Abstract
Control methods for tomato-potato psyllid (TPP; Bactericera cockerelli) are currently dominated by the use of synthetic biocides. A non-chemical alternative for TPP management is crop mesh, which forms a physical barrier between crop and pest. This study examined the ability of TPP adults to penetrate, or lay eggs through, 22 commercially available crop meshes using a laboratory bioassay. Adult TPP were prevented from moving through the mesh if the mesh complied with any one of the following criteria: shortest pore length <0.55 mm; longest pore length <0.60 mm; diagonal length <0.85 mm; area <0.3 mm2. Eggs were found on foliage only when TPP adults had penetrated the mesh, suggesting that eggs could not be laid through the mesh. The results indicate that crop meshes may provide non-chemical control for TPP that can be used by organic growers, and producers attempting to reduce chemical inputs as part of integrated pest management.
Introduction
The tomato-potato psyllid, (TPP) Bactericera cockerelli (Šulc) (Homoptera: Psyllidae), was confirmed as present in New Zealand in 2006 (Teulon et al. Citation2009). TPP attacks a wide range of fruit, vegetables and non-crop species within the Solanaceae, and causes plant damage due to the removal of photosynthates, introducing phytotoxic compounds in the saliva and the transmission of the bacterial pathogen Candidatus Liberibacter solanacearum, the cause of zebra chip disease in potatoes (Liefting et al. Citation2008; Thomas et al. Citation2011; Butler & Trumble Citation2012).
Research on direct management of TPP has primarily been based on synthetic insecticides, with some investigation of the potential of botanical pesticides and of biological control (Berry et al. Citation2009; Page-Weir et al. Citation2011; O’Connell et al. Citation2012; Jorgensen et al. Citation2013). While there has been some success achieved in the use of existing or newly registered synthetic insecticides, they are not considered an ideal (or permanent) solution in New Zealand because many growers of solanaceous field crops now utilise regimes of integrated pest management that aim to reduce the use of such chemicals (e.g. Cameron et al. Citation2009). Consequently, organic and other producers who do not wish to use, or are prohibited from using, synthetic insecticides currently have limited TPP management options.
Physical barriers to insects locating or settling on crop plants, such as mulches and kaolin dusts, provide a viable option for growers seeking an alternative to chemical pest control (Collier Citation2001, Citation2002; Peng et al. Citation2011). Mesh covers form a barrier between insect and crop, and are being developed in various regions of the world for the management of a wide range of invertebrate pests on a variety of vegetable crops, including Solanaceae (Keun et al. Citation2013; Saidi et al. Citation2013). Crop mesh is usually woven from monofilament materials such as nylon and polypropylene and is produced in a range of pore sizes depending on the target pest. Covers with smaller pores are heavier (per m2), more expensive and generally require more effort to handle. Therefore, in practice, mesh with the largest pore size that prevents entry of the target pest is often used, as this tends to be most economical, makes handling easier and minimises the effect of the cover on the crop microclimate.
Prior to full-scale field trials being performed on the efficacy of crop meshes to exclude TPP, it was desirable to establish which crop mesh covers, if any, could actually prevent movement of adult TPP on to foliage. The aim of this investigation was to assess the ability of a number of commercially available crop meshes to form a barrier to TPP, ascertain the maximum allowable pore dimensions (e.g. length, area) that still prevent movement of TPP, and determine the ability of TPP females to lay eggs through mesh that is in contact with leaf surfaces.
Materials and methods
Estimation of pore dimensions in cover meshes
The measurements of mesh pore size provided by suppliers vary in the type of measurement (e.g. thread gauge vs pore size) and we therefore assessed pore size independently. Four replicate pieces from 22 meshes (from 12 manufacturers) were examined microscopically (see for details). Cosio, Empak, Redpath and QuantumGrow meshes were sourced from New Zealand; CropSolutions, Gromax and Wondermesh were sourced from the UK; and LS Econet was sourced from the Netherlands. In each piece of cloth, measurements were taken of the shortest and longest side of four pores selected on an ad hoc basis. These values were averaged for each piece of cloth, and then an overall mean calculated from the four replicate pieces of each type of cloth. The pores were assumed to be rectangular or square so a simplistic measure of pore area was calculated as Area = (longest side × shortest side), and length of the diagonal estimated by Diagonal = √(longest2 + shortest2).
Table 1 Pore dimensions of commercial crop meshes and the number of TPP adults (x/5) and eggs found on the surface of a Capsicum leaf after 7 days’ exposure in a laboratory trial (see Methods for details) (mean ± sem; n = 4).
