Abstract
To better understand the role of nitric oxide (NO) in mango fruit ripening, fruit that was pretreated with salicylic acid (SA) and NO was stored at 25 °C for 11 days. Fruit firmness, ethylene production, total soluble solids (TSS), titratable acidity (TA) and vitamin C content were monitored. The expression of six genes involved in ethylene biosynthesis and signalling, namely, MiACO, MiACS, MiETR1, MiERS1, MiEIN2 and MiERF, was investigated using real-time qPCR. The results showed that the treatment caused significant inhibition of the firmness decrease and ethylene output, maintained relatively high levels of TSS and vitamin C, and delayed the decrease in the TA. Furthermore, MiACO and MiERS1 were significantly downregulated, whereas MiETR1 and MiEIN2 were upregulated, and MiACS and MiERF exhibited fluctuating expression patterns. The findings of the study suggested that MiACO, MiACS, MiETR1, MiERS1, MiEIN2 and MiERF might participate in the SA and NO signal induction pathways during ripening.
Introduction
Mango (Mangifera indica L.) is one of the most important tropical fruits in the world, and its worldwide annual production is 35.9 million t, worth US$ 2150 million (FAOSTAT Citation2010). Additionally, mango is a climacteric fruit that exhibits a characteristic rise in ethylene production and respiration rate during fruit ripening. These changes are accompanied by alterations in colour, aroma volatiles and softness (Singh & Zaharah Citation2010), thereby resulting in enhanced perishability during storage at ambient temperature. In practice, the fruit is generally harvested green and consumed after a period of storage. However, rapid fruit softening after harvest (Suntornwat et al. Citation2000) and poor handling practices during postharvest operations, such as tight fruit packing, inadequate transport and rough fruit handling (Zúñiga-Arias et al. Citation2009), lead to postharvest decay and affect mango quality. To extend storage life, avoid possible losses and improve the quality of mango fruit, it is necessary to impede fruit softening and ripening after harvest. Until now, various methods have been developed to slow the ripening process, including calcium treatment (Singh et al. Citation1993), edible coating (Feygenberg et al. Citation2005), heat treatment (Benitez et al. Citation2006), 1-methylcyclopropene (1-MCP) application (Wang et al. Citation2009), the use of controlled atmosphere (CA) (Singh & Zaharah Citation2010), nitric oxide (NO) fumigation (Zaharah & Singh Citation2011a,Citationb,Citationc), combination of 1-MCP treatment and CA storage (Sivakumar et al. Citation2012) and oxalate treatment (Zheng et al. Citation2012). However, there remains a need for more effective techniques to inhibit fruit softening and ripening during storage.
NO acts as a multifunctional signalling molecule in plants and is involved in many physiological processes, including the ripening of climacteric and non-climacteric fruit (Leshem & Wills Citation1998; Leshem & Pinchasov Citation2000). Postharvest exogenous application of NO has been demonstrated to delay fruit ripening in a range of horticultural crops, such as strawberries (Wills et al. Citation2000; Zhu & Zhou Citation2005), avocados (Leshem & Pinchasov Citation2000), carnations (Bowyer et al. Citation2003), mangoes (Zaharah & Singh Citation2010) and Chinese bayberries (Wu et al. Citation2012).
Salicylic acid (SA) is seen as an inducer of systemic acquired resistance and one of the important signal molecules involved in disease resistance in plants (Alvarez Citation2000; Desveaux et al. Citation2004). In addition, growing evidence suggests that NO interacts with SA in plants. For example, it has been demonstrated that the treatment of tobacco and Arabidopsis leaves with NO resulted in the induction of a substantial increase in endogenous SA (Durner et al. Citation1998; Huang et al. Citation2004). In addition, the effect of SA in inducing NO in Arabidopsis was also observed (Zottini et al. Citation2007).
An increase in ethylene production is observed before the initiation of ripening, and ethylene is a trigger of the ripening process in climacteric fruits (Oeller et al. Citation1991). The ethylene biosynthesis pathway has been well established in higher plants. Ethylene is biosynthesized by 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO), which catalyse the reactions from S-adenosylmethionine (SAM) to ACC and from ACC to ethylene, respectively (Yang & Hoffman Citation1984). After synthesis, ethylene is perceived by a family of membrane-localized receptors, and then the signal is mediated downstream by members of different genes families, including CTR1, EIN2, EIN3/EILs and ERFs (Chang & Stadler Citation2001; Chen et al. Citation2005), and then the signal passes through a partially elucidated cascade that ultimately influences a myriad of ethylene-associated plant growth and development processes (Wang et al. Citation2002).
