ABSTRACT
The ripe fruit of Pyrus ussuriensis ‘Nanguoli’ is favoured by people for its attractive colour, exquisite flesh and pleasant flavour. However, its flavour is negatively affected by early harvest. Therefore, this study aimed to determine whether exogenous methyl jasmonate (MJ) can help to regulate aromatic volatiles biosynthesis in pre-climacteric ‘Nanguoli’ fruit at different maturity stages (135 days after full bloom (DAFB) or ‘early harvested’ and 145 DAFB or ‘normal harvested’). MJ promoted aromatic volatile biosynthesis in normal harvested fruit, with the number of volatile compounds increasing from 43 in untreated fruit to 51 and 57 in fruit that had been treated with 1 and 5 mM MJ, respectively. By contrast, MJ had little effect on early harvested fruit, which exhibited an increase from 41 to 49 and 44 volatile compounds following treatment with 1 and 5 mM MJ, respectively. The total content of aromatic volatiles also increased by 53.75% and 66.82% in normal harvested fruit treated with 1 and 5 mM MJ, respectively, whereas early harvested fruit exhibited only slight increases. There was no significant difference between the effects of 1 and 5 mM MJ on aromatic volatile biosynthesis, indicating that the observed effects were dependent on the maturity stage of the fruit rather than the exogenous MJ concentration within the range tested. The ethylene production rate exhibited similar changes to those observed for the aromatic volatile content and there were no significant differences in the aromatic volatile contents of ‘Nanguoli’ fruits that had been treated with 1-methylcyclopropene (1-MCP) + MJ at different maturity stages. Therefore, it is deduced that ethylene may trigger aromatic volatile biosynthesis.
Aroma plays an important role in fruit quality, and has a large effect on its acceptance by consumers and value. Aroma is also considered a characteristic of ripe fruit. Pyrus ussuriensis ‘Nanguoli’ is a cultivar that has been extensively planted in Liaoning Province, China, due to its excellent flavour and sensory attributes. This climacteric fruit requires approximately 10 days after harvest to reach full ripeness and optimal quality. The fully ripened fruit has a golden yellow peel, exquisite flesh, abundant juice and a sweet flavour. Its aromatic volatiles mainly include esters, alcohols, aldehydes, ketones and carbohydrates, with esters that have a fruity note predominating (Chen Citation2005; Zhuang et al. Citation2007; Dong et al. Citation2010; Feng et al. Citation2010; Qin et al. Citation2012a).
Methyl jasmonate (MJ) is considered a naturally occurring plant growth regulator (Fan et al. Citation1998a), and has been reported to have large effects on chlorophyll degradation, anthocyanin formation (Rudell et al. Citation2002; Rudell & Mattheis Citation2008; de la Peña Moreno et al. Citation2010a), ethylene production (Lalel et al. Citation2003; Kondo et al. Citation2005; Mukkun & Singh Citation2009) and fruit aroma development (Fan et al. Citation1997; Lalel et al. Citation2003; Ayala-Zavala et al. Citation2005; Kondo et al. Citation2005; de la Peña Moreno et al. Citation2010b). Transient increases in the concentrations of jasmonates occur during the onset of fruit ripening in apple and tomato, suggesting their involvement in modulating the early steps of climacteric fruit ripening (Creelman & Mullet Citation1997; Lalel et al. Citation2003), which has been reported to occur through the stimulation of ethylene biosynthesis (Fan et al. Citation1998b; Kondo et al. Citation2000; Lara et al. Citation2003) – although in apple and pear, the impact of MJ application on ethylene production differed between cultivars, according to their biological characteristics (Kondo et al. Citation2005). In climacteric fruit, exogenous MJ enhances ethylene emission in the pre-climacteric stage, but inhibits it in the climacteric and post-climacteric stages (Fan et al. Citation1997, Citation1998a; Kondo et al. Citation2007).
