Abstract
Resveratrols and flavonoids have important beneficial roles in plants and to humans. The grape family (Vitaceae) is one of several families that can biosynthesise both resveratrols and flavonoids. Previous research has shown that UV-C may induce the biosynthesis of resveratrols in the fruit of grape. But little is known about the UV-C-induced biosynthesis of resveratrols and flavonoids in grape leaves. This study demonstrated that the biosynthesis of resveratrol in grape leaves strongly increased in response to UV-C irradiation. The largest contribution to total resveratrols was trans-resveratrol. The expression of related genes, including PAL, C4H, 4CL and STS, increased and peaked 6–12 h after treatment, earlier than the peak of total resveratrol. In contrast, total flavonoid content and the expression of the key gene CHS was not affected by UV-C radiation, staying at a low level after UV-C irradiation. In summary, there is a differential response to UV-C radiation for the biosynthesis of stilbenes and flavonoids in grape leaves.
Keywords:
Introduction
Phenylpropanoid metabolism is comprised of a complex series of branching biochemical reactions that provide the plant with a host of important phenolic compounds. The general phenylpropanoid pathway leads from phenylalanine to coumaroyl-CoA and is catalysed by the enzymes phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H) and coumaroyl-CoA ligase (4CL). From coumaroyl-CoA, there are two branches, one to flavonoids, whose first committed step is conducted by chalcone synthase (CHS). The other branch of the pathway is to trans-resveratrol (trans-res), which is catalysed by stilbene synthase (STS). Flavonoids include anthocyanin, flavone, flavonol, isoflavone and proanthocyanidin. Trans-res may be isomerised to cis-resveratrol (cis-res) or transformed to trans-piceid (trans-pd) and cis-piceid (cis-pd) by resveratrol 3-O-β-glycosyltransferases (O-3-GT) (Donnez et al. Citation2009). Trans-res and its derivatives form stilbenes.
Almost all plants can synthesise flavonoids; however, only a few plants can synthesise stilbenes. In stilbene-synthesising plants, STS competes with CHS in the synthesis of resveratrol (Lanz et al. Citation1991). Due to their antioxidant activity, flavonoids and stilbenes from plants are used as food additives, as dietary products and for medicinal use. One of the most relevant and extensively studied stilbenes is trans-res, which has been reported to have a number of beneficial health properties, such as high antioxidant capacity, cardiovascular protective effects, antimutagenic properties, as well as oestrogenic and cancer chemopreventive activities (Jang et al. Citation1997; Lu & Serrero Citation1999; Gusman et al. Citation2001). In addition to trans-res, trans-pd also shows high inhibitory activity against tumours and metastasised carcinoma (Kimura & Okuda Citation2000). The grape family Vitaceae is one of several families that can biosynthesise both resveratrols and flavonoids. In grapevines, accumulation of stilbenes can be induced by stresses such as fungal attack (Langcake Citation1981) and wounding (Langcake & Pryce Citation1976).
UV-B radiation (280–320 nm), which filters through the ozone layer, makes up only a small amount of the radiation that reaches the Earth's surface. UV-C radiation (200–280 nm), which is the most hazardous type of UV light, is physiologically insignificant, because these wavelengths are almost completely absorbed by the atmosphere (Coohill Citation1989; Frohnmeyer & Staiger Citation2003). Moderate levels of supplemental UV-B can stimulate transcription of genes involved in protective responses (Brosché & Strid Citation2003), and relatively high levels of solar UV-B can enhance the accumulation of UV-absorbing compounds such as stilbenes (Berli et al. Citation2008). These compounds are thus postulated to reduce UV-B transmittance and protect the photosynthetic apparatus in the leaf mesophyll (Burger & Edwards Citation1996; Mazza et al. Citation2000). However, little is known about the response of flavonoid biosynthesis to UV-C. Grapevines accumulate stilbenes in response to UV-C irradiation (Douillet-Breuil et al. Citation1999; Adrian et al. Citation2000). The influence of UV-C depends on the dose, the sensitivity of the plant species and its ability to attenuate the irradiation (Lavola et al. Citation2003). Grape leaves are an important possible source of stilbenes and flavonoids. Liu et al. (Citation2013) reported that grape leaves of about 75 cultivars (or species) have stilbenes. It would be very useful to produce stilbenes and flavonoids from full grape leaves, by either physical or chemical methods. In the past, studies of UV-C have mainly focused on the fruit of grape. The response of stilbene and flavonoid biosynthesis in grape leaves to UV-C irradiation is not well understood. In this study, the response of biosynthesis of stilbenes and flavonoids to UV-C irradiation in grape leaves was investigated.