The ‘Zill’ cultivar of mango, grown in Southern China, is considered to be one of the best mango varieties as it has a colourful skin, is fibreless, has a delicious taste and aroma, and has a very high pulp content. However, the consumption of this variety is limited, because of rapid ripening after harvest. Ethylene plays a key role in promoting fruit ripening, so alteration of its biosynthesis/signalling could be an important means of delaying this process. NO as a signal molecule has been shown to interfere with ethylene's effects to direct and to significantly influence fruit ripening (Manjunatha et al. Citation2010). Although previous studies have indicated that NO treatment could delay the fruit ripening of ‘Kensington Pride’ mangoes during storage at 5 ± 1 °C (Zaharah & Singh Citation2011a), to the best of our knowledge, no research work has reported the influences of pre- and postharvest SA and NO on the ripening and quality attributes of postharvest mango fruit (cv. Zill) stored at ambient temperature. Hence, one of the objectives of the present work was to evaluate the effect of combining the two treatment technologies (SA + NO) on the softening, ethylene production and quality of fruit. Another objective of this work was to analyse the transcriptional regulation of the genes involved in the ethylene biosynthesis (MiACO and MiACS) and signalling pathway (MiETR1, MiERS1, MiEIN2 and MiERF) in fruit treated with SA + NO during storage at 25 °C.
Materials and methods
Plant material and experimental design
Nine 8-year-old mango trees (Mangifera indica L. cv. Zill) from an orchard in the South Subtropical Crops Research Institute, Zhanjiang, China were chosen for this experiment. Approximately 400 pieces of fruit picked 100 days after anthesis (DAA) and located in different areas of each tree were selected on the basis of size and the absence of physical damage. The selected pieces of fruit were tagged and treated with SA. Each piece of fruit was sprayed with a solution of 150 µM SA with a hand-sprayer until the fruit was wet to runoff. Control fruits were sprayed with distilled water. Fruit treated with either SA or water were collected separately at 130 DAA and divided further into four groups (100 pieces of fruit each) and immersed for 30 min in a solution containing of 0 (control) or 100 µM sodium nitroprusside (SNP) (NO donor) (Sigma-Aldrich Corp, St Louis, MO, USA), respectively. Pre- and postharvest treatments were conducted as described in .
Table 1 Treatments of mango fruit with pre- and postharvest SA and NO.
All the fruit were allowed to dry for 30 min at room temperature and then placed into unsealed plastic bags (0.04 mm thick). Each bag contained five individual pieces of fruit with 20 bags per group and these were stored in four incubators (Sanyo MIR 553 Model, Gunma, Japan) held at 25 °C (with 90% − 95% RH) for 11 days. All assessments were conducted with three replicates.
Flesh firmness
The firmness of the fruit flesh was measured every day using a handheld ‘penetrometer’ (FT-327; UC Fruit Firmness Tester, Milano, Italy) equipped with a probe 8 mm in diameter. A small slice of fruit skin was removed and the firmness was recorded from three different pieces of fruit with three different points per fruit. The means were expressed in (kg cm−2).
Determination of ethylene production rate
The ethylene production rate was measured by enclosing four pieces of mango fruit in an airtight container equipped with a rubber stopper for 2 h at 25 °C. A 1 mL gas sample was withdrawn from the headspace of the containers with a syringe and then injected into a gas chromatograph (Model GC-17A, Shimadzu Co, Kyoto, Japan) fitted with a flame-ionization detector and an activated alumina column (200 × 0.3 cm) with an injector temperature of 120 °C, a column temperature of 60 °C and a detector temperature of 60 °C. Helium was used as a carrier gas at a flow rate of 30 mL min−1. Three replicates were used and the ethylene production rate was expressed as µL C2H4 kg−1 h−1.
RNA extraction and RT-PCR
Mango peels that were frozen in liquid nitrogen were ground using a mortar and pestle. Total RNA was extracted using the hot borate method of Wan & Wilkins (Citation1994). Potentially contaminating DNA was eliminated by treatment with DNA-free DNase I digestion (Takara Biotechnology, Dalian, China). The DNA-free total RNA was then used as a template for RT-PCR. The first-strand cDNA of the product was subjected to PCR amplification.
Quantitative real-time PCR analysis
Total RNA from the samples was isolated and the first-strand cDNA was synthesized as described above. For real-time PCR, oligonucleotide primers were designed on the basis of the sequence of each gene using Primer 5.0 software. All primers were tested with melting peaks and dissociation curves to confirm that there was only one product for each pair of primers. To verify that primers were specific for amplification of the target genes, all PCR products were purified and resequenced. The sequences of all primers used for qPCR are described in . Miactin in mango fruit was used as the housekeeping gene to quantify the cDNA abundance.
Table 2 Primer sequences of genes related to ethylene biosynthesis and signalling used for quantitative real-time PCR.
The synthesized cDNA was diluted 1:40 with water, and 2 µL of the diluted cDNA was used as a template for quantitative real-time PCR analysis. PCR was performed in a total volume of 20 µL, 1 µL for each primer (10 µM, final concentration 200 nM) and 10 µL for SYBR Green PCR Supermix (Bio-Rad Laboratories) on a Bio-Rad CFX96 Real-Time PCR System according to the manufacturer's instructions. The RT-qPCR programme included an initial denaturation step at 94 °C for 5 min, followed by 40 cycles of 10 s at 94 °C, 30 s at 55 °C and 30 s at 72 °C. No-template controls for each primer pair were included in each run. All RT-qPCR reactions were normalized using the Ct value corresponding to the reference gene. The relative expression levels of the target gene were calculated with the formula 2−ΔΔ CT (Livak & Schmittgen Citation2001). Values represented the average of three biological replicates.