Efficacy of TPP exclusion by different meshes
A laboratory experiment was conducted to determine the effect of mesh pore dimensions on penetration by adult TPP, and also examine whether psyllids were able to lay eggs on foliage through the mesh. Five adult TPP were placed in a lightproof plastic cup (70 mm diameter, 90 mm high). The cup was placed over a piece of mesh held between two metal plates with 40 mm diameter holes in their centre. Below the mesh, a second plastic container, of the same size, but transparent, housed a piece of phenolic floral foam (approx. 40 × 40 × 50 mm) saturated with water into which was inserted a single, freshly detached leaf from a bell pepper (Capsicum annuum L.) of approx. 400–900 mm2 surface area, placed so the leaf pressed against the underside of the mesh. No other food or water was present in either container. This setup was repeated four times for each of the 22 types of mesh. The containers were housed in a controlled environment room at 25 ± 1 °C, 50%–60% RH, and a photoperiod of 16:8 hours L:D, for a period of 7 days, after which the number of TPP that had penetrated through the mesh, both alive and dead, and the number of eggs (on the leaf, mesh, cup sides etc.) were counted.
Statistical analysis
All statistical analyses were performed using Genstat (v16, VSN International, Hemel Hempstead, UK). The numbers of TPP passing through the mesh in each replicate chamber were analysed by logistic regression (of binomial proportions). The length of the shortest pore side, longest side, diagonal and area were each used as explanatory variables, and in each case a probit transformation was applied to the response variable and the dispersion parameter of each generalised linear model was estimated as part of the analysis. From the models produced, estimates were made of the dimensions that would exclude 50%, 95% and 99% of TPP, along with their 95% confidence intervals.
Results
From the original 88 containers, four replicates were lost (due to the lightproof cups being dislodged due to earthquakes). Of the remaining 84 chambers, TPP eggs were found on seven leaves, all of which also had TPP adults present. Thus there were no occasions when eggs were discovered in the absence of TPP adults, indicating TPP cannot, or preferred not to, lay eggs through the mesh (see ). Eggs were also not found on any other substrate in the experimental arenas (e.g. mesh, walls of the container or floral foam).
TPP adults passed through the mesh cover on 17 occasions but for only six types of mesh (). The number of TPP passing through the mesh exhibited fairly clear logistic relationships with all mesh dimensions measured (). Estimates of the maximum allowable pore dimensions to exclude 99% of TPP are given in , and can be rounded to approximate values of: short length <0.55 mm; long length <0.60 mm; diagonal <0.85 mm; area <0.3 mm2.
Table 2 Maximum pore dimensions (±95% CI) estimated by probit regression that would allow a given proportion of TPP to penetrate a mesh crop cover.
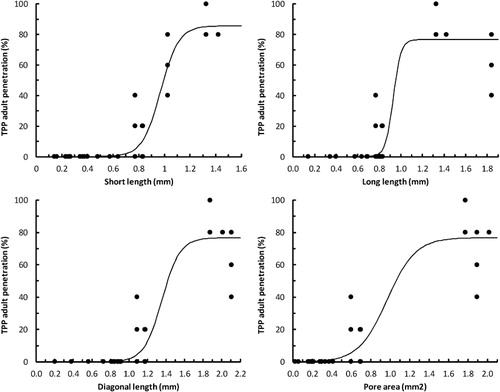
Only three of the meshes tested complied with the maximum allowable dimensions for all four parameters, which were all produced by LS Econet and all demonstrated 100% exclusion (). The six meshes that allowed TPP to penetrate complied with none of the maximum allowable dimensions, suggesting that if a mesh meets any of the necessary criteria it may be capable of excluding TPP.
Another mesh, Wondermesh WM32, also met none of the maximum allowable dimension criteria but had no TPP penetration, suggesting some other factor(s) we have not measured may have a role in TPP exclusion.
Discussion
The results of the study indicate that crop meshes with certain pore size dimensions can be a highly effective barrier to TPP adults and that eggs were only laid directly on to leaves and not through (or on) the mesh, even though the leaf was in contact with the mesh. Extrapolated to a field situation, this suggests that once in place and the edges ‘sealed’ by burying in soil, meshes could provide a complete barrier to TPP accessing crops and provide 100% exclusion until the crop is harvested. Other control methods, such as use of chemical sprays and the use of biocontrol organisms (e.g. entomopathic fungi and insect predators such as Coccinellidae; Lacey et al. Citation2009; O’Connell et al. Citation2012) are mostly curative, i.e. they kill TPP once it has infested a crop, and therefore after crop damage has occurred. In comparison, crop meshes prevent TPP accessing the crop, thereby preventing any damage occurring in the first place. In addition, once the mesh is in position there should be no need for resetting or ‘reapplication’ as is often the case with chemical insecticides.
Currently, purpose-designed woven crop mesh covers retail between NZ$6000–9000 per ha depending on the amounts purchased and mesh size (Wondermesh, pers. comm.). Spread over the expected 10 year working life of the mesh, this represents a $600–$900 annual cost.
These costs are not dissimilar to the costs of agrichemical control, with growers spending an additional NZ$300–1200 ha−1 ($700/ha on average) on additional agrichemicals (Ogden Citation2011). Initial field trials at the Biological Husbandry Unit, Canterbury, indicate that the covers can increase the yield of marketable potatoes (compared with uncovered control plots) by approximately 9 tonne ha−1 which, at current prices of NZ$800 tonne ex. field (Scott Lawson, Lawson’s Organic Farms Ltd, pers. comm.), equals NZ$7200 to the grower, giving a considerable return on investment. In addition, in warmer cropping areas, such as Hawke’s Bay on the North Island of New Zealand, untreated potato crops can yield zero marketable tubers (Scott Lawson, Lawson’s Organic Farms Ltd, pers. comm.), so the expected economic return could be higher still in such locations.