During pear production in China, most of the fruits, including those of ‘Nanguoli’, are harvested early to extend their shelf life. However, our previous research on the aromatic constituents of fully ripened ‘Nanguoli’ fruit showed that early harvested fruit had a lower ester note (i.e. were less fruity) and a poorer flavour than normal harvested fruit (Qin et al. Citation2012b). Therefore, it is desirable to improve the aroma of early harvested pear fruit.
Previous research has demonstrated that exogenous MJ has an important effect on the production of aromatic volatiles. However, the effect of MJ on the biosynthesis of aromatic volatiles at different stages of maturity in pre-climacteric fruits remains unknown. Therefore, this study investigated the effects of exogenous MJ on aromatic volatile biosynthesis in pre-climacteric ‘Nanguoli’ pear fruit at different stages of maturity.
Materials and methods
Materials
Pyrus ussuriensis ‘Nanguoli’ fruits were harvested from Liaoning Institute of Pomology (Xiongyue, Liaoning Province, China) at two different stages of maturity: early harvested fruit, which were harvested at 135 days after full bloom (DAFB), and normal harvested fruit, which were harvested at 145 DAFB. Only fruit of uniform size and colour, and which were free from defects, were used in the experiment.
MJ treatments
Immediately after harvest, 180 fruit from each maturity stage were randomly separated into three groups of 60 fruit and given the following treatments: (1) 0 mM MJ, (2) 1 mM MJ and (3) 5 mM MJ. Postharvested ‘Nanguoli’ fruit were washed with tap water and air-dried. In each treatment, fruit were immersed in a 2-L solution of 0.177% (v/v) Tween® 20 with 0 (control), 1 or 5 mM MJ (Sigma-Aldrich Chemical Company, USA) for 5 min (Kondo et al. Citation2005). All fruit were then stored at 20 ± 1°C until reaching full ripeness (approximately 20 days for the 1-MCP treatment group and 10 days for the treatment group without 1-MCP; see below), which was determined by measuring the firmness. There were three replicates per treatment, each of which contained 20 fruit.
1-MCP treatments
Sixty fruit from each maturity stage were treated with 0.2 μL L−1 1-methylcyclopropene (1-MCP) for 24 h at 20°C according to Gamrasni et al. (Citation2010), following which they were treated with MJ according to the above methods. All fruit were then stored at 20 ± 1°C until reaching full ripeness (approximately 20 days after treatment), which was determined by measuring the firmness.
Measurement of ethylene production rate and quality parameters
The soluble solids content (SSC), titratable acid and ethylene production rate were measured in the fully ripened fruit. SSC (%) was estimated using a refractometer (WYT-4; Shanghai Precision Instrument Ltd Co., China). Titratable acid was measured by homogenising 2 g of flesh in 20 mL distilled water and then titrating 10 mL of juice with 0.02 M NaOH. The titration was monitored using a pH indicator dye and titratable acid was expressed as malic acid content (%).
The ethylene production rate was measured by gas chromatography (GC). A fruit of known weight (g) was placed in a sealed desiccator (approximately 2 L volume) for 1 h at 25°C, following which 1.0 mL of gas in the head space was withdrawn with a syringe and injected into the injector port of the gas chromatograph (GC5890C; Nanjing Kejie Analytical Instruments Co. Ltd, China), which was equipped with a flame ionisation detector. An activated alumina column (0.53 mm × 30 m; Nanjing Jianuo Instrument Co. Ltd, China) was used to separate ethylene and N2 was used as the carrier gas at a rate of 45 mL min–1. The column, injector and detector temperatures were 45, 150°C and 220°C, respectively. Five replicates were taken and the results were expressed as the mean (μL g−1 h−1) of these. Ethylene samples of known concentration (0.5 μL L−1) were routinely used for calibration. An external standard was used to calculate the ethylene concentration.
Analysis of aromatic volatiles
The volatile compounds were analysed using a modified version of the method previously described by Chen (Citation2005) and Qin et al. (Citation2012a, Citation2012b).