Materials and methods
Plant material and treatment
Vines of Vitis vinifera ‘Hongbaladuo’ (table grape) were used in our experiments and were grown in the vineyard at the Institute of Botany, Chinese Academy of Sciences, Beijing. As in Wang et al. (Citation2013), mature (30-day-old), healthy leaves of similar size were detached from the shoot between 0800 h and 0900 h; the leaf petioles were immediately inserted into water, recut under water into 6 cm pieces and rapidly transferred from the water to triangular flasks containing ddH2O. All leaves were incubated in the dark at 25 °C for 30 min, and then the leaf abaxial surfaces were exposed to 10 min of 6 W m−2 UV-C irradiation from a UV-C lamp (Model ZW30S26W, Beijing Lighting Research Institute, Beijing, China). The distance between the leaf blade and the UV-C lamp was 40 cm. The leaves remained in the flasks until sampling. Control leaves were not irradiated. Samples were collected at 0, 3, 6, 12, 24 and 48 h after the initiation of treatments. All treatments and controls were replicated three times, and each replication consisted of six leaves. After sampling, the leaves were ground into powder in liquid nitrogen and stored at –80 °C until analysis.
Extraction and determination of total resveratrol
The resveratrol in leaves was extracted as in Liu et al. (Citation2013). Briefly, 1 g of leaf tissue was extracted with 15 mL of methanol/ethyl acetate (1:1, v/v) for 24 h at room temperature in the dark. After centrifugation at 10,000 g at 4 °C for 10 min, the supernatant was evaporated at 40 °C until dry and then dissolved in 2 mL methanol. The extract was filtered through a 0.45 μm PTFE membrane before HPLC analysis.
All samples were analysed using a Dionex P680 HPLC system (Dionex Corporation, Sunnyvale, CA, USA) equipped with a reverse-phase C18 column of Atlantis® T3 (5 µm particle sizes, 4.6 × 250 mm ID; Waters Corporation, Milford, MA, USA) and a C18 Nova Pack guard pre-column (Waters). The injection volume was 10 μL and the column temperature was 30 °C. Cis-isomers were detected at 288 nm and trans-isomers at 306 nm, and photodiode array spectra were recorded from 240 to 600 nm. Separation was performed at a flow rate of 1.0 mL min−1 with the mobile phase consisting of acetonitrile (A) and ddH2O (B). The solvent gradient was as follows: 0 min 10% solvent A; 5 min 17% solvent A; 12 min 18% solvent A; 22 min 22% solvent A; 30 min 33% solvent A; 45 min 38% solvent A; and 58 min 100% solvent A. The fluorimetric detection for cis-isomers was at 288 nm, while that for trans-isomers was at 306 nm. The maximum excitation wavelength was 240 nm and emission was measured at 600 nm.
Extraction and determination of total flavonoids
Flavonoids in leaves were extracted as in Chen et al. (Citation2012). Briefly, 1 g of fresh weight frozen leaves was extracted with 30 mL of methanol/water (70:30, v/v) at 4 °C for 36 h in the dark. The extract was centrifuged at 20,000 g for 10 min, the supernatant was collected, and the tissues were re-extracted as above two additional times. The combined supernatant solutions were evaporated until dry in a rotary vacuum at 35 °C and then reconstituted in 2 mL of 100% methanol. After centrifugation at 10,000 g for 10 min, 50 μL of the supernatant was diluted to 500 μL for further analysis. All samples were analysed using the ultraviolet visible spectrophotometer (UV-VIS) (SPECORD 200, Analytik Jena, Jena, Thuringia, Germany) at 350 nm.