Measurements of the TSS, TA and vitamin C content of fruit flesh
The TSS of each sample of fruit flesh was measured by a digital refractometer (ATC-20E; Atago, Yokyo, Japan) and expressed as a percentage.
The TA of the fruit flesh tissue (2 g) was measured by titration to pH 8.1 with 0.1 M NaOH using phenolphthalein as an indicator. The TA was expressed as % citric acid equivalent.
The vitamin C levels of the fruit flesh were measured based on the method described by Hong et al. (Citation2012). The results were expressed as mg per 100 g fresh weight (FW).
Statistical analysis
The data were subjected to Sigmastat (release 3.5; Systat Software Inc, Point Richmond, California, USA) using two-way analysis of variance (ANOVA) including treatments and storage time. The effects of various treatments and storage time were assessed using two-way ANOVA, and Fisher's least significant difference (LSD) test was calculated using a significant value of P ≤ 0.05. The LSD value was indicated in each case. The data values were expressed as the mean ± SE (n = 3).
Results
Fruit firmness
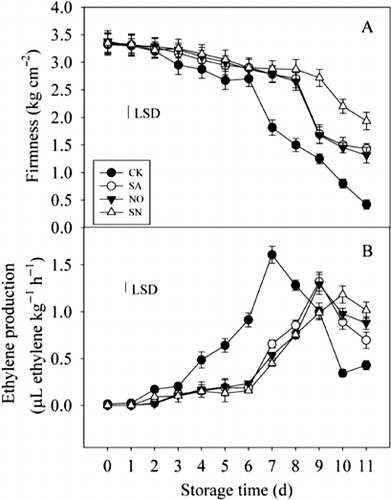
Fruit firmness is one of the most common physical parameters used to assess the process of fruit softening and ripening. Changes in mango flesh firmness with four different treatments, comprising control, SA, NO and a combination of SA and NO (SN) are shown in . The untreated control fruit softened rapidly within the first 7 days during storage at 25 °C, with a significant difference noticed between treated fruit and untreated control samples on day 3. The decrease in firmness was approximately 1.82-fold from 3.32 kg cm−2 on day 0 to 1.82 kg cm−2 on day 7, and the firmness decreased further to 0.42 kg cm−2 on day 11. However, fruit softening was greatly inhibited by the SA, NO and SN treatment within the first 7 days of storage, only declining by 1.20-, 1.22-, 1.16-fold, respectively, compared with the initial values of samples, with no significant difference observed among them. However, the firmness in fruit that received the SN treatment showed a notable effect in comparison with samples treated with SA and NO after 8 days in storage, and the SN treatment resulted in significantly higher fruit firmness than did other treatments at the end of storage. Additionally, the untreated control fruit began to soften sharply on day 7 in storage, whereas softening in the fruit that received SA, NO and SN treatments was delayed by 2, 2 and 4 days, respectively. The interactions between treatments and storage time were significant for fruit firmness.
Ethylene production
The ethylene production rate exhibited a typical climacteric pattern during mango fruit ripening at 25 °C (). Production in untreated control fruit increased rapidly and reached the maximum value on day 7, approximately 115-fold compared with day 0, and then decreased quickly. Ethylene production in SA-, NO- and SN-treated fruit was detectable from day 2 after treatment. Ethylene production in fruit treated with SA, NO or SN peaked on days 9, 9 and 10, respectively, and was approximately 82%, 80% and 74% of the values of the control fruit at day 7. Applications of SA, NO and SN treatments not only delayed the onset of ethylene releases but effectively suppressed the ethylene peak values. Moreover, the SN treatment showed a pronounced reduction in ethylene production at 9 days of storage in comparison with the SA treatment, with no significant difference between the NO and SA treatment during storage. There was a significant interaction between treatments and storage time for ethylene production.
Expressions of the ethylene biosynthesis of the genes MiACS and MiACO
Expression patterns of MiACS and MiACO in mango peels after different treatments were analysed by RT-qPCR. As shown in , the expression of MiACS in control fruit continuously increased, peaking on day 9, then decreased rapidly, and a significant increase in MiACS gene expression levels was found during fruit ripening. The expression level of MiACS in SA-treated fruit was lower than that of untreated control fruit during the storage period, with the exception of day 5. With increasing expression of MiACS, no significant difference was found from day 3 to day 7 between NO-treated and untreated control fruit. The expression level of MiACS was reduced during later storage; however, its expression level increased significantly from day 3 to day 5 with SN treatment compared with untreated control fruit.
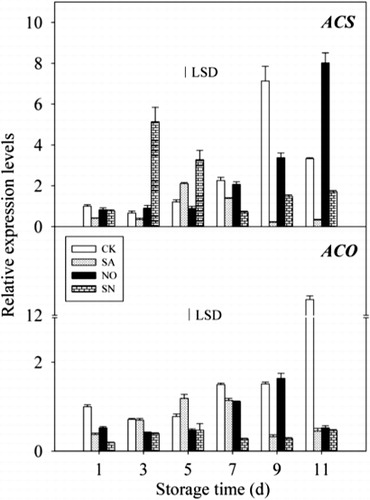
Unlike the expression pattern of MiACS, the transcript level of MiACO in control fruit increased during the whole storage period. The transcript levels of MiACO in SA-treated fruit slightly increased from day 1 to day 5 of storage and then decreased. The transcript levels of MiACO in fruit treated with NO and SN were lower than that in untreated control fruit, with significant difference observed between them. In addition, the transcript level of MiACO in fruit treated with SN was the lowest among the different conditions and remained almost unchanged over the storage period. These results indicated that NO, especially SN treatment, significantly inhibited the transcription of MiACO in fruit ().
