the following morning. The materials were treated with a saturated solution of p-dichlorobenzene for 5 h, fixed for 22 h in fresh Cannoy's I fixative, and then transferred to 70% ethanol for preservation. Root tips were softened with 1 M hydrochloric acid at 60 °C for 8 min and stained for 15 min with basic fuchsin before being squashed between a glass slide and a cover slip. For each genotype, 10 best cells of good chromosomal dispersion were selected, counted and photographed under a Nikon microscope.
Photographs of selected cells from eight genotypes () were enlarged for karyotyping. The lengths of the short arm (s) and long arm (l) of each chromosome were measured and the following karyotype parameters were calculated: chromosome relative length (Li & Chen Citation1985); ratio of longest chromosome length to shortest chromosome length;range of arm ratio (r = l/s); average arm ratio (AAR); percentage of chromosome with arm ratio > 2 (PCA); and index of karyotype asymmetry (As.K) (Arano Citation1963). Chromosomes were classified on the basis of arm ratio according to Stebbins (Citation1971) and the karyotype formulae were given according to Levan et al. (Citation1964) based on centromere position: m, median (r = 1.01–1.69); sm, submedian (r = 1.70–3.00); st, subterminal (r = 3.01–7.00); t, terminal (r = 7.01–8). Morphological characters such as leaf length, leaf width, plant height, crown diameter and flower stem thickness were measured at the flowering stage. Each trait was measured from five individuals of each genotype and correlations between ploidy and different characters were calculated and a T-test was employed to determine the significant difference of the results.
Table 2 Daylily genotypes used for karyotype analysis.
Results
Chromosome number and ploidy in Hemerocallis
The results showed that among the 149 daylily cultivars examined, 79 were diploids (2n = 2x = 22), 22 were triploids (2n = 3x = 33) and 48 were tetraploids (2n = 4x = 44), accounting for 53.1%, 14.7% and 32.2% of the total, respectively (). For introduced cultivars from the United States and New Zealand, 42% were triploids, 50% were diploids and 8% were tetraploids. For the Chinese cultivars, most were diploids except ‘Baltimore Oriole’ which was a tetraploid. For the wild genotypes from the Taihang Mountain range, 13 were triploids, 16 were diploids and no tetraploid was found.
For the 82 cultivars introduced from the United States, ploidy of 31 cultivars was known. Our repeated experiments confirmed that 22 (71%) were consistent with the original chromosome number, while the rest showed lower ploidy than claimed ().
Table 3 Comparison of known ploidy and detected ploidy of Hemerocallis spp. from the United States.
Classification of karyotype
Karyotypes of eight daylily genotypes were investigated, which included three diploids, three triploids and two tetraploids. The idiograms and the karyotype measurements are presented in and .
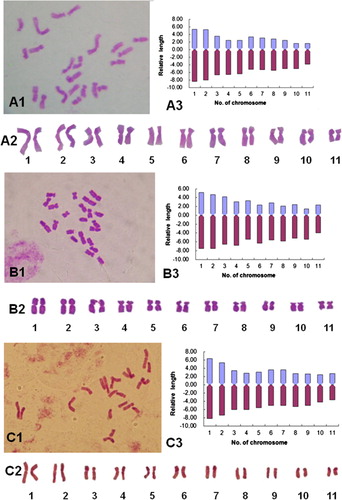
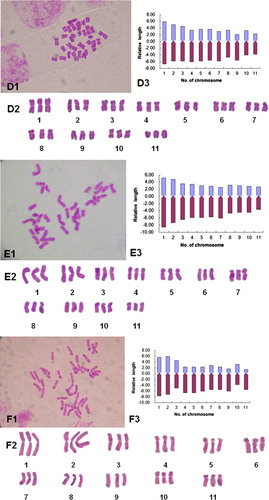
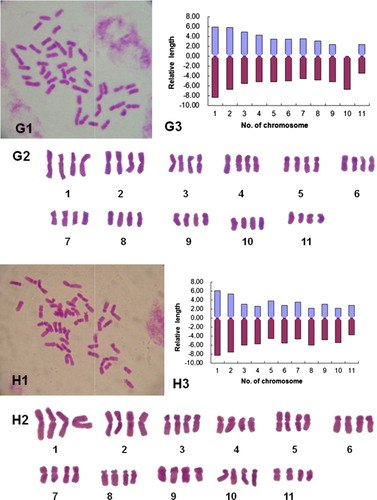
Figure 1 Chromosome morphology and karyotypes of some daylily genotypes. A1–H1, Somatic chromosomes; A2–H2, karyotype images; A3–H3, karyotype idiograms. A, White Temptation; B, NZ–Q; C, Zuoquan No.1; D, Lishan No.1; E, Strawberry Fields Forever; F, NZ–D; G, Diane Taylor, H, NZ–Y.Table 4 Karyometric data of the some daylily genotypes (Hemerocallis spp.).