Total RNA isolation and RT-PCR analysis
Total RNA was extracted from leaves using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and was treated with DNase I (Promega Corporation, Fitchburg, WI, USA) to avoid DNA contamination. The quantity and quality of total RNA was determined by spectrophotometry and by 1% formaldehyde denaturing gel electrophoresis. The samples with bright bands of ribosomal 28S to 18S RNA in a ratio >1.5:1 were used for RT-PCR analysis. 1 μg of RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen) with an oligo(dT)15 primer according to the manufacturer's instructions (Tiangen Biotech, Beijing, China). qRT-PCR experiments were conducted using Real Master Mix (SYBR Green) (Tiangen Biotech). Reactions were performed on a StepOnePlus Real-Time PCR system (Life Technologies Corporation, Carlsbad, CA, USA). The following standard thermal profile was used for all PCR experiments: −94 °C for 5 min; 40 cycles of 95 °C for 15 s; and 60 °C for 60 s. Fluorescence signals were captured at the end of each cycle, and a melting curve analysis was performed from 68 to 95 °C to check the specificity of the PCR reaction. Gene-specific primers were designed using the Primer 5 software (). The enzyme STS is pivotal to the biosynthesis of resveratrol. Analyses of the preliminary grape genome sequence confirmed the large size of the multigene families with an estimated number of 48 STS genes (Vannozzi et al. Citation2012). The primers used here matched 13 STS mRNA sequences. They included VvSTS7, VvSTS9, VvSTS10, VvSTS15, VvSTS21, VvSTS27, VvSTS29, VvSTS33, VvSTS37, VvSTS41, VvSTS45, VvSTS47 and VvSTS48. This may reflect the expression of the STS family. PAL, CHS and O-3-GT are also multi-gene families, and have 10, three and three members, respectively, based on PN40024 12X V1 coverage (). The primers used for qRT-PCR in this study matched VvPAL1-10, VvCHS1-2 and VvO-3-GT1-2.
Table 1 Primers used in RT-PCR.
Table 2 PAL, C4H, 4CL, CHS members identified based on the grapevine PN 40024 12X V1 coverage.
The amplification curves were analysed with the LinRegPCR software and the amplification efficiency of the primers was at 90%–110%. The actin gene was used as the internal control for all of the qRT-PCR data. Analyses of qRT-PCR data used the classic (1 + E)−ΔΔCT method (CT is the threshold cycle of one gene, E is the amplification efficiency). ΔCT is equal to the difference in threshold cycles for target (X) and reference (R) (CT,X–CT,R), while the ΔΔCT is equal to the difference of ΔCT for the control (C) and the treatment (T) (ΔCT,T–ΔCT,C). The amplification system (e.g. primer and template concentrations) was properly optimised, and the efficiency was close to 1. Therefore, the amount of the target product, scaled to an endogenous reference and relative to a calibrator, was given by the equation:
Statistical analysis
Statistical analyses were performed using SPSS 14.0 and were tested for statistical significance using the paired Student's t-test. Differences were considered significant at P < 0.05.
Results
Accumulation of resveratrol and flavonoids in leaves after UV-C irradiation
Our results showed that trans-pd, cis-pd and trans-res were detectable, but cis-res was undetectable. Total resveratrol (sum of the above three forms) in the control leaves showed no significant (P < 0.05) change and were at trace levels (0–10 μg g−1). In contrast, the total amount in UV-C-irradiated leaves changed significantly. Before treatment, the concentration of total resveratrol was very low (0.97 μg g−1 FW). At 3 h after treatment, the total amount of resveratrol began to increase, and accumulated slowly until 6 h post-irradiation. Thereafter, total resveratrol content sharply increased and peaked (18.18 μg g−1 at 6 h post-irradiation and 467.62 μg g−1 at 24 h post-irradiation). Total resveratrol at the peak was 480.8 times higher than levels in control leaves. Levels then declined to 199.55 μg g−1 by 48 h post-irradiation. The contribution of the three forms of resveratrol to the total amount differed significantly. Trans-res made the greatest contribution and showed a similar trend as total resveratrol. Concentration of trans-pd continually rose, reaching 18.72 μg g−1 FW at 48 h after UV-C irradiation. Concentration of cis-pd always maintained lower levels after UV-C irradiation (). In contrast to the strong response of resveratrol to UV-C, biosynthesis of flavonoids did not respond significantly in the 48 h after UV-C irradiation ().