Although these results indicate that mesh crop covers show promise for controlling TPP, there are a number of issues not addressed by these laboratory assays. For example, although the mesh may deter TPP and other insects from accessing the crop, they may not bar access by micropathogens such as fungal spores. Similarly, if meshes alter aspects of the microclimate in the crop, such as temperature and relative humidity, this may influence the development of fungal diseases of solanaceous crops such as Phytophthora infestans and Alternaria solani (although a reduction in the incidence of disease is also possible; see Diez et al. Citation1999). As yet we have no information on the durability and longevity of meshes under New Zealand field conditions, or if the efficacy of meshes change as they age. Future work will examine the ability of meshes tested in this study to exclude TPP (from potato crops) under field conditions, and monitor any side effects of the mesh cover on crop microclimate and the development of crop diseases.
References
- Berry NA, Walker MK, Butler RC 2009. Laboratory studies to determine the efficacy of selected insecticides on tomato/potato psyllid. New Zealand Plant Protection 62: 145–151.
- Butler CD, Trumble JT 2012. The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera:Triozidae): life history, relationship to plant diseases, and management strategies. Terrestrial Arthropod Reviews 5: 87–111. 10.1163/187498312X634266
- Cameron PJ, Walker GP, Hodson AJ, Kale AJ, Herman TJB 2009. Trends in IPM and insecticide use in processing tomatoes in New Zealand. Crop Protection 28: 421–427. 10.1016/j.cropro.2009.01.002
- Collier RH 2001. Crop covers offer considerable opportunities for pest control. The Vegetable Farmer, December 2001, 18–20.
- Collier RH 2002. Using crop covers and mulches as an aid to pest control. The Vegetable Farmer, December 2002, 16–19.
- Diez MJ, Rosello S, Nuez F, Costa J, Lacasa A, Catala MS 1999. Tomato production under mesh reduces crop loss to tomato spotted wilt virus in some cultivars. HortScience 34, 634–637.
- Jorgensen N, Butler RC, Vereijssen J 2013. Biorational insecticides for control of the tomato potato psyllid. New Zealand Plant Protection 66: 333–340.
- Keun CB, Su LH, Bong KY 2013. Establishment of 60 mesh nets to reduce crop loss by Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) in tomato greenhouse. Korean Journal of Applied Entomology 52: 23–27. 10.5656/KSAE.2013.01.1.082
- Lacey LA, de la Rosa F, Horton DR 2009. Insecticidal activity of entomopathogenic fungi (Hypocreales) for potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae): development of bioassay techniques, effect of fungal species and stage of the psyllid. Biocontrol Science and Technology 19: 957–970. 10.1080/09583150903243904
- Liefting LW, Perez-Egusquiza ZC, Clover GRG, Anderson JAD 2008. A new ‘Candidatus Liberibacter’ species in Solanum tuberosum in New Zealand. Plant Disease 92: 1474–1474. 10.1094/PDIS-92-10-1474A
- O’Connell DM, Wratten SD, Pugh AR, Barnes AM 2012. ‘New species association’ biological control? Two coccinellid species and an invasive psyllid pest in New Zealand. Biological Control 62: 86–92. 10.1016/j.biocontrol.2012.03.011
- Ogden SC 2011. Tomato potato psyllid and Liberibacter in New Zealand – impacts and research programme overview. Proceedings of the 11th annual 2011 zebra chip reporting session, San Antonio, Texas, USA. Pp. 173–177.
- Page-Weir NEM, Jamieson LE, Chhagan A, Connolly PG, Curtis C 2011. Efficacy of insecticides against the tomato/potato psyllid (Bactericera cockerelli). New Zealand Plant Protection 64: 276–281.
- Peng L, Trumble JT, Munyaneza JE, Liu T-X 2011. Repellency of a kaolin particle film to potato psyllid, Bactericera cockerelli (Hemiptera: Psyllidae), on tomato under laboratory and field conditions. Pest Management Science 67: 815–824. 10.1002/ps.2118
- Saidi M, Gogo EO, Itulya FM, Martin T, Ngouajio M 2013. Microclimate modification using eco-friendly nets and floating row covers improves tomato (Lycopersicon esculentum) yield and quality for small holder farmers in East Africa. Agricultural Sciences 4: 577–584. 10.4236/as.2013.411078
- Teulon DAJ, Workman PJ, Thomas KL, Nielsen M-C 2009. Bactericera cockerelli: incursion, dispersal and current distribution on vegetable crops in New Zealand. New Zealand Plant Protection 62: 136–144.
- Thomas KL, Jones DC, Kumarasinghe LB, Richmond JE, Gill GSC, Bullians MS 2011. Investigation into the entry pathway for tomato potato psyllid Bactericera cockerelli. New Zealand Plant Protection 64: 259–268.