Expressions of ethylene receptors of genes MiETR1 and MiERS1
The expressions of two ethylene receptor of genes MiETR1 and MiERS1 in mango peel are presented in . Although MiETR1 in all fruit showed a fluctuating expression pattern, the levels of MiETR1 mRNA in untreated control fruit almost remained lower than those in fruit treated with SA, NO and SN throughout the storage period. In addition, significant differences were observed between NO- and SN-treated fruit and untreated controls from day 1 to day 5.
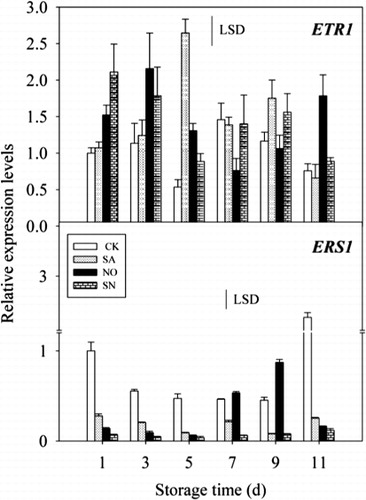
As shown in , a decreasing level of MiERS1 transcript was found in control fruit from day 1 to day 5, reached a minimum at day 7 when ethylene production attained its maximum, and then increased. Moreover, decreasing transcripts of MiERS1 in treated fruit were also noticed during the same storage period. Additionally, the amount of MiERS1 transcript in treated fruit was lower than that in untreated controls, with a significant difference occurring between them apart from that in NO-treated samples on days 7 and 9.
Expressions of the genes MiEIN2 and MiERF
MiEIN2 and MiERF are the two important transcription factors in the ethylene-signalling pathway. The effect of SA, NO and SN treatments on mRNA accumulation was investigated in mango peel ().
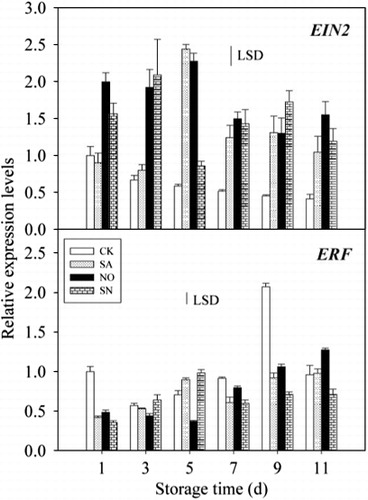
The amount of MiEIN2 mRNA in untreated control fruit remained almost unchanged during the storage period. The levels of MiEIN2 mRNA in NO- and SN-treated fruit first increased and then decreased, but the expression levels were obviously higher than those in untreated control fruit over the storage period. However, the SA treatment only significantly influenced the expression of MiEIN2 compared with untreated control fruit from day 5 to day 11.
The accumulation of MiERF transcripts in untreated control fruit first increased from day 3 to day 9 of storage and then rapidly decreased. MiERF transcripts gradually increased in fruit treated with NO, but the amount expressed was lower than that of untreated controls, with a significant difference observed between them. MiERF transcripts in SN-treated fruit were also markedly lower than those in untreated controls, except for days 3 and 5 during storage.
Changes of the TSS, TA and vitamin C in fruit flesh
TSS is often used as an indicator of the fruit quality and maturity level. Changes in the TSS in mango fruit with different treatments and storage at 25 °C for 11 days are shown in . The TSS content in untreated control fruit increased rapidly, peaking on day 7, and then declined. However, the TSS content in treated fruit increased slowly up to 9 days. There was a significant difference observed between untreated and treated fruit on day 3. After 11 days of storage, the value of TSS in SN-treated fruit remained the highest among the treated fruit, and there was a significant difference between fruit treated with SA and SN.
Table 3 Changes in TSS (oBrix), TA (as % citric acid) and vitamin C (mg 100g−1 FW) of mango (Mangifera indica cv. Zill) fruit treated with pre- and postharvest SA and NO during storage at 25 °C for 11days.
The TA values in untreated control fruit increased, reaching a maximum on day 3, and then declined until the end of the storage period. The TA content in SA-, NO- and SN-treated fruit slowly increased, peaking on days 7, 5 and 7, respectively, and thereafter decreased. With significant changes in the TA in untreated and treated fruit, a significant difference occurred between them on day 5 ().
Vitamin C in fruit flesh first increased and then decreased in all fruit as the storage time increased and this reduction was effectively inhibited by the SA, NO and SN treatments. As shown in , the loss of vitamin C was delayed in treated fruit compared with the untreated controls during storage and a significant difference between them was found after 7 days of storage. In addition, fruit treated with SN retained a higher vitamin C content than those with SA and NO during storage.