Table 3 The content of resveratrols and flavonoids in grape leaves treated by UV-C irradiation.
Expression of the general phenylpropanoid pathway genes, STS and CHS
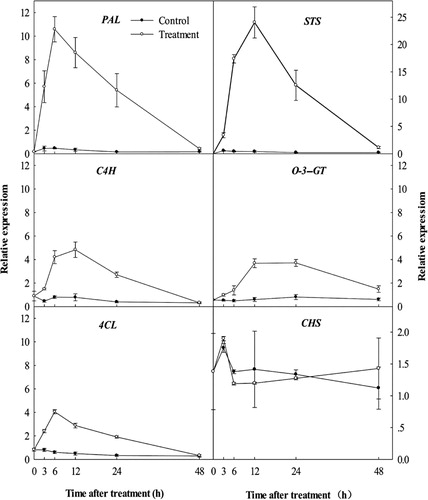
In order to analyse the relationship between total resveratrol and total flavonoid accumulation in the leaves after treatment and the expression of related biosynthetic genes, we performed qRT-PCR analyses of PAL, C4H, 4CL, CHS, STS and O-3-GT (). After UV-C irradiation, PAL and 4CL exhibited a similar trend. Their expression first increased, peaked and then declined. The expression of PAL and 4CL peaked 6 h after UV-C irradiation, but the expression of C4H peaked 12 h after UV-C irradiation. The expression of PAL reached a higher peak compared with C4H and 4CL. We designed the primers in the conserved region of 20 STS mRNA sequences, which may reflect the expression of the STS family. STS expression was low in the control leaves (). At 3 h after UV-C irradiation, the expression of STS slowly increased, then rose sharply, and peaked 12 h after UV-C irradiation. The relative expression at its peak was more than 5000 times that before irradiation. After the peak, the expression continued decreasing, and declined to a low level by 48 h. In contrast, the relative expression of CHS showed no significant difference between the leaves of the treatment and the control (). The expression of O-3-GT gradually rose after UV-C irradiation, plateaued from 12 to 24 h, and then declined.
Discussion and conclusion
UV-C induced the accumulation of resveratrol and the expression of related genes in leaves
Previous studies showed that salicylic acid, methyl jasmonate (JAMe), cyclodextrin, mechanical damage and plant diseases can promote the accumulation of resveratrol (Bavaresco et al. Citation1997; Krisa et al. Citation1999; Vitrac et al. Citation2002; Bru et al. Citation2006; Vezzulli et al. Citation2007; Kiselev et al. Citation2010). Moreover, some studies have investigated transcription levels of resveratrol synthesis-related genes. Application of JAMe resulted in an increase in the expression of PAL, C4H, 4CL and STS in calluses of berries and leaf petioles (Tassoni et al. Citation2005; Belhadj et al. Citation2008; Lijavetzky et al. Citation2008). In addition, the expression of PAL and STS was induced by salicylic acid in a cell suspension of grape leaves (Kiselev et al. Citation2010). Previous studies of the synthesis of UV-C-induced resveratrol were focused on fruits of grapes at different developmental stages and from different cultivars (Adrian et al. Citation2000; Cantos et al. Citation2002, Citation2003). Grape leaves have a relatively remarkably high content of resveratrol (Petit et al. Citation2009; Wang et al. Citation2010). However, less attention has been paid to the influence of abiotic and biotic factors on the biosynthesis of resveratrol in leaves. This study showed that irradiation by UV-C induced an increase in resveratrol content in grape leaves, and the expression of PAL, C4H, 4CL and STS was increased. The expression of STS sharply increased, with a peak value at about 5000-fold of the control. Trans-res is the product of the STS enzyme. The start and peak times of trans-res accumulation were later than those of STS expression ( and ). Resveratrol O-glucosyltransferase (O-3-GT) may convert trans-res into trans-pd (Jeandet et al. Citation1997; Belhadj et al. Citation2008). From 3 to 24 h after UV-C treatment, trans-pd content and the expression of O-3-GT constantly increased, which indicates that O-3-GT has an important role for trans-pd accumulation. Leaf tissue may make a better study system than fruits for studying the mechanism of synthesis of resveratrol. Leaves are easily treated with UV-C, and using leaves excludes the possibility of transportation of resveratrol between the skin and pulp in fruit when studying the accumulation of resveratrol.