Extraction and concentration of volatiles: Headspace solid-phase microextraction (HS-SPME) was used for the extraction and concentration of volatiles in this study. First, 10 g of pulp was combined with 3.6 g of NaCl (to accelerate the emission of volatiles) and 50 μL of 0.04 g mL–1 3-nonanone (internal standard). Then, 65 µm polydimethylsiloxane–divinylbenzene (PDMS/DVB; Supelco Co., Bellefonte, PA, USA) was used to adsorb the volatiles. The extraction was performed in a constant-temperature water bath at 40°C and was stirred by a magnetic follower on a magnetic stirrer. Adsorption occurred over 30 min, following which the extraction fibre was introduced into the heated injector port of the gas chromatograph for desorption at 250°C for 5 min in splitless mode.
GC–mass spectrometry (MS) analysis: Volatiles were identified and quantified by GC–MS using an Agilent 5973B mass detector coupled to an Agilent 7890A gas chromatograph, which was equipped with a 30 m × 0.25 mm × 1.0 mm HP-5 mass spectrometer (5% phenyl-polymethylsiloxane) capillary column. Helium was used as carrier gas at a rate of 1.0 mL min–1, and the injector and detector temperatures were 250°C and 280°C, respectively. The oven temperature was kept at 35°C for 8 min, and then increased by 2°C min–1 to reach 140°C, at which it was maintained for 2 min, following which it was increased by 10°C min–1 to reach 260°C, at which it was maintained for 5 min. Mass spectra were recorded at 70 eV in electron impact ionization mode. The quadrupole mass detector and ion source temperatures were 150°C and 230°C, respectively, and the transfer line temperature was 280°C. Mass spectra were scanned in the m/z range of 33–350 amu at 1-s intervals.
The volatile components were tentatively identified by comparing the mass spectra of the samples with authentic standards and the data system library (NIST 98). Only reproducible peaks across three biological replicates were extracted as data for analysis. The internal standard method was used for quantitative analysis of the volatile components, whereby the content of each compound was normalised to the concentration of 3-nonanone.
Statistical analysis
Analysis of variance and Tukey’s multiple range test were performed to compare the means and least significant differences of the aromatic contents and ethylene production data between different treatments using the SPSS statistical software package. The values in this study were the means of three replicates and a significance level of p < .05 was used.
Results and discussion
Quality parameters of ‘Nanguoli’ fruit under different MJ treatments
The SSC and titratable acid contents are important quality parameters of fruit. There were no significant differences in these quality parameters between MJ treatments ().
Table 1. Quality parameters in ‘Nanguoli’ fruit treated with methyl jasmonate.
Effect of MJ treatment on aromatic volatile biosynthesis
Flavour plays an important role in consumer satisfaction and generally influences further consumption of the fruit (Pelayo et al. Citation2003). Esters are the main aromatic volatile of ‘Nanguoli’, which are perceived by people as a rich fruity note. ‘Nanguoli’ fruit treated with MJ experienced the largest increase in ester production, leading to a fruitier flavour of the fruit. Similarly, MJ has previously been shown to stimulate fruit flavour in apple and mango fruits (Fan et al. Citation1997; Ayala-Zavala et al. Citation2005; Kondo et al. Citation2005).
It is important to recognise that fruit aroma is a complex trait that is controlled by numerous volatiles that are present at particular concentrations and proportions (Ayala-Zavala et al. Citation2005). In the present study, although MJ treatment affected the production of aromatic volatiles, their proportions remained similar to the control fruit (; Figure S1), with esters remaining as the main group, followed by alcohols, ketones or aldehydes. However, the contents of aldehydes and hydrocarbons, which represented less than 20% of the total aromatic volatile content, differed between treatments, with normal harvested fruit treated with 1 and 5 mM MJ exhibiting a 1.91- and 2.29-fold increase in aldehydes and a 3.90- and 3.34-fold increase in hydrocarbons, respectively, compared with the control fruit. By contrast, for early harvested fruit, the total content and specific contents of each kind of aromatic volatile were only slightly affected by MJ treatment.