Discussion
Firmness is one of the most important physiochemical parameters in fruit during storage, which is significantly influenced by ripening. The results presented in this study indicate that mango fruit treated with SA, NO or SN maintained a higher level of firmness compared with untreated control samples at all storage intervals. In particular, the SN treatment was more effective in delaying fruit softening during storage at 25 °C, suggesting that the SN treatment was useful for delaying mango fruit ripening during storage at ambient temperature.
The ripening processes of climacteric fruit were delayed by restraining ethylene biosynthesis (Feng et al. Citation2004; Hong et al. Citation2004). In the present study, we observed that NO, especially the SN treatment, effectively inhibited the ethylene production and delayed ripening and senescence in mango fruit. Similarly, the inhibition of ethylene synthesis with NO treatment has been reported in different fruits, such as strawberries (Zhu & Zhou Citation2007), peaches (Flores et al. Citation2008), bananas (Cheng et al. Citation2009), tomatoes (Eum et al. Citation2009), mangoes (Zaharah & Singh Citation2011b) and papayas (Li et al. Citation2014). NO could effectively prevent the autocatalytic ethylene biosynthesis. For example, Zaharah & Singh (Citation2011b) reported that the activities of ACS and ACO in ‘KP’ mango pulp were inhibited by NO-fumigation at ambient temperature and during cool storage. It has been suggested that the probable mechanism involves the binding of NO to ACO to form a binary ACO-NO complex, which is chelated by ACC to produce a stable ACC-ACO-NO. This ternary complex, in turn, causes a decrease in the ethylene production (Zhu, Liu & Zhou Citation2006). Yet another mode of the NO regulation of ethylene is through regulation of the effect of H2O2, the latter being an effective inducer of ethylene biosynthetic gene transcription (Jakubowicz et al. Citation2010). To investigate the regulatory mode of NO in ethylene biosynthesis in mango fruit, we examined the expression of ACO and ACS genes by RT-qPCR.
As reported, ACO and ACS are two critical enzymes involved in the ethylene biosynthetic pathway (Yang & Hoffman Citation1984) and both are encoded by multigene families in various plant organs. The expression of ACS and ACO genes is regulated by many factors, including hormones, senescence and ripening. The present data showed that the accumulation of MiACS mRNA was not affected, but MiACO mRNA was reduced by the NO and SN treatments. The suppression of ethylene production with NO treatment may possibly be attributed to a reduced accumulation of MiACO mRNA. Similar results have been obtained with tomatoes (Eum et al. Citation2009) and bananas (Cheng et al. Citation2009) in which NO delayed the expressions of homologues of ACO but not ACS. Therefore, it is possible that the ACC-ACO-NO mechanism of the NO-mediated inhibition of ethylene biosynthesis, according to a previous report in peaches (Zhu, Liu & Zhou Citation2006), also exists in mango fruit; however, the precise mode of NO action in this process is not clear and merits further study.
Ethylene receptors function as negative regulators of ethylene responses, and hormones inactivate them (Bleecker & Kende Citation2000). Genetic and biochemical studies using a number of mutants in Arabidopsis and tomato have shown that the receptors act redundantly to suppress ethylene responses in the absence of ethylene (Klee & Tieman Citation2002). Moreover, ethylene receptors genes have been identified in many plant species, and there is much evidence to show that ethylene receptors are regulated by tissues, developmental stage and external stimuli (Lashbrook et al. Citation1998; Sato-Nara et al. Citation1999). It was found that MiERS1 and MiETR1 were downregulated in the mesocarp of mango (cv. Kent) fruit during ripening (Ish-Shalom et al. Citation2011), whereas MdERS1 and MdERS2 in apple fruit (Malus domestica Borkh.) (Yang et al. Citation2013) and LeETR4 in tomatoes (Tieman et al. Citation2000) were upregulated during ripening. Examination of mango ethylene receptors in our experiment showed that the accumulation of MiETR1 mRNA was induced whereas MiERS1 mRNA was suppressed by SN treatment during the storage period. Similar accumulation patterns of MiETR1 and MiERS1 mRNA were found in fruit treated with NO from 1 to 5 days of storage; thereafter, their expressions showed a fluctuating pattern. However, Yang et al. (Citation2010) reported that NO treatment significantly repressed the expression of LeETR4 in tomato fruit during ripening.
EIN2 has been proven to act as a bi-functional signal transducer that mediates the signal propagation between CTR1 and downstream components (EIN3/EIL). EIN2 is an integral membrane protein whose function is not understood (Alonso et al. Citation1999). For example, in cut carnation flowers, the expression of DcEIN2 was enhanced by ethylene treatment, whereas in petunias, PhEIN2 was regulated by ethylene in a tissue-specific manner (Shibuya et al. Citation2004). In rice (Jun et al. Citation2004) and tomato fruit (Zhu et al. Citation2006), the expression of EIN2 was not regulated by ethylene. Our work has shown that no significant difference in the expression of MiEIN2 was found during ripening. This result is similar to the result of Yang et al. (Citation2013) in apple fruit. However, NO treatment enhanced the expression of the EIN2 gene compared with the untreated control. Thus, the precise regulation mechanism of NO on the MiEIN2 gene still needs to be explored further.