Cultivars ‘White Temptation’, ‘NZ-Q’ and ‘Zuoquan No.1’ showed a consistent chromosome number of 22. For ‘White Temptation’, the relative chromosome length (percentage of the total) ranged from 5.49 to 13.79, with a length ratio of 2.17. According to their centromeric positions, six chromosomes were metacentric, 14 were submetacentric and two were subtelocentric with a karyotype formula of 2n = 2x = 22 = 6m + 14sm + 2st. Chromosome arm ratio ranged from 1.54 to 3.17, with an average arm ratio of 2.12. The karyotype belongs to ‘2B’ type (–, ).
The karyotype of ‘NZ-Q’ was slightly more asymmetrical than that of ‘White Temptation’, with relative chromosome length ranging from 6.33 to 12.66 and a length ratio of the longest to the shortest chromosome of 2.00. The karyotype formula for ‘NZ-Q’ is 2n = 2x = 22 = 10m + 10sm + 2st. The chromosome morphology, karyotype diagram and type of the karyotype of ‘NZ-Q’ are shown in – and .
The karyotype of ‘Zuoquan No.1’ was similar to that of ‘White Temptation’ with relative chromosome length ranging from 6.35 to 14.55 and a ratio of the longest to the shortest chromosome of 2.29. The karyotype formula is 2n = 2x = 22 = 10m + 12sm. Chromosome arm ratio ranged from 1.29 to 2.21, with an average arm ratio of 1.67. The karyotype belongs to ‘2B’ type (–, ).
Triploid cultivar ‘Strawberry Fields Forever’ showed chromosome relative length of 6.35–13.74, with the ratio of the longest to the shortest chromosome of 2.16. Eighteen chromosomes were metacentric and 15 were submetacentric. Karyotype formula is 2n = 3x = 33 = 18m + 15sm. Chromosome arm ratio ranged from 1.39 to 2.41, with an average arm ratio of 1.75. The karyotype belongs to the ‘2B’ type (–, ).
Triploid cultivar ‘Lishan No.1’ showed chromosome relative length ranging from 6.09 to 12.64, with the ratio of the longest to the shortest chromosome of 2.07. Twenty-four chromosomes were metacentric and nine were submetacentric with a karyotype formula of 2n = 3x = 33 = 24m + 9sm. Chromosome arm ratio ranged between 1.15 and 2.78, with an average arm ratio of 1.62. The karyotype belongs to ‘2B’ type (–, ).
The karyotype of ‘NZ-D’ is slightly different from the rest with its chromosome relative length ranging from 6.91 to 13.38 and the ratio of the longest to the shortest chromosome of 1.94. Twelve of the 33 chromosomes were metacentric, 15 were submetacentric and 6 were subtelocentric with a karyotype formula of 2n = 3x = 33 = 12m + 15sm + 6st. Chromosome arm ratio ranged between 1.23 and 4.17 with an average arm ratio of 2.40. The karyotype belongs to ‘3A’ type (–, ).