Table 2. Aromatic volatile content (ng g–1) of ‘Nanguoli’ fruit treated with methyl jasmonate at different maturity stages.
Fan et al. (Citation1997) also found that MJ stimulated ester production in pre-climacteric fruits, but had no effect on ketone and aldehyde emissions regardless of the developmental stage. Similarly, in the present study, MJ was found to stimulate ester (particularly acetyl ester) biosynthesis in both normal harvested and early harvested ‘Nanguoli’ fruit (). However, ketone and aldehyde emissions were also affected by MJ in the normal harvested fruit.
The total content of esters increased by 689.3 and 822.3 ng g–1 following treatment with 1 and 5 mM MJ, respectively. However, individual esters were affected differently by exogenous MJ. The content of acetyl ester increased by 421.8 and 605.4 ng g–1, respectively, which represented 1.30- and 1.43-fold increases compared with the control, and the contents of ethyl butanoate, ethyl hexanoate and ethyl 2-hexenoate also greatly increased. By contrast, the contents of 3-hexylene hexanoate, ethyl (E,Z)-2,4-decadienoate and phenylmethyl acetate markedly decreased. Ethyl butanoate, ethyl hexanoate and ethyl 2-hexenoate are considered to be important aromatic compounds in pear fruit (Takeoka et al. Citation1992) and are very important for sensory responses, and so may be an important target for future research.
In the normal harvested ‘Nanguoli’ fruit, 51 and 57 volatile compounds were detected in the 1 and 5 mM MJ treatment groups, respectively, which included esters, alcohols, aldehydes, ketones and carbohydrates, while there were only 43 volatile compounds in the control group. By contrast, the number of volatile compounds in the early harvested fruit only increased from 41 to 49 and 44 following treatment with 1 and 5 mM MJ, respectively. Similarly, the total content of aromatic volatiles was greatly increased for normal harvested ‘Nanguoli’ fruit treated with MJ, exhibiting 53.75% and 66.82% increases with 1 and 5 mM MJ, respectively, while early harvested fruit experienced only slight increases following MJ treatment.
Kondo et al. (Citation2005) speculated that the effect of MJ on aromatic volatiles in apple fruit may depend on the growth stage of the fruit at the time of treatment. However, the present study is the first to examine the effects of exogenous MJ on aromatic volatile biosynthesis in pre-climacteric fruit at different maturity stages. The findings suggest that MJ has a greater effect on aromatic volatile biosynthesis in normal harvested fruit compared with early harvested fruit, indicating that the level of stimulation is dependent on the fruit maturity stage.
Different concentrations of MJ have previously been shown to have different effects on aromatic volatile biosynthesis, with the optimal concentration depending on the type of fruit and treatment method (Perez et al. Citation1997; Mukkun & Singh Citation2009). However, the two concentrations of MJ tested in this study (1 and 5 mM) produced similar effects. Thus, it can be concluded that only a small amount of exogenous MJ is required to trigger the biosynthesis of aromatic volatiles.
Effect of MJ treatment on ethylene production
Ethylene plays an essential role in regulating ripening and interrelated physiological activities in climacteric fruits. Increased ethylene production has previously been observed in ‘La France’ pear fruit treated with n-propyl dihydrojasmonate (PDJ), which is a jasmonic acid derivative (Kondo et al. Citation2007). Therefore, the present study investigated the effect of exogenous MJ on the ethylene production rate () in pre-climacteric fruit. Ethylene production rates were promoted by exogenous MJ in pre-climacteric ‘Nanguoli’ fruit harvested at different maturity stages, with a marked increase being observed in normal harvested fruit compared with only a slight increase in early harvested fruit ().
Figure 1. Ethylene production rate of ‘Nanguoli’ fruit treated with methyl jasmonate (MJ) in the presence or absence of 1-MCP. Values are the means ± SD of three replicates.