The ERF families of transcription factors act as positive controllers of the ethylene response and potentially are involved in cross talk with other signals (Guo & Ecker Citation2004). Our result indicated that the expression of ERF was enhanced during ripening. Similar results were obtained with the earlier report of Li et al. (Citation2007) and Yang et al. (Citation2013) showing that in tomatoes, the over-expression of LeERF1 shortens fruit postharvest life, and in apples, MdERF1 and MdERF2 are expressed during ripening. The expression of the ERF gene in NO-treated fruit was downregulated during storage. Although the expression of the ERF gene in SN-treated fruit was slightly upregulated from day 3 to day 5, its expression was evidently downregulated in the late stage of storage.
We also observed the changes in other quality parameters, including TSS, TA and vitamin C in treated mango fruit during storage. Our result indicated that there was a lower increase of the TSS content of mango treated with NO and SN during storage at 25 °C. Similar results have been reported by Zhu, Liu & Zhou (Citation2006, Citation2009) and Li et al. (Citation2014) who observed a delay in the increase of the TSS in peaches, Chinese winter jujubes and papayas treated with NO during storage at 25, 20 and 22 °C, respectively. However, Singh et al. (Citation2009) reported that NO had no significant effect on the TSS in Japanese plums during ripening.
Citric acid is one of the major organic acids in mango fruit. We noticed that the NO and SN treatments delayed the decrease in the TA in mango fruit. A controversy has developed around the effect of NO on the TA after harvest for different fruits. Duan et al. (Citation2007) reported that NO had no significant effect on the TA in longan fruit after 6 days of storage at 28 °C, whereas NO delayed the increase in the TA in Japanese plum during ripening (Singh et al. Citation2009) and did not significantly affect the citric acid concentration during ripening in cold-stored mango fruit (Zaharah & Singh Citation2011a).
Based on the result of our study, the NO and SN treatment maintained high levels of vitamin C in mango fruit, which is consistent with recent findings in other fruit systems, such as longan (Duan et al. Citation2007). In contrast, Zaharah & Singh (Citation2011a) reported that NO treatment did not affect the concentration of ascorbic acid during ripening in cold-stored mango fruit.
In conclusion, our results indicated that pre- and postharvest SA and NO treatments suppressed the expression of genes related to ethylene biosynthesis and signalling, including ACO and ERS1, while enhancing the expression of the genes ETR1 and EIN2 during storage. This effect, in turn, may have delayed and reduced ethylene production and the fruit ripening process. Moreover, the treatment could retard the increase in firmness, maintain high levels of TSS and vitamin C, and delay the decrease in TA. Thus, it is suggested that the application of a combination of SA and NO treatments may be a promising method for extending shelf life and maintaining the quality of mango fruit.
Acknowledgements
This research was supported by the Chinese Special Fund of Basic Scientific Research Projects for State Level and Public Welfare–Scientific Research Institutes (1251022011003, 1630062013018) and the Hainan Province Science Foundation (No. 310100).
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR 1999. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152. 10.1126/science.284.5423.2148
- Alvarez ME 2000. Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Molecular Biology 44: 429–442. 10.1023/A:1026561029533
- Benitez MM, Acedo Jr AL, Jitareerat P, Kanlavanarat S 2006. Mango fruit softening response to postharvest heat treatment. Acta Horticulturae 712: 811–816.
- Bleecker AB, Kende H 2000. Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biology 16: 1–18. 10.1146/annurev.cellbio.16.1.1
- Bowyer MC, Wills RBH, Badiyan D, Ku VVV 2003. Extending the postharvest life of carnations with nitric oxide–comparison of fumigation and in vivo delivery. Postharvest Biology and Technology 30: 281–286. 10.1016/S0925-5214(03)00114-5
- Chang C, Stadler R 2001. Ethylene hormone receptor action in Arabidopsis. BioEssays 23: 619–627. 10.1002/bies.1087
- Chen YF, Etheridge N, Schaller GE 2005. Ethylene signal transduction. Annals of Botany 95: 901–915. 10.1093/aob/mci100
- Cheng G, Yang E, Lu W, Jia Y, Jiang Y, Duan X 2009. Effect of nitric oxide on ethylene synthesis and softening of banana fruit slice during ripening. Journal of Agricultural and Food Chemistry 57: 5799–5804. 10.1021/jf901173n
- Desveaux D, Subramaniam R, Despres C, Mess JN, Levesque C 2004. A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Developmental Cell 6: 229–240. 10.1016/S1534-5807(04)00028-0
- Duan XW, Su XG, You YL, Qu HX, Li YB, Jiang YM 2007. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chemistry 104: 571–576. 10.1016/j.foodchem.2006.12.007
- Durner J, Wendehenne D, Klessig DF 1998. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proceedings of the National Academy of Sciences 95: 10328–10333. 10.1073/pnas.95.17.10328
- Eum HL, Kim HB, Choi SB, Lee SK 2009. Regulation of ethylene biosynthesis by nitric oxide in tomato (Solanum lycopersicum L.) fruit harvested at different ripening stages. European Food Research and Technology 228: 331–338. 10.1007/s00217-008-0938-3
- FAOSTAT 2010. Area harvested and production of mango (including mangosteen and guava). http://faostat.fao.org/ (accessed 30 July 2012).