The karyotype of tetraploid ‘Diane Taylor’ showed a chromosome relative length of 5.90–14.34, with the length ratio of the longest to the shortest of 2.43. Thirty-six of the 44 chromosomes were metacentric, four were submetacentric and four were subtelocentric with a karyotype formula of 2n = 4x = 44 = 36m + 4sm + 4st. The karyotype belongs to ‘2B’ type (–, ).
The karyotype of tetraploid ‘NZ-Y’ showed a chromosome relative length of 6.53–14.44, with the ratio of the longest to the shortest chromosome of 2.21. Chromosomes 1–2 were large (L), 3–10 were medium-small (M1) and 11 was small (S). Twenty-four of the 44 chromosomes were metacentric and 20 were submetacentric with a karyotype formula of 2n = 4x = 44 = 24m + 20sm. Chromosome arm ratio ranged between 1.20 and 2.24, with an average arm ratio of 1.82. The karyotype belongs to ‘2B’ type (–, ).
Relationships between ploidy levels and plant morphology
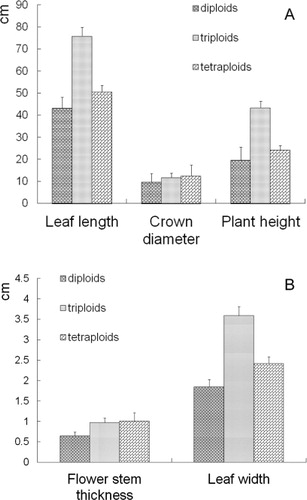
Ploidy of daylily correlated closely with some botanical characteristics of the plants (). Genotypes at different ploidy levels showed significant differences in leaf length, leaf width, plant height, crown diameter and flower stem thickness. T-test indicated that leaf length (75.77 cm) of the triploid was significantly greater than that of the tetraploid (50.47 cm, P < 0.01) and the tetraploid was significantly higher than the diploid (43.15 cm,P < 0.01). Leaf width (3.59 cm (3x), 2.42 cm (4x), 1.84 cm (2x), P < 0.01) and plant height (49.39 cm (3x), 24.27 cm (4x), 19.5 cm (2x), P < 0.01) followed a similar trend. Crown diameter of the triploid (11.75 cm) and tetraploid (12.44 cm) were similar (P > 0.05) but significantly higher than that of the diploid (9.57 cm, P < 0.01). Flower stem thickness of the triploid (0.97 cm) and tetraploid (1.00 cm) were similar (P > 0.05), but significantly thicker than that of the diploid (0.64 cm, P < 0.01). In summary, with the increase of ploidy, flower stem thickness and crown diameter gradually increased, but plant height, leaf length and leaf width of the triploid were the largest, and significantly larger than those of the tetraploid or diploid.
Discussion
Conventional cross-breeding is an important way to create new daylily cultivars. However, through this approach, the genetic diversity of cultivars decreases because of the utilization of limited parents and the selection of desirable traits (Tomkins et al. Citation2001). Although many new cultivars are released, this trend has been increasing (Sakhanokho et al. Citation2004). Therefore, screening and using parents with a wider genetic background is an effective way to address this problem. Correct identification of ploidy of parents to be used in breeding programmes is important to ensure breeding efficiency. The results of our experiment show that the chromosome base number of daylily has remained x = 11 in its long evolution history, but different ploidy levels such as diploid, triploid and tetraploid have evolved. This conclusion is supported by previous research (Wang et al. Citation2009). However, ploidy of some cultivars did not match with previous reported results, such as ‘Baltimore Oriole’ (Wang et al. Citation2009) which was reported as 2n = 3x = 33, whereas our result showed it is a tetraploid with 2n = 4x = 44. It is important to note that ‘Baltimore Oriole’ is an American variety but it has been introduced and cultivated in China for many years compared with the newly introduced cultivars. The authors do not know the real reason for this discrepancy in ploidy but postulate that it could be either a misidentification of the material or a human error in counting the chromosomes. The chance of a within-cultivar variation is minimal. Our research has also shown that this type of mismatch is rather common. Among the 31 cultivars with known ploidy, nine of them (29%) were either misidentified or miscounted (). This serious issue needs to be dealt with carefully by breeders when selecting parents for their breeding programmes. At the same time, the results showed that in the wild daylily from the Taihang Mountains, triploids accounted for 45% and diploids 55%; no tetraploid genotypes were found. This result suggests that triploidy is common in the wild and the triploids probably originated from unreduced gametes, but were not produced by the hybridization of a diploid and a tetraploid since no tetraploid has yet been identified in the natural habitat.