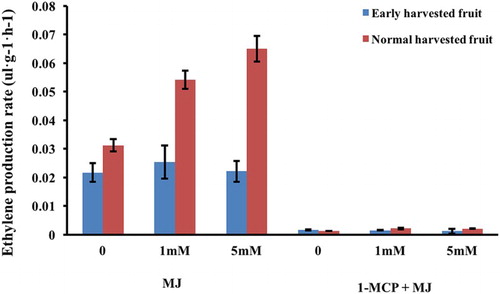
It has been reported that the effect of MJ on volatile production is related to its effect on the internal ethylene concentration, and that the effect of MJ on aromatic volatiles in apple fruit may be mediated by ethylene (Kondo et al. Citation2005). 1-MCP, a strong inhibitor of ethylene has been shown to inhibit ethylene production and inhibit the production of aroma volatile compounds in several climacteric fruit (Watkins, Citation2002). In the present study, exogenous MJ did not trigger the biosynthesis of ethylene and aromatic volatiles in ‘Nanguoli’ pear fruit that had been treated with 1-MCP ( and ), indicating that MJ stimulation of ‘Nanguoli’ pear fruit aromatic biosynthesis is associated with endogenous ethylene production, and so ethylene may trigger aromatic volatile biosynthesis.
Table 3. Aromatic volatile content of ‘Nanguoli’ fruit treated with I-MCP+ MJ at different maturity stages.
Figure S1 The main peak masses of the volatile compounds and the standard.
Download MS Word (679.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayala-Zavala JF, Wang SY, Wang CY, González-aguilar GA. 2005. Methyl jasmonate in conjunction with ethanol treatment increases antioxidant capacity, volatile compounds and postharvest life of strawberry fruit. European Food Research and Technology. 221:731–738. doi: 10.1007/s00217-005-0069-z
- Chen JL. 2005. Study on the aroma analysis, variation and physicochemical characteristic index of pears [graduation thesis]. Beijing: China Agricultural University.
- Creelman RA, Mullet JE. 1997. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 48:355–381. doi: 10.1146/annurev.arplant.48.1.355
- Dong P, Xin G, ZHang B, Feng F, Li TY. 2010. Effects of 1-MCP on aroma of Nanguo pear during storage at 20°C. Food Science. 31:477–479.
- Fan X, Mattheis JP, Fellman JK. 1998a. Responses of apples to postharvest jasmonate treatments. Journal of the American Society for Horticultural Science. 123:421–425.
- Fan X, Mattheis JP, Fellman JK. 1998b. A role for jasmonates in climacteric fruit ripening. Planta. 204:444–449. doi: 10.1007/s004250050278
- Fan X, Mattheis JP, Fellman JK, Patterson M. 1997. Effect of methyl jasmonate on ethylene and volatile production by summerred apples depends on fruit developmental stage. Journal of Agricultural and Food Chemistry. 45:208–211. doi: 10.1021/jf9603846
- Feng F, Xin G, ZHang B, Li TC, Li SQ. 2010. Changes in aroma components of Nanguo pear during post-harvest storage at 20°C. Food Science. 31:266–269.
- Gamrasni D, Ben-Ariea R, Goldway M. 2010. 1-Methylcyclopropene (1-MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biology and Technology. 58:104–112. doi: 10.1016/j.postharvbio.2010.05.007
- Kondo S, Setha S, Rudell DR, Buchanan DA, Mattheis JP. 2005. Aroma volatile biosynthesis in apples affected by 1-MCP and methyl jasmonate. Postharvest Biology and Technology. 36:61–68. doi: 10.1016/j.postharvbio.2004.11.005
- Kondo S, Tomiyama A, Seto H. 2000. Changes of endogenous jasmonic acid and methyl jasmonate in apples and sweet cherries during fruit development. Journal of the American Society for Horticultural Science. 125:282–287.