- Feng X, Apelbaum A, Sisler EC, Goren R 2004. Control of ethylene activity in various plant systems by structural analogues of 1-methylcyclopropene. Plant Growth Regulation 42: 29–38. 10.1023/B:GROW.0000014900.12351.4e
- Feygenberg O, Hershkovitz V, Ben-Arie R, Jacob S, Pesis E, Nikitenko T 2005. Postharvest use of organic coating for maintaining bio-organic avocado and mango quality. Acta Horticulturae 682: 507–512.
- Flores F, Sánchez-Bel P, Valdenegro M, Romojaro F, Martínez-Madrid M, Egea M 2008. Effects of a pretreatment with nitric oxide on peach (Prunus persica L.) storage at room temperature. European Food Research and Technology 227: 1599–1611. 10.1007/s00217-008-0884-0
- Guo H, Ecker JR 2004. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology 7: 40–49. 10.1016/j.pbi.2003.11.011
- Hong JH, Cowan AK, Lee SK 2004. Glucose inhibits ACC oxidase activity and ethylene biosynthesis in ripening tomato fruit. Plant Growth Regulation 43: 81–87. 10.1023/B:GROW.0000038248.54232.6a
- Hong KQ, Xie JH, Zhang LB, Sun DQ, Gong DQ 2012. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Scientia Horticulturae 144: 172–178. 10.1016/j.scienta.2012.07.002
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J 2004. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 218: 938–946. 10.1007/s00425-003-1178-1
- Ish-Shalom M, Dahan Y, Maayan I, Irihimovitch V 2011. Cloning and molecular characterization of an ethylene receptor gene, MiERS1, expressed during mango fruitlet abscission and fruit ripening. Plant Physiology and Biochemistry 49: 931–936. 10.1016/j.plaphy.2011.05.010
- Jakubowicz M, Galganska H, Nowak W, Sadowski J 2010. Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. Journal of Experimental Botany 61: 3475–3491. 10.1093/jxb/erq177
- Jun SH, Han MJ, Lee S, Seo YS, Kim WT, An G 2004. OsEIN2 is a positive component in ethylene signaling in rice. Plant and Cell Physiology 45: 281–289. 10.1093/pcp/pch033
- Klee H, Tieman D 2002. The tomato ethylene receptor gene family: form and function. Physiologia Plantarum 115: 336–341. 10.1034/j.1399-3054.2002.1150302.x
- Lashbrook CC, Tieman DM, Klee HJ 1998. Differential regulation of the tomato ETR gene family throughout plant development. The Plant Journal 15: 243–252. 10.1046/j.1365-313X.1998.00202.x
- Leshem YY, Pinchasov Y 2000. Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripeneing of strawberries Fragaria anannasa (Dutch.) and avocados Persea americana (Mill.). Journal of Experimental Botany 51: 1471–1473. 10.1093/jexbot/51.349.1471
- Leshem YY, Wills RBH 1998. Harnessing senescence delaying gases nitric oxide and nitrous oxide, a novel approach to postharvest control of fresh horticultural produce. Biologia Plantarum 41: 1–10. 10.1023/A:1001779227767
- Li XP, Wu B, Guo Q, Wang JD, Zhang P, Chen WX 2014. Effects of nitric oxide on postharvest quality and soluble sugar content in papaya fruit during ripening. Journal of Food Processing and Preservation 38: 591–599. 10.1111/jfpp.12007
- Li YC, Zhu BZ, Xu WT, Zhu HL, Chen AJ, Xie YH et al. 2007. LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Reports 26: 1999–2008. 10.1007/s00299-007-0394-8
- Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. 10.1006/meth.2001.1262
- Manjunatha G, Lokesh V, Neelwarne B 2010. Nitric oxide in fruit ripening: trends and opportunities. Biotechnology Advances 28: 489–499. 10.1016/j.biotechadv.2010.03.001
- Oeller PW, Min-Wong L, Taylor LP, Pike DA, Theologis A 1991. Reversible inhibition of tomato fruit senescence by antisense RNA. Science 254: 437–439. 10.1126/science.1925603
- Sato-Nara K, Yuhashi KI, Higashi K, Hosoya K, Kubota M, Ezura H 1999. Stage- and tissue-specific expression of ethylene receptor homolog genes during fruit development in muskmelon. Plant Physiology 120: 321–330. 10.1104/pp.120.1.321
- Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, et al. 2004. The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiology 136: 2900–2912. 10.1104/pp.104.046979
- Singh SP, Singha Z, Swinny EE 2009. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biology and Technology 53: 101–108. 10.1016/j.postharvbio.2009.04.007
- Singh BP, Tandon DK, Kalra SK 1993. Changes in postharvest quality of mangoes affected by preharvest application of calcium salts. Scientia Horticulturae 54: 211–219. 10.1016/0304-4238(93)90089-9
- Singh Z, Zaharah SS 2010. Controlled atmosphere storage of mango fruit–an overview. IX International Mango Symposium 992: 481–492.