The difference of karyotype between the wild daylilies and horticultural cultivars from outside China is considerably large. In the diploid genotypes, the karyotype asymmetry coefficient of wild genotype ‘Zuoquan No.1’ is 61.6%, which is significantly lower than the horticultural cultivars ‘White Temptation’ (66.3%) and NZ-Q (66.1%). In the triploid genotypes, the karyotype asymmetry coefficient of wild collection ‘Lishan No.1’ (60.3%) is also less than horticultural cultivars ‘Strawberry Fields Forever’ (63.4%) and ‘NZ-D’ (66.3%). Karyotype symmetry or asymmetry can be used to infer the relationship of species (Stebbins Citation1971). Karyotype asymmetry does not vary significantly between diploid and triploid wild genotypes. For example, the karyotype asymmetry coefficient of wild diploid genotype ‘Zuoquan No.1’ is similar to that of the wild triploid genotype ‘Lishan No.1’. This suggests that diploids and triploids in nature have not diverged much. However, karyotype asymmetry of tetraploid cultivars ‘Diane Taylor’ and ‘NZ-Y’ were similar (61.2% and 62.9% respectively), which were between triploid and diploid cultivars, and more similar to wild collections. Indeed, the karyotype of tetraploid ‘NZ-Y’ has high similarity with wild diploid genotype ‘Zuoquan No.1’ apart from the ploidy difference. This might suggest that some tetraploids such as ‘NZ-Y’ might be the direct doubling of diploid genomes.
Generally, increase in ploidy leads to increased gene copy number which may change the transcription product and result in quantity and quality changes of metabolites, hence the change of traits. Our results suggest that plant height, leaf length, leaf width, crown diameter and flower stem thickness of the triploid and tetraploid were significantly higher than those of the diploid. Our results also found that, different materials with the same ploidy level showed a considerable difference, such as that of plant height of triploid ‘Spacecoast Gator Eye’ from the United States, which was significantly lower than that of the triploid ‘Licheng No.3’ from China. This kind of inconsistency was also observed in maize (Zhu Citation1979) and ramie (Yan et al. Citation2003). The variations in plant height, leaf length, leaf width, crown diameter and flower stem thickness among wild types are generally greater than those among cultivars. This may be due to the fact that cultivars were directionally selected for these traits during the breeding process, whereas the wild type is more natural.
The results also showed that leaf length, leaf width and plant height of triploids were greater than tetraploids. This may mean that the number of genomes cannot indefinitely increase through polyploidization, i.e. there is an optimal ploidy level. The expression of the traits cannot be strengthened simply with the ploidy linearly (Song Citation1989). This limitation is also observed in other crops (Zhao et al. Citation1994; Yan et al. Citation2003).
Acknowledgements
This study was supported by grants from Shanxi Provincial Science and Technology Programme of China (20110311017-2 and 20080311010-1) and Shanxi Provincial Hundred Talent Programme.
References
- Arano H 1963. Cytological studies in subfamily Carduoideae (Compositae) of Japan IX. Botanical Magazine 76: 32–39.
- Balao F, Herrera J, Talavera S 2011. Phenotypic consequences of polyploidy and genome size at the micro evolutionary scale: A multivariate morphological approach. New Phytologist 192: 256–65. 10.1111/j.1469-8137.2011.03787.x
- Chen X 1980. Flora Reipublicae Popularis Sinicae 14. Beijing, Science Press. Pp. 52–62. ( in Chinese)
- Hiroyuki S, Keiko M, Shigefumi T, Yukiko A, Masaru N 2003. Ploidy estimation in Hemerocallis species and cultivars by flow cytometry. Scientia Horticulturae 97: 185–192. 10.1016/S0304-4238(02)00150-4
- Huang QC, Qin GY 2008. Research of geminating chromosome sets in Gramineous plants. Beijing, Atomic Energy Press.