UV-C did not induce the synthesis of flavonoids and the expression of related genes in leaves
Flavonoids and resveratrol are two branches of the phenylpropanoid pathway, which involves the enzymes PAL, C4H and 4CL. STS and CHS use the same substrate. Therefore, there should be some relationship between the synthesis of resveratrol and flavonoids. UV-B has also been found to increase flavonoids in apple skins (Solovchenko & Schmitz-Eiberger Citation2003) and in fruits of grape (Schultz Citation2000; Cantos et al. Citation2001; Kolb et al. Citation2003). Some key enzymes involved in the phenylpropanoid (PAL) and flavonoid (CHS) biosynthetic pathways are up-regulated by UV-B (Pontin et al. Citation2010). Moreover, UV-B induced an increase in resveratrol content in grape skin (Cantos et al. Citation2001; Berli et al. Citation2008). Similar to effects seen from UV-B, UV-C induced the synthesis of resveratrol in the present study as well as in other studies. Different from effects from UV-B irradiation, UV-C irradiation did not result in a significant change in total flavonoid content and the expression of CHS in this study ( and ). Zhang et al. (Citation2012) reported that 1.8 kJ m−2 UV-C exposure slightly increased the level of various anthocyanins in the skins of the 11 w grape berries. Erkan et al. (Citation2008) reported that doses of 0.43 and 2.15 kJ m−2 UV-C irradiation had little effect on anthocyanin accumulation, and 4.30 kJ m−2 showed markedly reduced concentrations in strawberry fruit. The effects of UV-C irradiation on anthocyanins were found to depend on dosage and cultivar (Perkins-Veazie et al. Citation2008). Li et al. (Citation2014) reported that 4.1 kJ m−2 UV-C irradiation suppressed anthocyanin and flavonoid accumulation in strawberry plants. The effects of UV-C irradiation on flavonoids were found to depend on dosage and cultivar (Perkins-Veazie et al. Citation2008).
A dramatic increase in the expression and activity of PAL after UV-C irradiation was also reported by Pombo et al. (Citation2011). This increase was found to be beneficial to increasing total phenolic content. A study by Li et al. (Citation2014) showed that 4CL activity was inhibited in strawberry plants by UV-C radiation, and the effects of UV-C radiation on C4H were not obvious. In the current study, PAL gene expression was strongly up-regulated after UV-C treatment, whereas C4H and 4CL gene expression was only relatively weakly up-regulated. This should provide enough substrates for enzymes of STS and CHS. High STS expression is an important cause of resveratrol accumulation. However, relatively stable CHS expression is also an important cause of resveratrol accumulation; this is because of the decline in substrate competition. Vannozzi et al. (Citation2012) found that UV-C resulted in a dramatic increase in STS transcription, but the expression of all three CHS genes was strongly suppressed relative to the untreated leaf discs. Our results support this result for the expression of CHS genes and total flavonoid content ( and ). UV-B and UV-C only differ in wavelength, but their effects differ greatly. This may be associated with different signal transduction components between UV-B and UV-C, and is worthy of further investigation.
In summary, these results demonstrated that the biosynthesis of resveratrol in grape leaves strongly increased in response to UV-C irradiation. Total resveratrols peaked 24 h after UV-C treatment. The biggest contributor to total resveratrols was trans-resveratrol. The expression of related genes, including PAL, C4H, 4CL and STS, increased and reached a peak at 6–12 h after treatment, which is earlier than the peak of total resveratrol. In contrast, total flavonoids and the expression of CHS were not affected by UV-C irradiation. In conclusion, there is a differential biosynthetic response to UV-C irradiation for stilbenes and flavonoids in grape leaves.
Additional information
Funding
References
- Adrian M, Jeandet P, Douillet-Breuil AC, Tesson L, Bessis R 2000. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. Journal of Agricultural and Food Chemistry 48: 6103–6105. 10.1021/jf0009910
- Bavaresco L, Petegolli D, Cantü E, Fregoni M, Chiusa G, Trevisan M 1997. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 36: 77–83.
- Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S et al. 2008. Effect of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiology and Biochemistry 46: 493–499.