- Sivakumar D, Deventer FV, Terry LA, Polantad GA, Korstenb L 2012. Combination of 1-methylcyclopropene treatment and controlled atmosphere storage retains overall fruit quality and bioactive compounds in mango. Journal of the Science of Food and Agriculture 92: 821–830. 10.1002/jsfa.4653
- Suntornwat O, Lertwikoon N, Bungaruang L, Chaimance P, Speirs J 2000. Cloning and characterization of a putative endopolygalacturonase cDNA from ripening mango (Mangifera indica Linn cv, Nam Dok Mai). Acta Horticulturae 509: 153–158.
- Tieman DV, Taylor MG, Ciardi JA, Klee HJ 2000. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proceedings of the National Academy of Sciences 97: 5663–5668. 10.1073/pnas.090550597
- Wan CY, Wilkins TA 1994. A modified hot borate method significantly enhances the yield of high quality RNA from cotton (Gossypium hirsutum L.). Analytical Biochemistry 223: 7–12. 10.1006/abio.1994.1538
- Wang KLC, Li H, Ecker JR 2002. Ethylene biosynthesis and signaling networks. Plant Cell 14: S131–S151.
- Wang B, Wang J, Feng X, Lin L, Zhao Y, Jiang W 2009. Effects of 1-MCP and exogenous ethyleneonfruit ripening andantioxidants in stored mango. Plant Growth Regulation 57: 185–192. 10.1007/s10725-008-9335-y
- Wills RBH, Ku VVV, Leshem YY 2000. Fumigation with nitric oxide to extend the postharvest life of strawberries. Postharvest Biology and Technology 18: 75–79. 10.1016/S0925-5214(99)00061-7
- Wu FH, Yang HQ, Chang YZ, Cheng JY, Bai SFX, Yin JY 2012. Effects of nitric oxide on reactive oxygen species and antioxidant capacity in Chinese Bayberry during storage. Scientia Horticulturae 135: 106–111. 10.1016/j.scienta.2011.12.011
- Yang SF, Hoffman NE 1984. Ethylene biosynthesis and its regulation in higher plants.Annual Review of Plant Physiology 35: 155–189. 10.1146/annurev.pp.35.060184.001103
- Yang XT, Song J, Campbell-Palmer L, Fillmore S, Zhang ZQ 2013. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biology and Technology 78: 55–66. 10.1016/j.postharvbio.2012.11.012
- Yang HQ, Wu FH, Chang YZ 2010. Effects of nitric oxide on postharvest ripening and Le-ETR4 expression of tomato fruit. Acta Horticulturae Sinica 37: 1257–1263. ( in Chinese)
- Zaharah SS, Singh Z 2010. Nitric oxide fumigation delays mango fruit ripening. IX International Mango Symposium 992: 543–550.
- Zaharah SS, Singh Z 2011a. Postharvest nitric oxide fumigation alleviates chilling injury, delays fruit ripening and maintains quality in cold-stored ‘Kensington Pride’ mango. Postharvest Biology and Technology 60: 202–210. 10.1016/j.postharvbio.2011.01.011
- Zaharah SS, Singh Z 2011b. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biology and Technology 62: 258–266. 10.1016/j.postharvbio.2011.06.007
- Zaharah SS, Singh Z 2011c. Postharvest fumigation with nitric oxide at the pre-climacteric and climacteric-rise stages influences ripening and quality in mango fruit. Journal of Horticulture Science and Biotechnology 86: 645–653.
- Zheng XL, Ye LB, Jiang TJ, Jing GX, Li JR 2012. Limiting the deterioration of mango fruit during storage at room temperature by oxalate treatment. Food Chemistry 130: 279–285. 10.1016/j.foodchem.2011.07.035
- Zhu HL, Zhu BZ, Shao Y, Wang XG, Lin XJ, Xie YH, et al. 2006. Tomato fruit development and ripening are altered by the silencing of LeEIN2 gene. Journal of Integrative Plant Biology 48: 1478–1485. 10.1111/j.1744-7909.2006.00366.x
- Zhu SH, Liu MC, Zhou J 2006. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biology and Technology 42: 41–48. 10.1016/j.postharvbio.2006.05.004
- Zhu SH, Sun LN, Zhou J 2009. Effects of nitric oxide fumigation on phenolic metabolism of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao) in relation to fruit quality. LWT - Food Science and Technology 42: 1009–1014. 10.1016/j.lwt.2008.12.012
- Zhu SH, Zhou J 2005. Effect of nitric oxide (NO) on ripening and senescence of strawberry. Science and Agricultural Sinica 38: 1418–1424. ( in Chinese)
- Zhu SH, Zhou J 2007. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chemistry 100: 1517–1522. 10.1016/j.foodchem.2005.12.022
- Zottini M, Costa A, Michele RD, Ruzzene M, Carimi F, Schiavo FL 2007. Salicylic acid activates nitric oxide synthesis in Arabidopsis. Journal of Experimental Botany 58: 1397–1405. 10.1093/jxb/erm001
- Zúñiga-Arias G, Ruben R, Van Boekel M 2009. Managing quality heterogeneity in the mango supply chain: evidence from Costa Rica. Trends in Food Science and Technology 20: 168–179. 10.1016/j.tifs.2008.12.001