- Kong H 1998. A study on karyotype of Hemerocallis fulva var.oppositibracteata. Guihaia 18: 368–370.
- Kong H, Wang CR 1993. A study on karyotype of Hemerocallis in Gansu. Guihaia 13: 247–251.
- Levan A, Fredga K, Sanderg AA 1964. Nomenclacture for centromeric position on chromosomes. Hereditas 52: 197–200.
- Li MX, Chen RY 1985. On the standards of plant karyotyping. Journal of Wuhan Botanical Research 4: 297–302.
- Li ZW, Pinkham L, Campbell NF, Espinosa AC, Conev R 2009. Development of triploid daylily (Hemerocallis) germplasm by embryo rescue. Euphytica 169: 313–318. 10.1007/s10681-009-9958-8
- Li J, Zhang SA 1995. Comparative studies on karyotype of twenty two wild and horticultural species in Hemerocallis. Journal of Shanghai Agricultural College 13: 208–217.
- Masterson J 1994. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264: 421–424. 10.1126/science.264.5157.421
- Matsuoka M 1971. Spontaneous occurrence of triploid Hemerocallis in Japan. Japanese Journal of Breeding 21: 275–284. 10.1270/jsbbs1951.21.275
- Mayfield D, Chen ZJ, Pires JC 2011. Epigenetic regulation of flowering time in polyploids. Current Opinion in Plant Biology 14: 174–178. 10.1016/j.pbi.2011.03.008
- Plodeck J 2002. The origin of the daylily cultivar traits. Hemerocallis Letter 8: 22–28.
- Sakhanokho HF, Cheatham CL, Pounder Jr CT 2004. Evaluation of techniques to induce polyploid daylilies. Southern Nursery Association Proceedings 49: 591–594.
- Singsit C, Veilleux RE 1991. Chloroplast density in guard cells of leaves of anther derived potato plants grown in vitro and in vito. HortScience 26: 592–594.
- Song P, Wang ZA, Wang ZN 1989. The relationship between chromosomal ploidy and stomatal apparatus character in leaves of Gossypium L. Journal of Zhejiang Agricultural University 15: 39–44.
- Stebbins GL 1971. Chromosomal evolution in higher plants. London, Edward Aronld. Pp. 85–104.
- Tomkins JP, Wood TC, Barnes LS, Westman A, Wing RA 2001. Evaluation of genetic variation in the daylily (Hemerocallis spp.) using AFLP markers. Theoretical and Applied Genetics 102: 489–496. 10.1007/s001220051672
- Wang Z, Wang J, Chang G, Chen C. 2009. Chromosome number and karyotype analysis of five varieties of Hemerocallis hybrida Hort. Journal of Tangshan Teachers College 31: 72–74.
- Wang X, Wang H, Wang J, Sun R, Wu J, Liu S et al. 2011. The genome of the mesopolyploid crop species Brassica rapa. Nature Genetics 43: 1035–1039. 10.1038/ng.919
- Wu JH, Ferguson AR, Murray BG, Jia Y, Datson PM, Zhang J 2012. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Annals of Botany 109: 169–179. 10.1093/aob/mcr256
- Yan CG, Cao RF, Li ZD, Zheng SX, Cui GX 2003. Studies on morphological characters, yield and quality traits of different ploidy materials of ramie. Scientia Agricultura Sinica 36: 628–632.
- Zhao LN, Pan CL, Zang GG, Luo SY, Sun HL 1994. A comparative study on the fiber shape and quality between tetraploid and diploid ramie. China's Fiber Crops 16: 74–77.
- Zhu QL 1979. Induced single sex spores of maize and its characters. Journal of Northwest Agricultural College 4: 45–57.