- Berli F, D’angelo J, Cavagnaro B, Bottini R, Wuilloud R, Silva F 2008. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. Journal of Agricultural and Food Chemistry 56: 2892–2898. 10.1021/jf073421+
- Brosché M, Strid Å 2003. Molecular events following perception of ultraviolet-B radiation by plants. Physiologia Plantarum 117: 1–10.
- Bru R, Sellés S, Casado-Vela J, Belchí-Navarro S, Pedreño MA 2006. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. Journal of Agricultural and Food Chemistry 54: 65–71. 10.1021/jf051485j
- Burger J, Edwards GE 1996. Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant and Cell Physiology 37: 395–399. 10.1093/oxfordjournals.pcp.a028959
- Cantos E, Espin JC, Tomas-Barberan FA 2001. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: a new ‘functional’ fruit? Journal of Agricultural and Food Chemistry 49: 5052–5058. 10.1021/jf010366a
- Cantos E, Espin JC, Tomás-Barberán FA 2002. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. Journal of Agricultural and Food Chemistry 50: 5691–5696. 10.1021/jf0204102
- Cantos E, Tomás-Barberán FA, Martínez A, Espín JC 2003. Differential stilbene induction susceptibility of seven red wine grape varieties upon post-harvest UV-C irradiation. European Food Research and Technology 217: 253–258.
- Chen S, Fang LC, Xi HF, Guan L, Fang JB, Liu YL et al. 2012. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Analytica Chimica Acta 724: 127–135.
- Coohill TP 1989. Ultraviolet action spectra (280 to 380 nm) and solar effectiveness spectra for higher plants. Photochemistry and Photobiology 50: 451–457. 10.1111/j.1751-1097.1989.tb05549.x
- Donnez D, Jeandet P, Clément C, Courot E 2009. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends in Biotechnology 27: 706–713. 10.1016/j.tibtech.2009.09.005
- Douillet-Breuil AC, Jeandet P, Adrian M, Bessis R 1999. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. Journal of Agricultural and Food Chemistry 47: 4456–4461. 10.1021/jf9900478
- Erkan M, Wang SY, Wang CY 2008. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biology and Technology 48: 163–171.
- Frohnmeyer H, Staiger D 2003. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiology 133: 1420–1428. 10.1104/pp.103.030049
- Gusman J, Malone H, Atassi G 2001. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 22: 1111–1117. 10.1093/carcin/22.8.1111
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW et al. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275: 218–220. 10.1126/science.275.5297.218
- Jeandet P, Breuil AC, Adrian M, Debord S, Meunier P, Maume B et al. 1997. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Analytical Chemistry 69: 5172–5177.
- Kimura Y, Okuda H 2000. Effects of naturally occurring stilbene glucosides from medicinal plants and wine, on tumour growth and lung metastasis in Lewis lung carcinoma-bearing mice. Journal of Pharmacy and Pharmacology 52: 1287–1295.
- Kiselev KV, Dubrovina AS, Isaeva GA, Zhuravlev YN 2010. The effect of salicylic acid on phenylalanine ammonia-lyase and stilbene synthase gene expression in Vitis amurensis cell culture. Russian Journal of Plant Physiology 57: 415–421.
- Kolb CA, Kopecký J, Riederer M, Pfündel EE 2003. UV screening by phenolics in berries of grapevine (Vitis vinifera). Functional Plant Biology 30: 1177–1186.
- Krisa S, Larronde F, Budzinski H, Decendit A, Deffieux G, Mérillon JM 1999. Stilbene production by Vitis vinifera cell suspension cultures: methyl jasmonate induction and 13C biolabeling. Journal of Natural Products 62: 1688–1690.
- Langcake P 1981. Disease resistance of Vitis spp. and the production of the stress metabolites reveratrol, ε-Viniferin, a-Viniferin and pterostilbene. Physiological Plant Pathology 18: 213–226.
- Langcake P, Pryce RJ 1976. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiological Plant Pathology 9: 77–86.
- Lanz T, Tropf S, Marner FJ, Schröder J, Schröder G 1991. The role of cysteines in polyketide synthases. Site-directed mutagenesis of resveratrol and chalcone synthases, two key enzymes in different plant-specific pathways. Journal of Biological Chemistry 266: 9971–9976.