- Kondo S, Yamada H, Setha S. 2007. Effect of jasmonates differed at fruit ripening stages on 1-aminocyclopropane-1-carboxylate (ACC) synthase and ACC oxidase gene expression in pears. Journal of the American Society for Horticultural Science. 132:120–125.
- Lalel H, Singh Z, Tan S. 2003. The role of methyl jasmonate in mango ripening and biosynthesis of aroma volatile compounds. The Journal of Horticultural Science and Biotechnology. 78:470–484. doi: 10.1080/14620316.2003.11511652
- Lara I, Miró RM, Fuentes T, Sayez G, Graell J, López ML. 2003. Biosynthesis of volatile aroma compounds in pear fruit stored under long-term controlled-atmosphere conditions. Postharvest Biology and Technology. 29:29–39. doi: 10.1016/S0925-5214(02)00230-2
- Mukkun L, Singh Z. 2009. Methyl jasmonate plays a role in fruit ripening of ‘Pajaro’ strawberry through stimulation of ethylene biosynthesis. Scientia Horticulturae. 123:5–10. doi: 10.1016/j.scienta.2009.07.006
- Pelayo C, Ebeler S, Kadar A. 2003. Postharvest life and flavor quality of three strawberry cultivars kept at 5°C in air or air+ 20 kPa CO2. Postharvest Biology and Technology. 27:171–183. doi: 10.1016/S0925-5214(02)00059-5
- de la Peña Moreno F, Blanch GP, Flores G, Ruiz del Castillo ML. 2010a. Impact of postharvest methyl jasmonate treatment on the volatile composition and flavonol content of strawberries. Journal of the Science of Food and Agriculture. 90:989–994.
- de la Peña Moreno F, Monagas M, Blanch GP, Bartolomé B, Ruiz del Castillo ML. 2010b. Enhancement of anthocyanins and selected aroma compounds in strawberry fruits through methyl jasmonate vapor treatment. European Food Research and Technology. 230:989–999. doi: 10.1007/s00217-010-1243-5
- Perez AG, Olías R, Espada J, Olíias JM, Sanz C. 1997. Rapid determination of sugars, nonvolatile acids, and ascorbic acid in strawberry and other fruits. Journal of Agricultural and Food Chemistry. 45:3545–3549. doi: 10.1021/jf9701704
- Qin GH, Huang WJ, ZHang HP, Tao ST, Li JC, ZHang SL. 2012b. Effect of harvest time on aromatic components of fully ripe Nanguo pear fruits. Food Science. 33:248–252.
- Qin GH, Tao ST, Cao YF, Wu JY, ZHang HP, Huang WJ, ZHang SL. 2012a. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chemistry. 134:2367–2382. doi: 10.1016/j.foodchem.2012.04.053
- Rudell D, Mattheis J. 2008. Synergism exists between ethylene and methyl jasmonate in artificial light-induced pigment enhancement of ‘Fuji’ apple fruit peel. Postharvest Biology and Technology. 47:136–140. doi: 10.1016/j.postharvbio.2007.05.021
- Rudell D, Mattheis J, Fan X, Fellman J. 2002. Methyl jasmonate enhances anthocyanin accumulation and modifies production of phenolics and pigments in’Fuji’apples. Journal of the American Society for Horticultural Science. 127:435–441.
- Takeoka G, Buttery R, Flath R. 1992. Volatile constituents of Asian pear (Pyrus serotina). Journal of Agricultural and Food Chemistry. 40:1925–1929. doi: 10.1021/jf00022a040
- Watkins CB. 2002. Ethylene synthesis, mode of action, consequences and control. In: Knee M, editor. Fruit quality and its biological basis. Sheffield: Sheffield Academic Press; p. 180–224.
- Zhuang XH, Liu SY, Ma YS, Ma CL, Hu GJ, CHen X. 2007. Study on aroma component of pear cv. Nanguo. Storage & Process. 41:19–21.