- Lavola A, Aphalo PJ, Lahti M, Julkunen-Tiitto R 2003. Nutrient availability and the effect of increasing UV-B radiation on Scots pine. Environmental and Experimental Botany 49: 49–60.
- Li D, Luo Z, Mou W, Wang Y, Ying T, Mao L 2014. ABA and UV-C effects on quality, antioxidant capacity and anthocyanin contents of strawberry fruit (Fragaria ananassa Duch.). Postharvest Biology and Technology 90: 56–62. 10.1016/j.postharvbio.2013.12.006
- Lijavetzky D, Almagro L, Belchi-Navarro S, Martínez-Zapater JM, Bru R, Pedreño MA 2008. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Research Notes 1: 132. 10.1186/1756-0500-1-132
- Liu CY, Wang LJ, Wang JF, Wu BH, Liu W, Fan PG et al. 2013. Resveratrols in Vitis berry skins and leaves: their extraction and analysis by HPLC. Food Chemistry 136: 643–649. 10.1016/j.foodchem.2012.08.017
- Lu RQ, Serrero G 1999. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. Journal of Cellular Physiology 179: 297–304. 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL 2000. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology 122: 117–126. 10.1104/pp.122.1.117
- Perkins-Veazie P, Collins JK, Howard L 2008. Blueberry fruit response topostharvest application of ultraviolet radiation. Postharvest Biology and Technology 47: 280–285.
- Petit AN, Baillieul F, Vaillant-Gaveau N, Jacquens L, Conreux A, Jeandet P et al. 2009. Low responsiveness of grapevine flowers and berries at fruit set to UV-C irradiation. Journal of Experimental Botany 60: 1155–1162.
- Pombo MA, Rosli HG, Martinez GA, Civello PM 2011. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria xananassa Duch.). Postharvest Biology and Technology 59: 94–102.
- Pontin MA, Piccoli PN, Francisco R, Bottini R, Martinez-Zapater JM, Lijavetzky D 2010. Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biology 10: 224. 10.1186/1471-2229-10-224
- Schultz H 2000. Climate change and viticulture: a European perspective on climatology, carbon dioxide and UV-B effects. Australian Journal of Grape and Wine Research 6: 2–12. 10.1111/j.1755-0238.2000.tb00156.x
- Solovchenko A, Schmitz-Eiberger M 2003. Significance of skin flavonoids for UV-B-protection in apple fruits. Journal of Experimental Botany 54: 1977–1984.
- Tassoni A, Fornalè S, Franceschetti M, Musiani F, Michael AJ, Perry B et al. 2005. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytologist 166: 895–905. 10.1111/j.1469-8137.2005.01383.x
- Vannozzi A, Dry IB, Fasoli M, Zenoni S, Lucchin M 2012. Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biology 12: 130. 10.1186/1471-2229-12-130
- Vezzulli S, Civardi S, Ferrari F, Bavaresco L 2007. Methyl jasmonate treatment as a trigger of resveratrol synthesis in cultivated grapevine. American Journal of Enology and Viticulture 58: 530–533.
- Vitrac X, Krisa S, Decendit A, Vercauteren J, Nührich A, Monti JP et al. 2002. Carbon-14 biolabelling of wine polyphenols in Vitis vinifera cell suspension cultures. Journal of Biotechnology 95: 49–56.
- Wang LJ, Ma L, Xi HF, Duan W, Wang JF, Li SH 2013. Individual and combined effects of CaCl2 and UV−C on the biosynthesis of resveratrols in grape leaves and berry skins. Journal of Agricultural and Food Chemistry 61: 7135–7141. 10.1021/jf401220m
- Wang W, Tang K, Yang HR, Wen PF, Zhang P, Wang HL et al. 2010. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiology and Biochemistry 48: 142–152.
- Zhang ZZ, Li XX, Chu YN, Zhang MX, Wen YQ, Duan C-Q et al. 2012. Three types of ultraviolet irradiation differentially promote expression of shikimate pathway genes and production of anthocyanins in grape berries. Plant Physiology and Biochemistry 57: 74–83. 10.1016/j.plaphy.2012.05.005